Abstract
Some species of the genus Passiflora have leaf morphological adaptations that grow to influence the development of the plant in producing areas. Hence, the objective of this work is to quantify and characterize the leaf anatomy of passion fruit species distributed in the South American region, which can become an important strategy in the selection of species more adapted to the environment where they will be grown. This work evaluates the abaxial and adaxial cuticle thickness (ABCT and ADCT), abaxial and adaxial epidermis thickness (ABET and ADET), xylem diameter (XD), phloem diameter (PD), and thickness of the palisade parenchyma (TPP), of the species Passiflora quadrangularis L., Passiflora foetida L., Passiflora edulis Sims, Passiflora gibertii N.E Brown, Passiflora coccinea Aubl, Passiflora alata Curtis, Passiflora tenuifila Killip, Passiflora caerulea L., and Passiflora cincinnata Mast. Passion fruit species present differences in leaf anatomy, which may influence the plant’s development. The species Passiflora quadrangularis L. showed a greater thickness of cuticles, epidermis, conducting vessels, and palisade parenchyma. The species Passiflora edulis has higher density and stomatal functionality. All Passiflora species formed druses on their leaves.
1. Introduction
The South America region has a great diversity of plants, and among them, the genus Passiflora stands out, as some species have characteristics of pharmaceutical interest due to the production of substances with an antioxidant role [1], in the cosmetics industry, whose target is the production of essential oils, in the food industry for the production of pulps and derivatives, and even in local shops for the consumption of fresh fruits [2,3]. Considering the economic importance of the crop and the need to improve productivity and fruit quality, some species have leaf morphological adaptations that develop on the plant growth in producing areas [4].
One adaptive structure that stands out is the cuticles. These are depositions of waxes that allow for the impermeability of organs such as leaves and fruits, providing resistance to the penetration of phytopathogenic microorganisms and even to agrochemicals such as herbicides [5,6]. Some species have thicker cuticles, making it an important factor in the selection of matrices in species’ breeding programs [4] to ensure varieties that are more adapted to environments with a high population of pathogens and transmissible vectors of viruses [7].
The epidermis also gains prominence in the leaf anatomy of plants, as stomata are found in them. Stomata are structures responsible for the exchange of O2 and CO2 that directly influence photosynthetic processes, in addition to acting in the regulation of transpiration when plants are under water stress with the stomatal closure [8]. These physiological responses of plants may interfere with stomatal density and functionality, as it enhances gas exchange and leaf transpiration due to the greater number of stomata per area and the opening of the cleft, respectively [9,10]. Thicker epidermis guarantees greater resistance to the attacks of pests and also diseases. However, they can influence the passage of light that falls on the palisade parenchyma. Thus, the understanding of these structures is a strategy for the selection of more adapted species [11].
The palisade parenchyma is a tissue that has chloroplast-rich cells in the shape of sticks or rods, thus making the tissue that presents high metabolic reactions, especially in photosynthetic processes, where the demand for light, CO2, and especially water, which is mostly transported by conducting vessels [12]. The increase in the size of parenchyma cells can enhance the process of carbon fixation, and at the same time guarantee a greater development of the plant, mainly in the production of dry mass, fruit development, and the quality of the pulps, due to the greater rise in sugars originating from photosynthetic processes [13,14].
The development of the conducting vessels provided the higher vascular plants the conquest of the terrestrial environment, which potentiated the distribution of water and minerals to all the tissues of the plant organism, with less energy expenditure, because, with the rise in transpiration, there is a difference in the internal hydrostatic potential of the vessels, and thus the movement of water in the xylem vessels overcomes the gravitational force [10,15]. Another function of the conducting vessels is the transverse growth, which increases the stem diameter, making this organ more robust and capable of withstanding a high amount of dry mass of leaves, branches, flowers, and fruits, in addition to the weather conditions of the environment, such as wind and fire. This phenomenon is very common in Brazilian savannas, where many species of passion fruit originated [16]. It is important to highlight that the differences visible to the naked eye in the morphology of plant organs are, in fact, derived from modifications in the structures of the dermal, fundamental, or vascular tissues of plants, making it necessary to have in-depth knowledge of these transformations motivated by variations in the environment.
Hence, the objective of this work was to quantify and characterize the leaf anatomy of passion fruit species distributed in the South America region, which can become an important strategy in the selection of species more adapted to the environment where they will be grown.
2. Material and Methods
2.1. Area Characterization
The experiment was set in January 2021 on a farm located in the municipality of Adamantina in the state of São Paulo, Brazil, within the following geographic coordinates, 21°28′ 15.568″ S and 51°2′53.077″ W, at an approximate altitude of 414 m. The climate of the region is characterized as Aw according to Köppen and Geiger, with a rainy summer and dry winter, an average temperature of 22.1 °C, and rainfall of 1204 mm per year.
The soil of the area was classified as Dark-Red Argisol [17] with good drainage. The chemical attributes are shown in Table 1.

Table 1.
Chemical attributes of the soil in the experimental planting.
Dolomitic limestone was applied in the total area, and planting was carried out after 15 days of its application. Planting fertilization was carried out according to the requirements of the crop [18].
2.2. Statistical Design and Conduct of the Experiment
The experimental design used randomized blocks with nine species of passion fruit, as described in the following Table 2:

Table 2.
Description of the geographic distribution of passion fruit species in the South America region.
Each species had 4 replications, totaling 36 plants or plots. The planting spacing of the passion fruit seedlings was 2.0 × 3.0 m, totaling 1666 plants per hectare. The seedlings were purchased from a commercial nursery in the municipality. They were with five fully expanded leaves, conducted on a wire 2.0 m above ground level, and drip irrigated.
2.3. Morphological Analyzes
The morphology parameters were determined in the plants at the flowering stage, so a fragment of the first fully expanded leaf was collected from the apex of four plants. All fragments received the procedures regarding ethanolic dehydration, diaphanization with xylene, inclusion in histological paraffin, and packaging in histological paraffin. All of the chosen sections were fixed with Mayer adhesive, stained with 1% safranin, and mounted on slides and cover slips with Entellan adhesive. All of the slides were observed under an Olympus optical microscope with a coupled camera to measure the anatomical parameters using the CellSens Standard image analysis program, which was calibrated with a microscopic rule at the same zoom level as the photographs where the following tissues were measured: abaxial and adaxial cuticle thickness (ABCT and ADCT); abaxial and adaxial epidermis thickness (ABET and ADET); xylem diameter (XD); phloem diameter (PD); and thickness of the palisade parenchyma (TPP) [14]. The lower or abaxial epidermal impression of the fragments, collected using cyanoacrylate ester [22], was used to determine the stomatal density (SD) and stomatal functionality (SF) [10]. For all variables, ten measurements were made per slide, totaling forty measurements per species.
It was also observed, in the internal tissues of the leaves, whether there was the formation (yes or no) of druses (D), with the aid of an optical microscope of polarized light.
2.4. Statistical Analysis
For statistical evaluation, the variables were subjected to normality tests, where the Shapiro–Wilk test was used. After meeting the test precepts, an analysis of variance was performed using the F test (p < 0.05), and their means were grouped using the Scott–Knott method [23]. The RStudio statistical program R was also used [24].
3. Results
The species P. coccinea showed the greatest thickness of the abaxial cuticle, with approximately 44.09% superior thickness in relation to the species P. gibertii, which presented the smallest average value. For the thickness of the adaxial cuticle, the species that presented the highest average values were P. quadrangularis; P. alata, and P. caerulea, where the species P. quadrangularis was the one that stood out most among them, being 59.59% thicker in relation to the species P. tenuifila, which presented the lowest mean, as shown in Table 3.

Table 3.
Value of the means of the abaxial cuticle thickness (ABCT); adaxial cuticle thickness (ADCT); abaxial epidermis thickness (ABET); adaxial epidermis thickness (ADET) of passion fruit species.
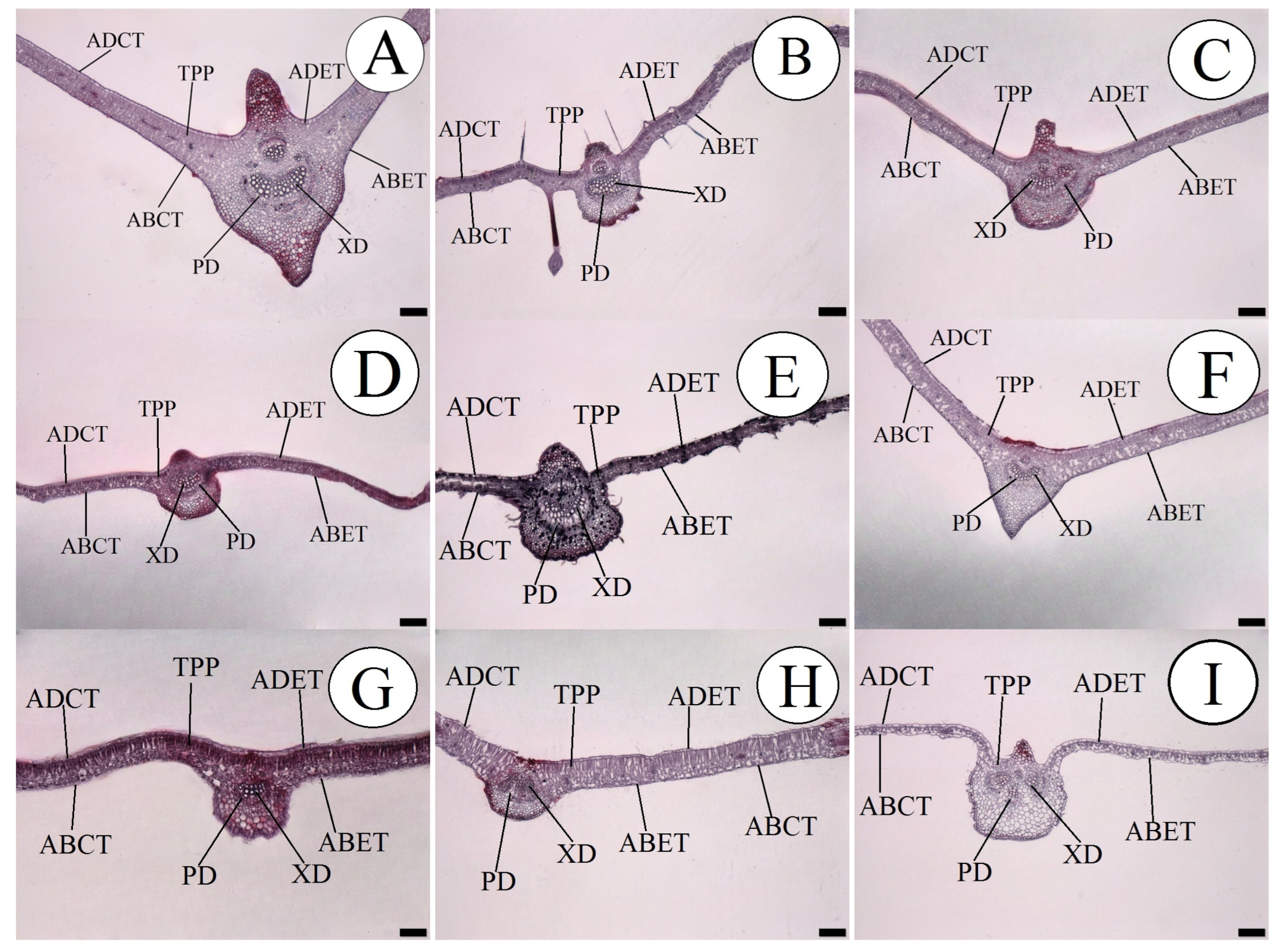
Greater development of the internal morphological structures of the leaf of the species P. quadrangularis was seen, as shown in Figure 1.
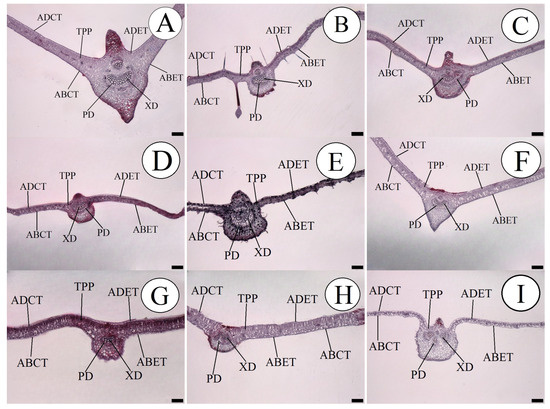
Figure 1.
Cross-section in leaves of the passion fruit species, showing the measured internal tissues. (A) P. quadrangularis; (B) P. foetida; (C) P. edulis; (D) P. gibertii; (E) P. coccínea; (F) P. alata; (G) P. tenuifila; (H) P. caerulea and (I) P. cincinnata. ABCT—Abaxial cuticle thickness; ADCT—Adaxial cuticle thickness; ABET—Abaxial epidermis thickness; ADET—Adaxial epidermis thickness; XD—Xylem diameter; PD—Phloem diameter; and TPP—Thickness of the palisade parenchyma. Bar = 200 µm.
Once more, the species P. quadrangularis, P. alata, and P. tenuifila stood out among the species for the thickness of the abaxial epidermis, and they were also similar for the thickness of the adaxial epidermis, where the species P. quadrangularis and P. alata were those with the highest mean values, as shown in Table 3.
A statistical difference was found between the passion fruit species in the xylem diameter, where P. quadrangularis, P. foetida, P. edulis, P. coccinea, and P. alata were the species that presented the highest mean values. The species P. quadrangularis was the one that presented the largest xylem diameter with 48.56%, which was higher than the species P. tenuifila, which showed the lowest mean. Similarly, differences were observed between the passion fruit species for the phloem diameter, where the species P. quadrangularis, P. coccinea, and P. alata presented the highest means, as shown in Table 4.

Table 4.
Mean values of xylem diameter (XD), phloem diameter (PD), and thickness of the palisade parenchyma (TPP), stomatal density (SD), and stomatal functionality (SF) of passion fruit species.
Once more, the species P. quadrangularis, together with P. cincinnata, stood out with the highest means for the thickness of the palisade parenchyma, where P. quadrangularis presented a difference of approximately 54.34% higher in relation to the species P. coccinea, as highlighted in Table 4.
Also, the species P. quadrangularis, P. alata, and P. tenuifila stood out among the species in the thickness of the abaxial epidermis, and also similarly for the thickness of the adaxial epidermis, where the species P. quadrangularis and P. alata were those with the highest average means, as shown in Table 3.
A statistical difference was found among the passion fruit species in the xylem diameter, where P. quadrangularis, P. foetida, P. edulis, P. coccinea, and P. alata were the species that presented the highest mean values. The species P. quadrangularis was the one that presented the largest xylem diameter, being 48.56% higher than the species P. tenuifila, which presented the lowest mean. Similarly, differences were observed between the passion fruit species for the phloem diameter, where the species P. quadrangularis, P. coccinea, and P. alata presented the highest means, as shown in Table 4.
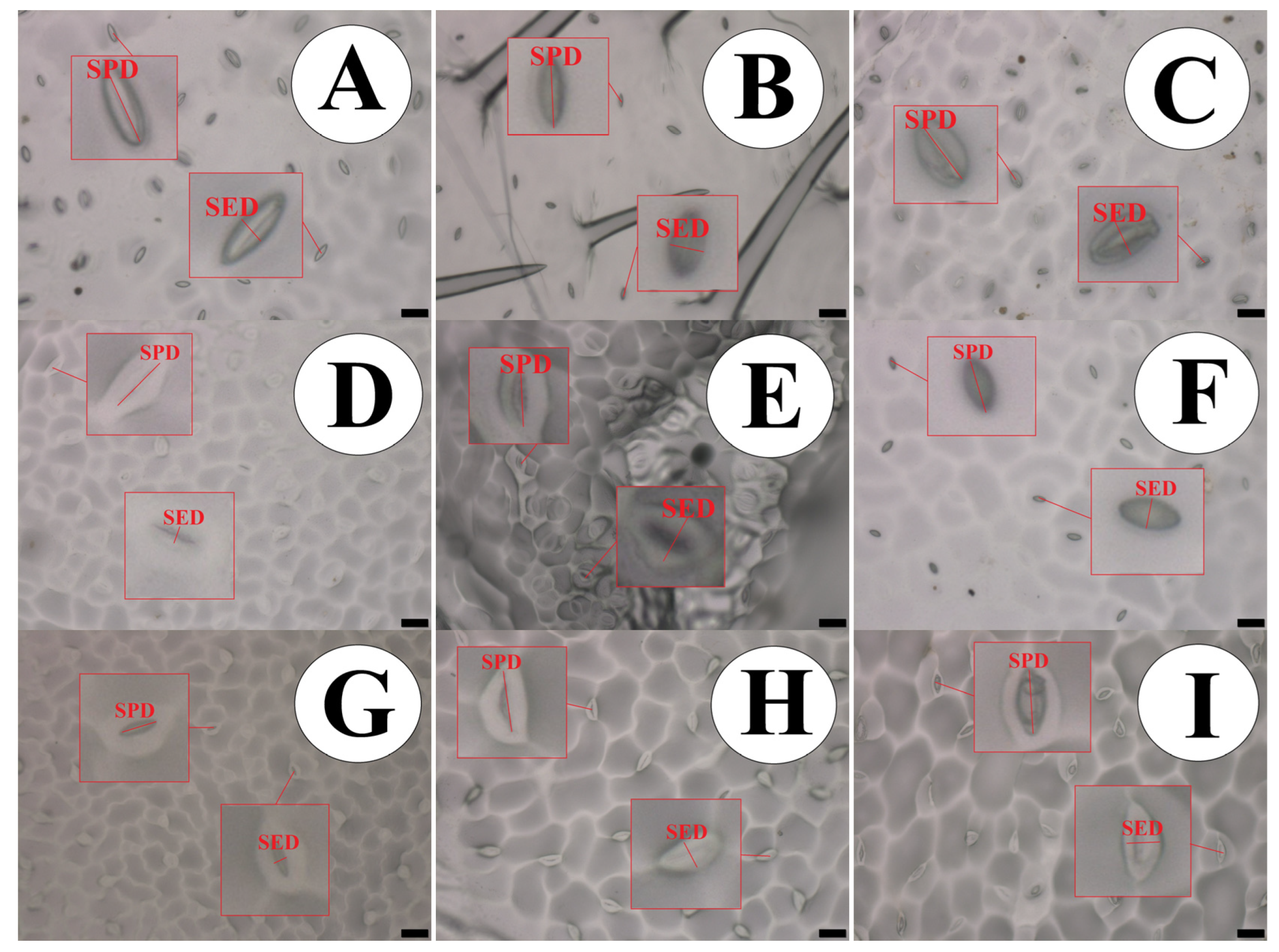
The species P. edulis presented the highest density and stomatal functionality, which was implied to be approximately 91.62% higher than the species P. caerulea, as shown in Table 4. This difference can be illustrated in Figure 2.
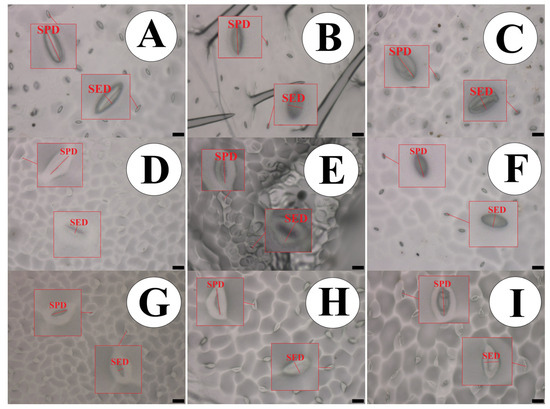
Figure 2.
Impression of the abaxial epidermis in leaves of the passion fruit species, showing the measured structures. (A) P. quadrangularis; (B) P. foetida; (C) P. edulis; (D) P. gibertii; (E) P. coccínea; (F) P. alata; (G) P. tenuifila; (H) P. caerulea and (I) P. cincinnata. Bar = 50 µm. SPD—Stomatal Polar Diameter and SED—Stomatal Equatorial Diameter. Bar = 20 µm.
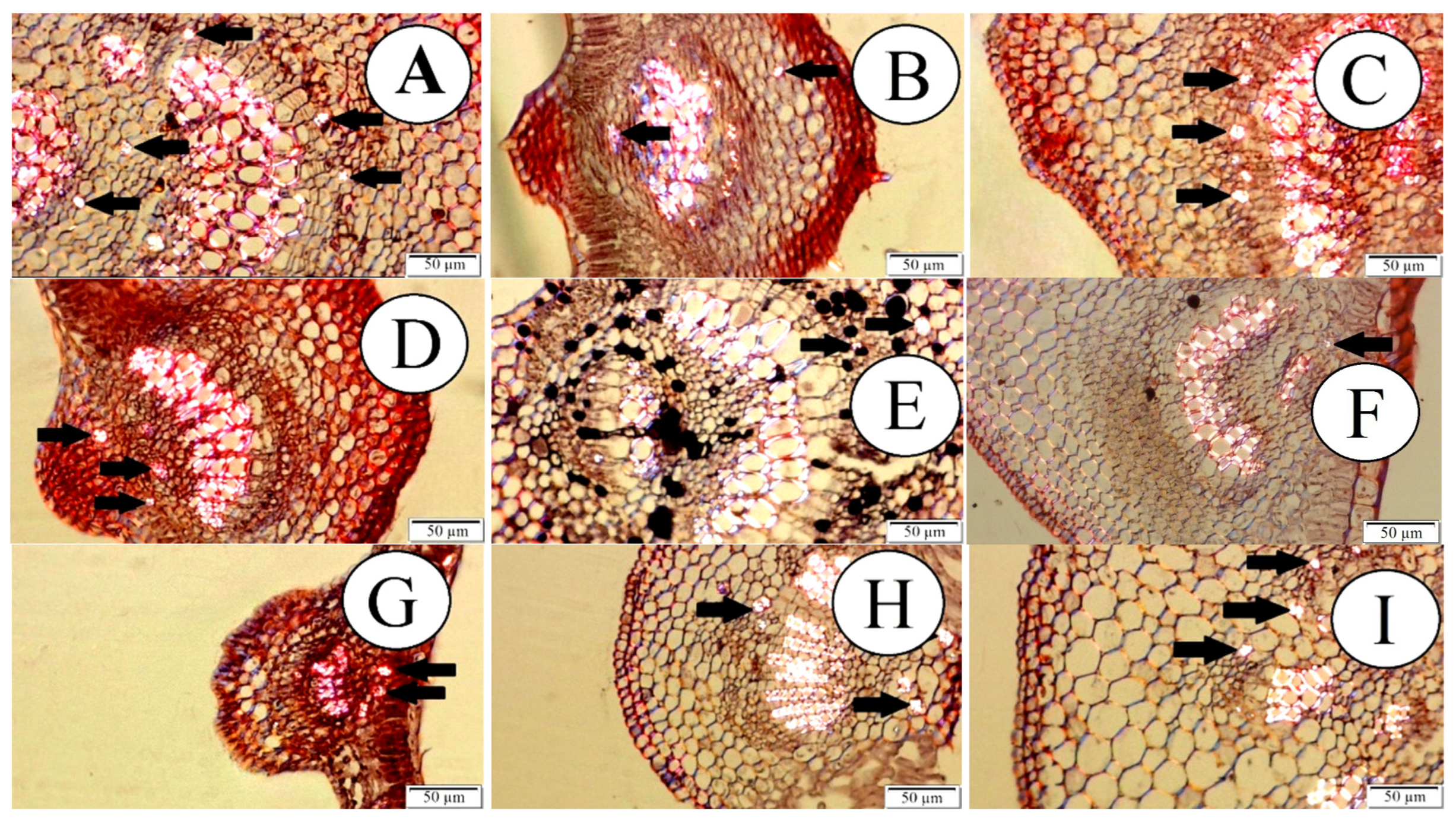
All passion fruit species showed the formation of druses in the internal tissues of their leaves, as shown in Figure 3.

Figure 3.
Internal tissues of passion fruit leaves observed in polarized optical microscopy. Black arrows point to druses formation. (A) P. quadrangularis; (B) P. foetida; (C) P. edulis; (D) P. gibertii; (E) P. coccínea; (F) P. alata; (G) P. tenuifila; (H) P. caerulea; (I) P. cincinnata. Bar = 50 µm.
Further investigations with native species of the genus Passiflora are needed in order to ensure a better understanding of the morphological responses of these plants to the production environment, and even to the selection of characteristics of economic interest for breeding programs for passion fruit species.
4. Discussion
These differences between cuticle thicknesses can eventually be a strategy in genetic improvement programs to obtain hybrids that aim at greater resistance to pest and disease attacks, as the cuticle composed of waxes is an adaptive structure of plants that acts as a mechanism of defense, providing impermeabilization to the leaves [6,25].
A thicker epidermis can provide a better balance in leaf transpiration, as many species are endemic to semi-arid environments where long periods of water shortage are common [26]. Fires can also occur in native vegetation in certain months, in which this tissue plays a defensive role as a physical barrier protecting the innermost tissues of the leaf limbo [27]. Even though the species P. cincinnata does not have the largest epidermal thicknesses, this species stands out as originating from this arid region.
Plant species with larger conducting vessels can demonstrate greater development due to the internal rise of the motive force of the liquids and also the more efficient distribution of sap to all the organs in the plant. This adaptive efficiency can be well observed when some species subjected to water stress show a prevalence, as observed by [26,28], and also about nutritional restriction, as pointed out by [14]. This difference can be well illustrated in Figure 1, demonstrating a thicker and more well-developed midrib of the leaf. In addition to the diameter of the conducting vessels, it should be seen that the number of vessels can influence the efficiency of transport of internal solutes, and so it is important to understand these morphological differences between species which direct which characteristic is the most important in plant breeding, selecting species that have a greater number or diameter of vessels.
A thicker epidermis can provide a better balance in leaf transpiration, as many species are endemic to semi-arid environments where long periods of water shortage are common [26]. Fires can also occur in native vegetation at certain months, in which this tissue plays a defensive role as a physical barrier protecting the innermost tissues of the leaf blade [27].
Morphological adaptations develop into an important strategy for plant species to survive the environment in which it was inserted, as the presence of well-developed palisade parenchyma can guarantee a higher rate of photosynthesis due to greater photon capture in the photosynthesis process; thus, absorption and accumulation of carbon in its dry mass may increase, therefore enhancing plant growth [29]. This increase in the need for greater assimilation of carbon gas demands a good stomatal apparatus in the leaves [28]. It is worth highlighting that the anatomical adaptations of the genus Passiflora are directly linked to the genetic phallus of each species, where polyploid individuals demonstrate more robust vegetative and reproductive vitality than their diploid relatives with notably larger organs [30,31]. It is worth noting that the Passiflora species is found in savannah regions, and has adaptations that help it survive in these environmental conditions. However, it does not have the largest leaf tissues [32].
This makes more studies necessary in the area of genetics and its actions on the anatomical adaptations of each species to the environment being cultivated. Beneficial phenotypes in wild species can be transferred to relatives through crossing, somatic hybridization, or even genetic engineering, with the production of transgenics [30]. Wild relatives can also be used as rootstocks for grafting, highlighting the species P. gibertii, which is widely used in Brazilian trade as a rootstock [26].
Density, distribution, and differentiation are important modes of stomatal function that may reflect on the growth and survival of plants when they are in the environment under either favorable conditions or water stress. These changes can affect the uptake of carbon dioxide and even the loss of water through stomatal conductance, which directly interferes with the internal temperature of the leaf. This thermal regulation is one of the functions of the cleft or opening of the stomata. This corroborates with [26], who studied passion fruit under water restriction. The stomata of the species were classified as anomocytic, while the trichomes were classified as tector type with a single cell [10,31]. When the plant is under water stress, the ratio between the polar and equatorial diameter of the guard cells makes them flaccid, therefore altering the elasticity of these cells [33,34], which results in a smaller equatorial diameter, and so the ostiole remains closed so that the leaf’s water potential is constant within the leaf [35].
The formation of these structures occurs through the deposition and accumulation of calcium silicates and oxalates, and after the formation of this structure, it presents hardness and resistance, providing greater protection for these plants to the attack of insects that have scraper and cutter mouthpieces [10,16]. The importance of adequate fertilization at the planting of the seedlings, which can be complemented with the addition of silicon, should be observed. The formation of drusen in these passion fruit species can be enhanced as a consequence.
5. Conclusions
The species Passiflora quadrangularis L. has a greater thickness of cuticles, epidermis, conducting vessels, and palisade parenchyma. The species Passiflora edulis has higher density and stomatal functionality. All Passiflora species formed druses on their leaves.
Author Contributions
Implementation and conduct of the experiment, J.C.C., T.d.S.F. and A.d.S.; analysis, data collection, data tabulation, initial manuscript writing, L.A.M.L. and J.C.C.; writing—review, P.A.M.d.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Konta, E.M.; Almeida, M.R.; Amaral, C.L.; Darin, J.D.C.; Rosso, V.V.; Mercadante, A.Z.; Antunes, L.M.G.; Bianchi, M.L.P. Evaluation of the antihypertensive properties of yellow passion fruit pulp (Passiflora edulis Sims f. flavicarpa Deg.) in spontaneously hypertensive rats. Phytother. Res. 2013, 28, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Miyake, R.T.M.; Furlaneto, F.P.B.; Narita, N.; Takata, W.H.S.; Creste, J.E. Economic evaluation of different types of nutritional management in yellow passion fruit vines (Passiflora edulis Sims.). Aust. J. Crop Sci. 2016, 10, 1572–1577. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Jorge, N. Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): Physical and chemical characteristics. Braz. Arch. Biol. Technol. 2012, 55, 127–134. [Google Scholar] [CrossRef]
- Martínez, M.A.; Morillo, A.C.; Reyes-Ardila, W. Characterization of the genetic diversity in Passiflora spp. in the Boyacá Department, Colombia. Chil. J. Agric. Res. 2020, 80, 342–351. [Google Scholar] [CrossRef]
- Carvalho, C.R.V.; Mapeli, A.M.; Oliveira, A.B. Anatomical characterization of Passiflora cincinnata Mast. fruit subjected to refrigeration. Rev. Bras. Frutic. 2021, 43, 1698. [Google Scholar] [CrossRef]
- Bhanot, V.; Fadanavis, S.V.; Panwar, J. Revisiting the architecture, biosynthesis and functional aspects of the plant cuticle: There is more scope. Environ. Exp. Bot. 2021, 183, 104364. [Google Scholar] [CrossRef]
- Ibaba, J.D.; Gubba, A. High-Throughput Sequencing Application in the Diagnosis and Discovery of Plant-Infecting Viruses in Africa, A Decade Later. Plants 2020, 9, 1376. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Montaña, P.A.; Sarmiento, F.; Mejía-Sequera, L.M.; Álvarez-Flórez, F.; Melgarejo, L.M. Physiological, biochemical and transcriptional responses of Passiflora edulis Sims f. edulis under progressive drought stress. Sci. Hortic. 2021, 275, 9655. [Google Scholar] [CrossRef]
- Lima, L.K.S.; Jesus, O.N.; Soares, T.L.; Santos, I.S.; Oliveira, E.J.; Coelho Filho, M.A. Growth, physiological, anatomical and nutritional responses of two phenotypically distinct passion fruit species (Passiflora L.) and their hybrid under saline conditions. Sci. Hortic. 2020, 263, 9037. [Google Scholar] [CrossRef]
- Castro, E.M.; Pereira, F.J.; Paiva, R. Histologia Vegetal: Estrutura e Função de Órgãos Vegetativos; UFLA: Lavras, Brazil, 2009; p. 234. [Google Scholar]
- Sorensen, H.K.; Fanourakis, D.; Tsaniklidis, G.; Bouranis, D.; Nejad, A.R.; Ottosen, C. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in Passiflora. Sci. Hortic. 2020, 267, 9354. [Google Scholar] [CrossRef]
- Gonçalves, Z.S.; Lima, L.K.S.; Soares, T.L.; Souza, E.H.; Jesus, O.N. Leaf anatomical aspects of CABMV infection in Passiflora spp. by light and fluorescence microscopy. Australas. Plant Pathol. 2021, 50, 203–215. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; p. 858. [Google Scholar]
- Lisboa, L.A.M.; Cavichioli, J.C.; Vitorino, R.; Figueiredo, P.A.M.; Viana, R.S. Nutrient suppression in passion fruit species: An approach to leaf development and morphology. Colloq. Agrar. 2021, 17, 89–102. [Google Scholar] [CrossRef]
- Brodersen, C.R. Finding support for theoretical tradeoffs in xylem structure and function. New Phytol. 2015, 209, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.S.; Soares, T.L.; Lima, L.K.S.; Gheyi, H.R.; Dias, E.A.; Jesus, O.N.; Coelho Filho, M.A. Effects of salinity on growth, physiological and anatomical traits of Passiflora species propagated from seeds and cuttings. Braz. J. Bot. 2021, 44, 17–32. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. Sistema Brasileiro de Classificação de Solos, 3rd ed.; EMBRAPA: Brasília, Brazil, 2013; p. 353. [Google Scholar]
- Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem para o Estado de São Paulo, 2nd ed.; IAC: Campinas, Brazil, 1996; p. 285. [Google Scholar]
- Killip, E.P. The American Species of Passifloraceae; Botanical Series; Field Museum of Natural History: Chicago, IL, USA, 1938. [Google Scholar] [CrossRef]
- Bernacci, L.C. Passifloraceae. In Flora Fanerogâmica do Estado de São Paulo; FAPESP: São Paulo, Brazil, 2003; pp. 247–248. [Google Scholar]
- Bernacci, L.C.; Meletti, L.M.M.; Soares-Scott, M.D. Maracujá-doce: O autor, a obra e a data da publicação de Passiflora alata (Passifloraceae). Rev. Bras. Frutic. 2003, 25, 355–356. [Google Scholar] [CrossRef]
- Segatto, F.B.; Bisognin, D.A.; Benedetti, M.; Costa, L.C.; Rampelotto, M.V.; Nicoloso, F.T. A technique for the anatomical study of potato leaf epidermis. Ciência Rural 2004, 34, 1597–1601. [Google Scholar] [CrossRef]
- Banzatto, D.A.; Kronka, S.N. Experimentação Agrícola, 4th ed.; Funep: São Paulo, Brazil, 2013; p. 237. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 1 January 2020).
- Ichiishi, K.; Ekino, T.; Kanzaki, N.; Shinya, R. Thick cuticles as an anti-predator defence in nematodes. Nematology 2021, 24, 11–20. [Google Scholar] [CrossRef]
- Contiero, L.F.; Cavichioli, J.C.; Lisboa, L.A.M.; Vitorino, R.A.; Ramos, S.B.; Figueiredo, P.A.M. Water stress in passion fruit cropping: An approach to its development. Rev. Eng. Agric. 2021, 29, 245–253. [Google Scholar] [CrossRef]
- Scremin-Dias, E.; Da Silva, J.R.; Catian, G.; Fabiano, V.S.; Arruda, R.C.O. Plant Morphoanatomical Adaptations to Environmental Conditions of the Pantanal Wetland. In Flora and Vegetation of the Pantanal Wetland. Plant and Vegetation; Damasceno-Junior, G.A., Pott, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 609–636. [Google Scholar] [CrossRef]
- Cavichioli, J.C.; Lisboa, L.A.M.; Vitorino, R.A.; Contiero, L.A.F.; Figueiredo, P.A.M.; Rocha, E.A. Physiological Parameters and Development of Passion Fruit Subjected to Water Stress and Propagation Methods. Braz. Arch. Biol. Technol. 2022, 65, 145. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Wai, M.H.; Rizwan, H.M.; Qarluq, A.Q.; Xu, M.; Wang, L.; Cheng, Y.; Aslam, M.; Zheng, P.; Wang, X. Advances in micropropagation, somatic embryogenesis, somatic hybridizations, genetic transformation and cryopreservation for Passiflora improvement. Plant Methods 2023, 19, 50. [Google Scholar] [CrossRef]
- Yockteng, R.; D’eeckenbrugge, G.C.; Souza-Chies, T.T. Passiflora. In Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin/Heidelberg, Germany, 2011; pp. 129–171. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 2nd ed.; Wiley: Hoboken, NJ, USA, 1965; p. 607. [Google Scholar]
- Souza, D.T.A. Biodiversidade de polinizadores em Passiflora cincinnata Mast. (Passifloraceae), em Ribeirão Preto, SP, Brasil. Zootec. Trop. 2011, 29, 17–27. [Google Scholar]
- Souza, E.V.; Andrade, G.C.; Araújo, H.H.; Pereira, J.D. Structural plasticity in leaves of Schinus terebinthifolius (Anacardiaceae) populations from three contrasting tropical ecosystems. J. Torrey Bot. Soc. 2022, 149, 187–193. [Google Scholar] [CrossRef]
- Nadal, M.; Roig-Oliver, M.; Bota, J.; Flexas, J. Leaf age-dependent elastic adjustment and photosynthetic performance under drought stress in Arbutus unedo seedlings. Flora 2020, 271, 1662. [Google Scholar] [CrossRef]
- Lavergne, A.; Sandoval, D.; Hare, V.J.; Graven, H.; Prentice, I.C. Impacts of soil water stress on the acclimated stomatal limitation of photosynthesis: Insights from stable carbon isotope data. Glob. Chang. Biol. 2020, 26, 7158–7172. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).