Abstract
Plant cell and tissue culture have been used as the alternative and potential renewable source for the production of valuable phytochemicals. Elicitation offers a reliable in vitro approach to produce or enhance potential phytochemicals. α-tocopherol, which is an isoform of vitamin E, is a potent fat-soluble phytochemical known in nature. The present study focused on enhancing the production of α-tocopherol in the cell suspension culture through an elicitation approach. Suspension cultures of Nicotiana tabacum were established from the leaf disk-derived callus. The cell suspension cultures were treated with different elicitors (methyl jasmonate, salicylic acid, and yeast extract) at the lag phase of the cell growth cycle. The effects of elicitors on cell cultures were determined in terms of biomass, and α-tocopherol enhancement was determined using high-performance liquid chromatography (HPLC). Different elicitors depending on the concentration exerted different effects on cell growth and α-tocopherol production. Methyl jasmonate treatment showed the significantly highest increase in α-tocopherol on the 6th day of treatment in tobacco suspension cultures. Methyl jasmonate at the concentration of 150 μM enhanced α-tocopherol content to 24-fold over the control. This study clearly shows that the elicitors had the potential to increase the accumulation of α-tocopherol considerably in tobacco cell cultures. The outcomes of this study could be of considerable importance to the nutraceutical and pharmaceutical industries.
1. Introduction
Plants produce a range of complex secondary metabolites that play major roles in their primary functions. Plants are the key source of many medicinally important compounds. Several phytochemical compounds are used as pharmaceuticals, pigments, agrochemicals, herbicides, flavors, and perfumes [1,2]. Plant cell suspension cultures have been used for the production of various valuable phytochemicals. Suspension cultures from different plant species have been used to produce various metabolites in a safe, affordable, and environmentally friendly manner [3]. Elicitors and precursor feeding are the important strategies employed in the in vitro cell cultures in order to enhance bioactive compounds in plants such as alkaloids, terpenes, flavonoids, steroids, phenolics, and other secondary metabolites within a short time [4,5]. The process of triggering and inducing the biosynthetic pathways for the production of metabolites is known as elicitation, and the substances used for the elicitation are called elicitors [4]. The application of elicitors boosts the accumulation of various bioactive compounds [6]. Earlier reports showed that the addition of elicitors like methyl jasmonate and salicylic acid in the culture medium enhanced the various metabolites production [7,8,9].
Tocopherols or vitamin E are lipid-soluble, essential dietary antioxidants for mammals, which are synthesized by photosynthetic organisms. Of the four known tocopherols, α-, β-, γ-, and δ, α-tocopherol is the major form of vitamin E present in plant cells, which exhibits the highest biological activity [10]. The production of tocopherol in plants is essential for their protection against oxidative stresses and provides nutritional benefits to humans. Vitamin E is also commercially used in the cosmetic and nutraceutical industries. As a member of plant secondary metabolites, tocopherols display diverse biological and physiological properties and are essential components of human health. The recommended dietary allowance for α-tocopherol is 15 mg for adults and 19 mg for lactating mothers. Studies have shown the role of vitamin E in delaying the onset of degenerative diseases and cancer. Hence, there is a great interest in its dietary supplements, and considerable efforts have been directed toward their biofortification in food crops. Due to its health-promoting properties and economic importance, extensive research has been performed to understand the vitamin E biosynthesis in plants, and the genes involved in the production of tocopherols have been elucidated. The demand for vitamin E has increased in recent times, and this rising market demand, in turn, has driven research on the sustainable production of vitamin E in plants. By utilizing metabolic engineering and precursor feeding, α -tocopherol production has been enhanced [11,12,13,14,15,16]. In this study, our main aim was to study the effect of different elicitors such as methyl jasmonate, salicylic acid, and yeast extract on the α-tocopherol accumulation in in vitro cultured cells of tobacco.
2. Materials and Methods
2.1. Suspension Culture
The callus was induced from tobacco leaf explants in Murashige and Skoog medium (MS) supplemented with 0.1 mg/L indole-3-acetic acid (IAA) + 2 mg/L Benzylaminopurine (BAP). The 2-months-old healthy callus of tobacco was used for initiating the suspension cultures. The suspension cultures were obtained by transferring (1 g) of crumbled callus culture into 50 mL of liquid MS medium with 0.1 mg/L IAA + 2 mg/L BAP and incubated in the rotary shaker at 110 rpm at 24 °C for 4 weeks under continuous light.
2.2. Growth Assessment of Suspension Culture
The growth analysis of cells in cell suspension cultures was assessed through fresh weight. A known volume of a uniformly dispersed suspension culture was transferred to a 15 mL marked centrifuge tube and centrifuged at 500 rpm for 5 min. After centrifugation, the supernatant was discarded, and the growth rate/growth index of the cell was assessed by the accumulated biomass. The accumulated biomass corresponds to the difference between the final and the initial mass in terms of fresh weight [17]. This was recorded as per the following formula:
2.3. Subculture
To obtain the fine cell suspension (single cells), subculturing was carried out every 20 days, and during maximum cell aggregation, 5 mL of packed cells were transferred to 45 mL fresh medium and further incubated. During the 4th subculture, different elicitors were supplemented at different concentrations on the 20th day, and 6 days later, the cells were harvested and α-tocopherol levels were quantified to determine the effects [15,18].
2.4. Preparation of Elicitors
Methyl jasmonate (MeJA) (Sigma Aldrich, St. Louis, MO, USA) was dissolved in 99% ethanol. A 10 mM stock solution was prepared. This solution was added to the autoclaved media after filter-sterilization (0.22 μm filter) to give a final concentration of 50, 100, 150, and 200 μM. Salicylic acid (SA) (Sigma Aldrich, St. Louis, MO, USA) was dissolved in deionized water, and a stock of 10 mM was prepared. This SA solution was added to the autoclaved media after filter-sterilization (0.22 μm filter) to give final concentrations of 50, 100, 150, and 200 μM. Yeast extract (YE), (Sigma Aldrich, St. Louis, MO, USA) was prepared by dissolving in distilled water, and it was autoclaved and added to the culture medium to give final concentrations of 50, 100, 150, and 200 mg/L.
2.5. Extraction of α-Tocopherol
The suspension culture was harvested by transferring the culture to an oak ridge tube and centrifuged at 3000 rpm for 15 min. The supernatant was discarded, and the pellet was briefly dried and weighed. The cells were suspended in methanol (2 mL) and sonicated for about 5 min at 20 Hz for cell disruption. The methanolic extract was filtered through a 0.22 μm filter and used for quantification using HPLC (Shimadzu, Kyoto, Japan).
2.6. Quantification of α-Tocopherol by HPLC
An HPLC system with the photodiode array detector (PDA) was used for the analysis. The samples were separated in a column, phenomenex C18, 5 μm (250 × 4.6 mm). Methanol (Merck, Darmstadt, Germany; HPLC grade) was used as the mobile phase. The flow rate was 0.5 mL/min, the injection volume was 20 μL, and the chromatogram was monitored at 292 nm. The peak purity of the tested sample was determined by comparing its ultraviolet (UV) spectra to those of the reference standards. Quantification was performed on the basis of the corresponding peak area recorded by chromatopac c-R6A (Shimadzu, Kyoto, Japan). α-tocopherol and γ-tocopherol (Sigma Aldrich, St. Louis, MO, USA) were used as the reference standards.
3. Results
3.1. Effect of Different Elicitors on Cell Growth
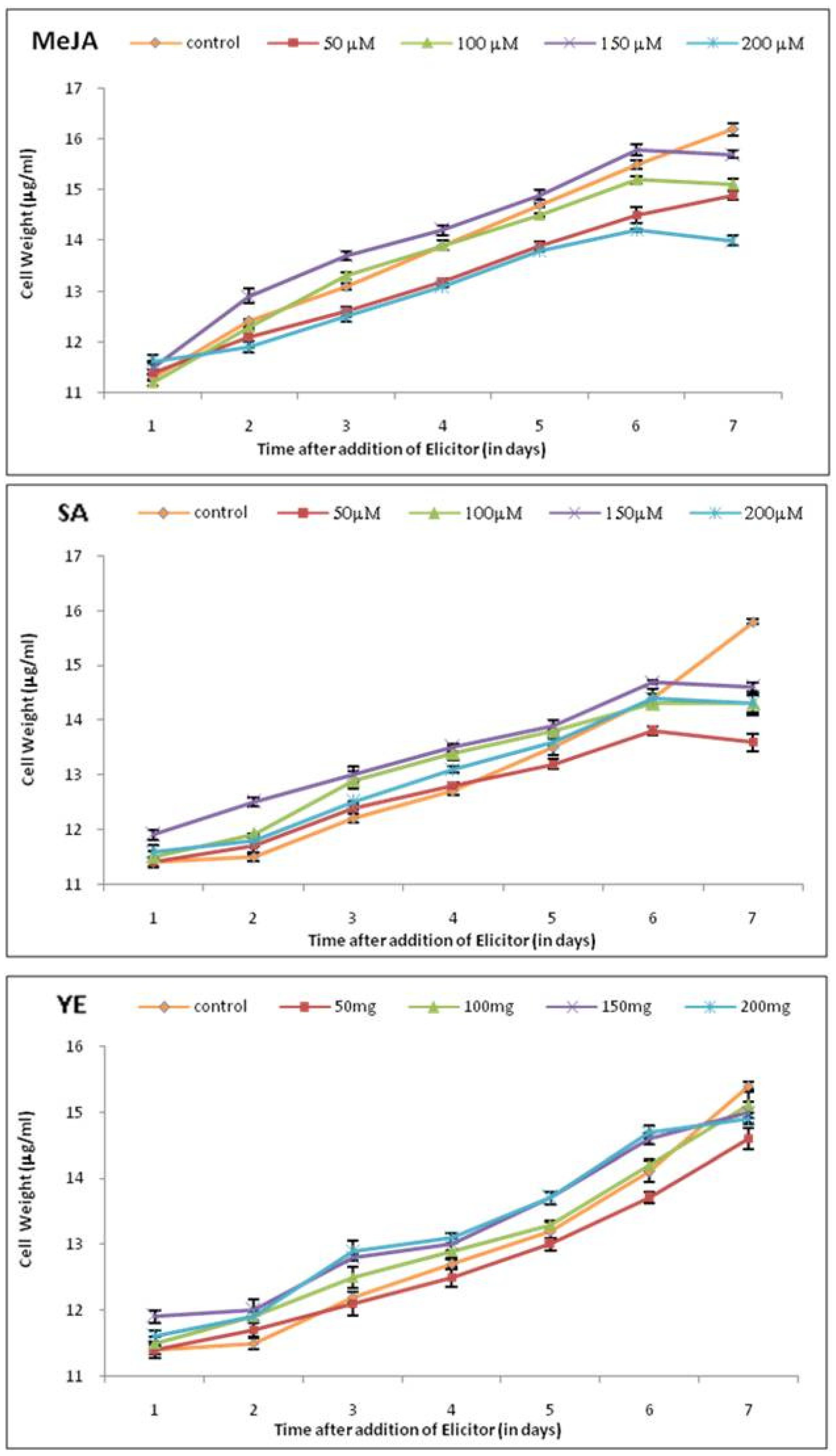
MeJA, SA, and YE were added to the cell suspension cultures of tobacco on the 20th day, corresponding to the lag phase of cell growth, and the effect of different concentrations of elicitors was investigated. The addition of MeJA, SA, and YE had no effects on cell growth till the 6th day. After the 6th day of treatment with elicitors, cell growth was almost uniformly decelerated for all the tested elicitors. A decrease in cell growth of up to 8% for tobacco cultures was noted over their controls (Figure 1 and Figure 2).
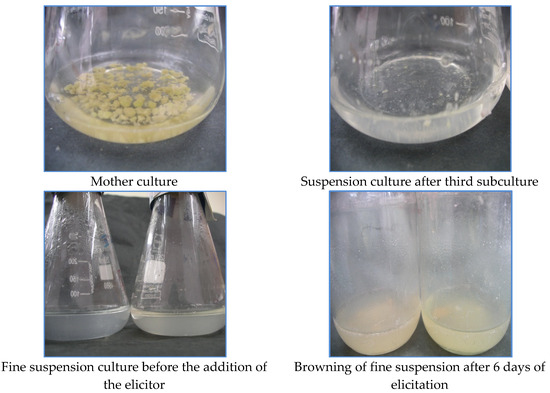
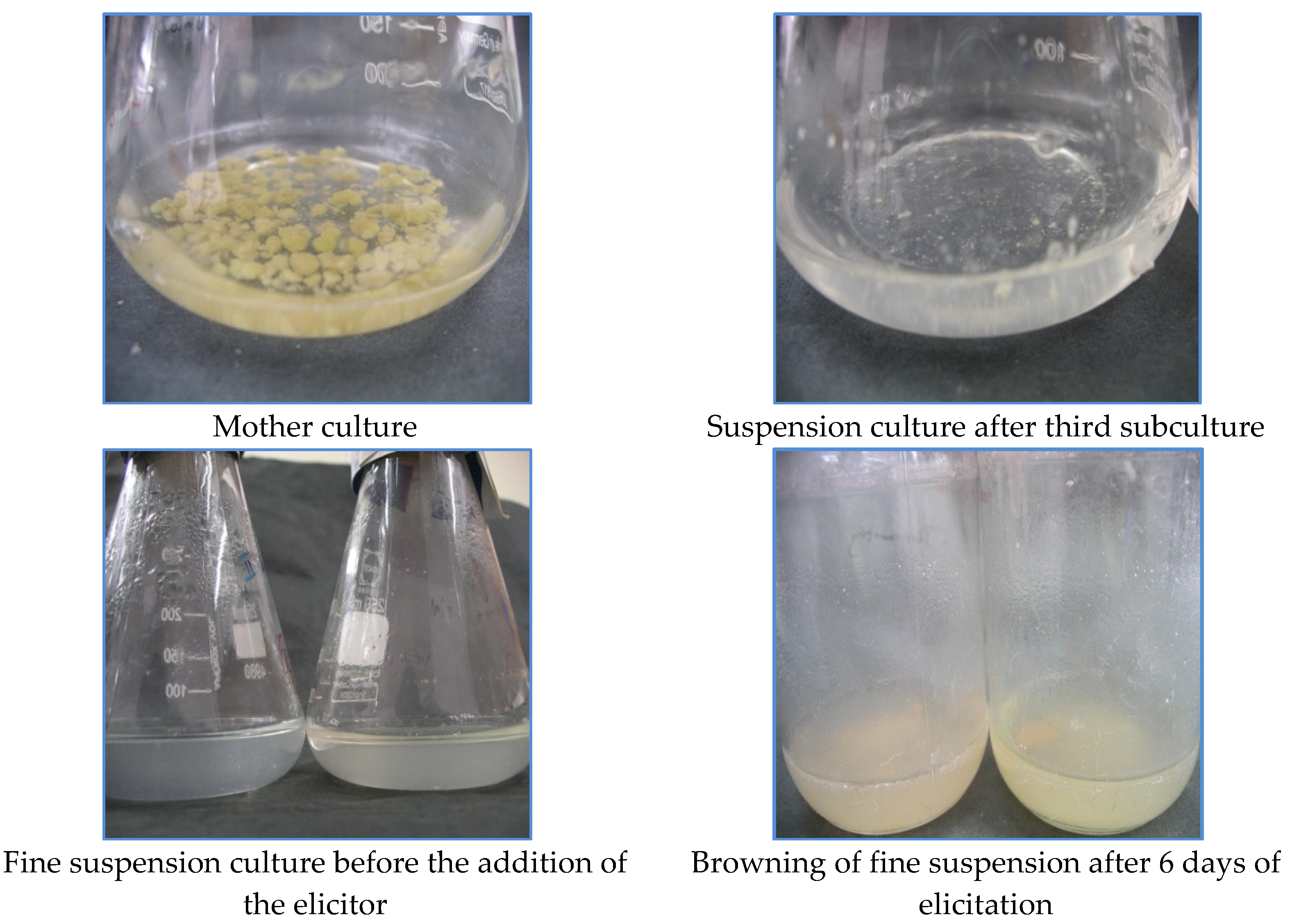
Figure 1.
Different stages of tobacco cell suspension culture.
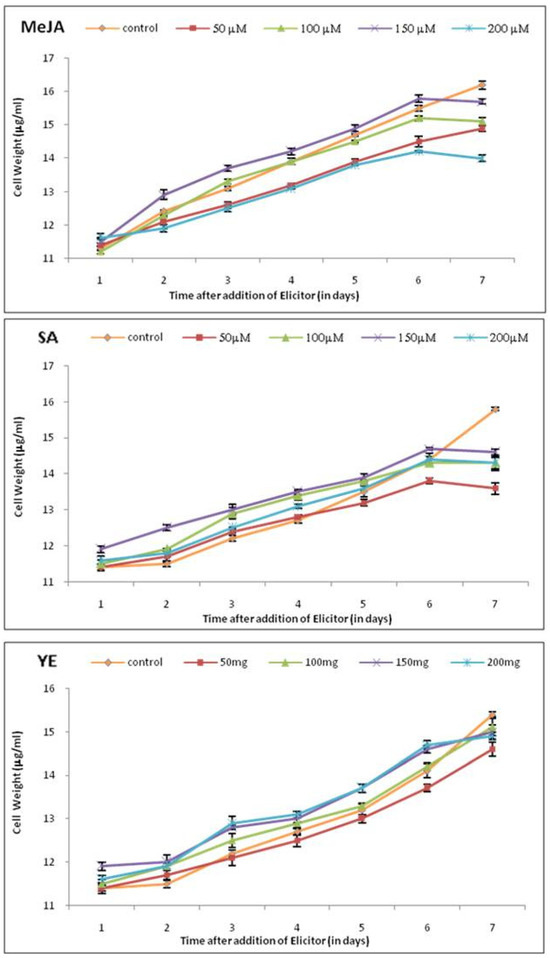
Figure 2.
Effect of elicitors at various concentrations on cell growth of tobacco cell suspension. Each value represents the mean of three independent experiments; the error bar indicates the standard deviation.
3.2. Effect of Elicitors on α-Tocopherol Accumulation in Tobacco Cells
The effect of elicitors on the intracellular accumulation of α-tocopherol was examined. The elicitors were added on the 20th day to the suspension culture, and the cultures were harvested on the 6th day, after which browning of the cultures was observed.
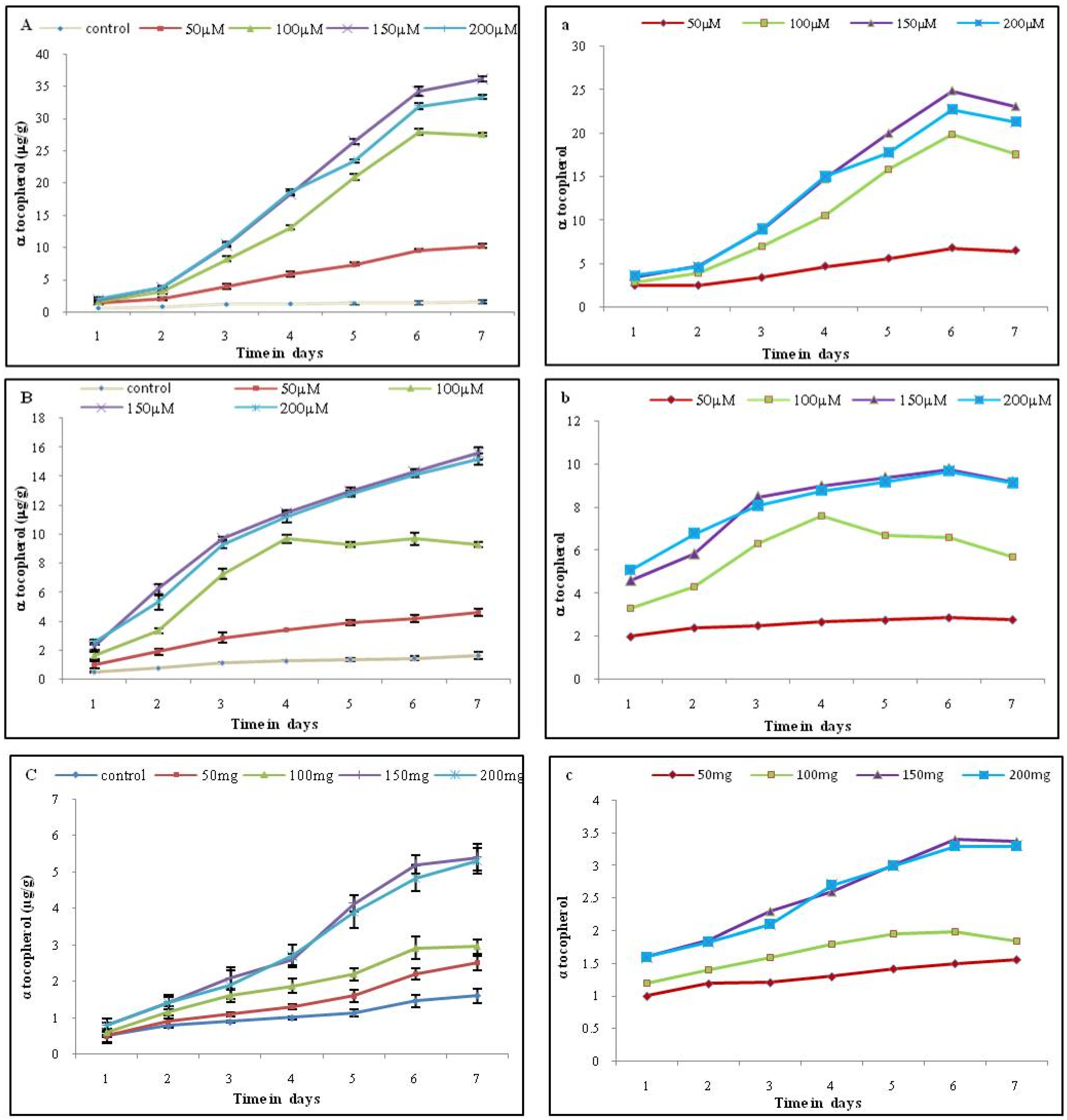
The maximum level of α-tocopherol was recorded on the 6th day of treatment with 150 μM MeJA (34.2 μg/g FW, which represents a 24-fold increase) compared to the control (1.4 μg). A similar kind of response was observed for SA at the 150 μM concentration, which is about 9.8 fold (14.3 μg/g FW) compared to the control (1.46 μg/g FW) (Figure 3, Supplementary Materials).
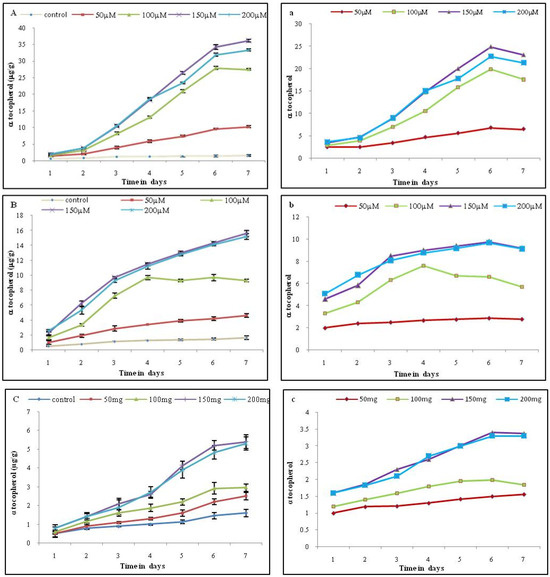
Figure 3.
Effect of different elicitors on α -tocopherol level on tobacco cell suspension culture. Each value represents the mean of three independent experiments; the error bar indicates the standard deviation. The left panel (A) MeJA (B) SA (C) YE represent levels of α-tocopherol (μg/g); the right panel (a) MeJA (b) SA (c) YE represent the fold increase in α -tocopherol content with respect to the control.
The addition of YE did not show any impact on α-tocopherol levels when compared with MeJA and SA. The content of α-tocopherol in cultures treated with 150 and 200 mg/L showed elicitation up to the 6th day. A concentration of 150 mg/L YE showed the highest accumulation of 5.2 μg/g FW, which was about 3.4 fold compared to the control (1.46 μg) (Figure 3).
4. Discussion
Plant suspension cultures are employed as bioreactors for the production of useful compounds. It is well known that MeJA regulates gene expression, including genes involved in the synthesis of tocopherols [19,20]. Munné-Bosch et al. [21] demonstrated that tocopherols may play a role in cellular signaling by modulating jasmonate levels and inducing changes in endogenous phytohormone content. Thus, it is evident that tocopherols have multiple functions in plants and are part of an intricate signaling network in which jasmonic acid, MeJA, and phytohormones play a crucial role. MeJA, a methyl ester of jasmonic acid, occurs in all plants in low amounts, but higher concentrations can inhibit cell growth and lead to plant cell death [22].
This study describes the production of α-tocopherol in cell suspension cultures of tobacco in response to elicitors. The accumulation of α-tocopherol in the elicited cultures was higher than that of the control. The effects of elicitors depended on their concentration, nature of culture used (in terms of cell growth), and duration of treatment.
Among the elicitors tested, the inductive effect of MeJA was the most efficient in enhancing α-tocopherol synthesis in tobacco cell cultures. An increase in MeJA concentration from 50 to 200 μM increased α-tocopherol production, and a 150 μM concentration produced the highest α-tocopherol accumulation. Our results are in accordance with the earlier reports. Jasmonic acid treatment promoted α-tocopherol production in cell suspension cultures of sunflower in its stationary phase (27 day old) and resulted in a significant increase in α-tocopherol content (50%), with 5 μM jasmonic acid after 72 h of its addition, which indicated that jasmonic acid exerts a stimulatory effect on α-tocopherol steady-state levels in sunflower cell cultures [23]. The same ability to enhance α-tocopherol production was also proven for Arabidopsis cell suspension cultures in the stationary phase (18 day old), which showed an increase of 66%. Antognoni et al. [24] reported an increase in α-tocopherol content up to 5-fold in Amaranthus caudatus callus culture with 100 μM MeJA. Sandorf and Hollander-Czytko [20] reported that MeJA induces the nuclear gene expression, which is involved in the regulation of tocopherol biosynthesis in plants.
Treatment with SA and YE in tobacco cell cultures was also tested to study the potential for α-tocopherol production. The results showed enhanced α-tocopherol production in response to these elicitors. However, the elicitation of α-tocopherol by SA and YE was less effective compared to MeJA. YE and SA are frequently used elicitors for metabolite elicitation in cultured cells in vitro. These elicitors trigger the accumulation of endogenous jasmonic acid, which subsequently regulates the gene expression. The results of this study indicated that YE supplementation had a lesser effect on tocopherol production compared to MeJA and SA, possibly due to differences in the concentration and duration of exposure. Further transcriptomic, metabolomic, and proteomic studies are essential to understand the regulation of their biosynthesis under elicitation conditions and to optimize the tocopherol production in suspension cultures. There are previous reports on the efficiency of SA and YE in enhancing the production of compounds such as sesquiterpenes in Hyoscyamus muticus root cultures [25], catharanthine in Catharanthus roseus culture [26], paclitaxel in Taxus cultures [27], anthraquinone production in Morinda elliptica culture [28], cannabinoid biosynthesis in cultures of Cannabis sativa L. [29], and oleanolic acid in Calendula officinalis [18].
In conclusion, we observed an increased production of α-tocopherol by elicitation in tobacco suspension cultures with different concentrations of elicitors. MeJA was observed to be the most effective elicitor for a 150 μM concentration culture with a 24-fold enhancement of α-tocopherol levels. From the present study, it is evident that the elicitors, when introduced into the medium, play a vital role in enhancing the production of desired metabolites by involving in various biosynthetic pathways. Overall, the present study indicates the potential of using a cell suspension culture based approach for the effective production of naturally synthesized nutritional components.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb15030040/s1, Tables: Effect of elicitors on α-tocopherol accumulation and fold increase in α-tocopherol in tobacco cell suspension culture.
Author Contributions
Conceptualization, M.C.H., S.B. and R.S.; literature review, S.B. and M.C.H.; writing—original draft preparation, S.B.; writing—review and editing S.B., M.C.H. and R.S.; supervision, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available upon request.
Acknowledgments
The authors are very thankful to The Department of Biotechnology, Bharathiar University, Coimbatore, Tamil Nadu, India. The authors would like to express appreciation to Thiruvalluvar University for infrastructural support, and to the Department of Biotechnology, Karpagam Academy of Higher Education for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alamgir, A.N.M. Biotechnology, In Vitro Production of Natural Bioactive Compounds, Herbal Preparation, and Disease Management (Treatment and Prevention). In Therapeutic Use of Medicinal Plants and their Extracts: Volume 2—Phytochemistry and Bioactive Compounds; Springer: Berlin/Heidelberg, Germany, 2018; Volume 74, pp. 585–664. [Google Scholar] [CrossRef]
- Fais, A.; Era, B. Phytochemical Composition and Biological Activity. Plants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Bulaon, C.J.I.; Phoolcharoen, W. Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical Production. Plants 2020, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, B.; Praveen, N. Elicitor and precursor-induced approaches to enhance the in vitro production of L-DOPA from cell cultures of Mucuna pruriens. Ind. Crops Prod. 2022, 188, 115735. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, W.; Yu, X. A combination of elicitation and precursor feeding leads to increased anthocyanin synthesis in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult. 2011, 107, 261–269. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: An updated review. Plant Cell Tissue Organ Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef] [PubMed]
- Vergara Martínez, V.M.; Estrada-Soto, S.E.; Arellano-García, J.J.; Rivera-Leyva, J.C.; Castillo-España, P.; Flores, A.F.; Cardoso-Taketa, A.T.; Perea-Arango, I. Methyl jasmonate and salicylic acid enhanced the production of ursolic and oleanolic acid in callus cultures of Lepechinia Caulescens. Pharmacogn. Mag. 2018, 13, S886–S889. [Google Scholar]
- Caretto, S.; Nisi, R.; Paradiso, A.; De Gara, L. Tocopherol production in plant cell cultures. Mol. Nutr. Food Res. 2010, 54, 726–730. [Google Scholar] [CrossRef]
- Harish, M.C.; Dachinamoorthy, P.; Balamurugan, S.; Bala Murugan, S.; Sathishkumar, R. Overexpression of homogentisate phytyltransferase (HPT) and tocopherol cyclase (TC) enhances α-tocopherol content in transgenic tobacco. Biol. Plant. 2013, 57, 395–400. [Google Scholar] [CrossRef]
- Sundararajan, S.; Rajendran, V.; Sivakumar, H.P.; Nayeem, S.; Mani Chandra, H.; Sharma, A.; Ramalingam, S. Enhanced vitamin E content in an Indica rice cultivar harbouring two transgenes from Arabidopsis thaliana involved in tocopherol biosynthesis pathway. Plant Biotechnol. J. 2021, 19, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Sundaram, V.; Vidya Muthulakshmi, M.; Srivastava, S. Multi-fold enhancement in vitamin E (alpha-tocopherol) production via integration of bioprocess optimisation and metabolic engineering in cell suspension of sunflower. J. Plant Biochem. Biotechnol. 2022, 31, 154–167. [Google Scholar] [CrossRef]
- Srinivasan, A.; S, V.; Raman, K.; Srivastava, S. Rational metabolic engineering for enhanced alpha-tocopherol production in Helianthus annuus cell culture. Biochem. Eng. J. 2019, 151, 107256. [Google Scholar] [CrossRef]
- Harish, M.C.; Dachinamoorthy, P.; Balamurugan, S.; Bala Murugan, S.; Sathishkumar, R. Enhancement of α-tocopherol content through transgenic and cell suspension culture systems in tobacco. Acta Physiol. Plant. 2013, 35, 1121–1130. [Google Scholar] [CrossRef]
- Qin, P.; Chen, P.; Zhou, Y.; Zhang, W.; Zhang, Y.; Xu, J.; Gan, L.; Liu, Y.; Romer, J.; Dörmann, P.; et al. Vitamin E biofortification: Enhancement of seed tocopherol concentrations by altered chlorophyll metabolism. Front. Plant Sci. 2024, 15, 1344095. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Hernández, G.; Vázquez-Flota, F.A. Growth Measurements. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Vázquez-Flota, F., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 51–58. [Google Scholar] [CrossRef]
- Wiktorowska, E.; Długosz, M.; Janiszowska, W. Significant enhancement of oleanolic acid accumulation by biotic elicitors in cell suspension cultures of Calendula officinalis L. Enzym. Microb. Technol. 2010, 46, 14–20. [Google Scholar] [CrossRef]
- Lopukhina, A.; Dettenberg, M.; Weiler, E.W.; Holländer-Czytko, H. Cloning and characterization of a coronatine-regulated tyrosine aminotransferase from Arabidopsis. Plant Physiol. 2001, 126, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Sandorf, I.; Holländer-Czytko, H. Jasmonate is involved in the induction of tyrosine aminotransferase and tocopherol biosynthesis in Arabidopsis thaliana. Planta 2002, 216, 173–179. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Weiler, E.W.; Alegre, L.; Müller, M.; Düchting, P.; Falk, J. Alpha-tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 2007, 225, 681–691. [Google Scholar] [CrossRef]
- Sudha, G.; Ravishankar, G.A. Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Organ Cult. 2002, 71, 181–212. [Google Scholar] [CrossRef]
- Gala, R.; Mita, G.; Caretto, S. Improving alpha-tocopherol production in plant cell cultures. J. Plant Physiol. 2005, 162, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Faudale, M.; Poli, F.; Biondi, S. Methyl jasmonate differentially affects tocopherol content and tyrosine amino transferase activity in cultured cells of Amaranthus caudatus and Chenopodium quinoa. Plant Biol. 2009, 11, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Gavrieli, J.; Oakey, J.S.; Curtis, W.R. Interaction of methyl jasmonate, wounding and fungal elicitation during sesquiterpene induction in Hyoscyamus muticus in root cultures. Plant Cell Rep. 1998, 17, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, W.; Hu, Q. Selection of fungal elicitors to increase indole alkaloid accumulation in catharanthus roseus suspension cell culture. Enzym. Microb. Technol. 2001, 28, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-H.; Wu, J.-Y. Ethylene inhibitors enhance elicitor-induced paclitaxel production in suspension cultures of Taxus spp. cells. Enzym. Microb. Technol. 2003, 32, 71–77. [Google Scholar] [CrossRef]
- Chong, T.M.; Abdullah, M.A.; Lai, O.M.; Nor’Aini, F.M.; Lajis, N.H. Effective elicitation factors in Morinda elliptica cell suspension culture. Process Biochem. 2005, 40, 3397–3405. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Peč, J.; Fei, J.; Choi, Y.H.; Dušek, J.; Verpoorte, R. Elicitation studies in cell suspension cultures of Cannabis sativa L. J. Biotechnol. 2009, 143, 157–168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).