Light Quality Influence on Growth Performance and Physiological Activity of Coleus Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
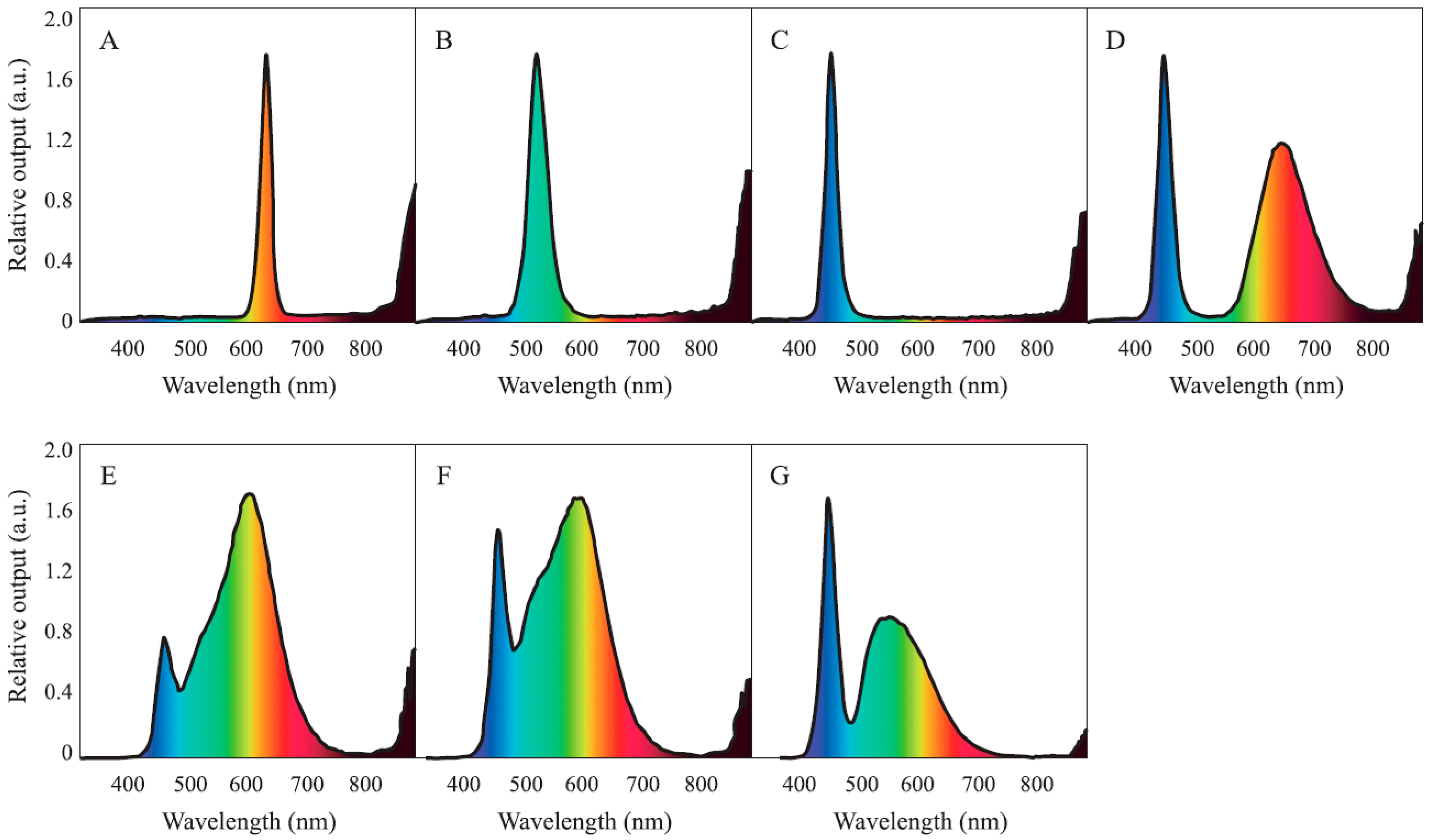
2.2. Experimental Design and Treatments
2.3. Growth Conditions and Experimental Management
2.4. Data Gathered
2.4.1. Plant Growth Parameters
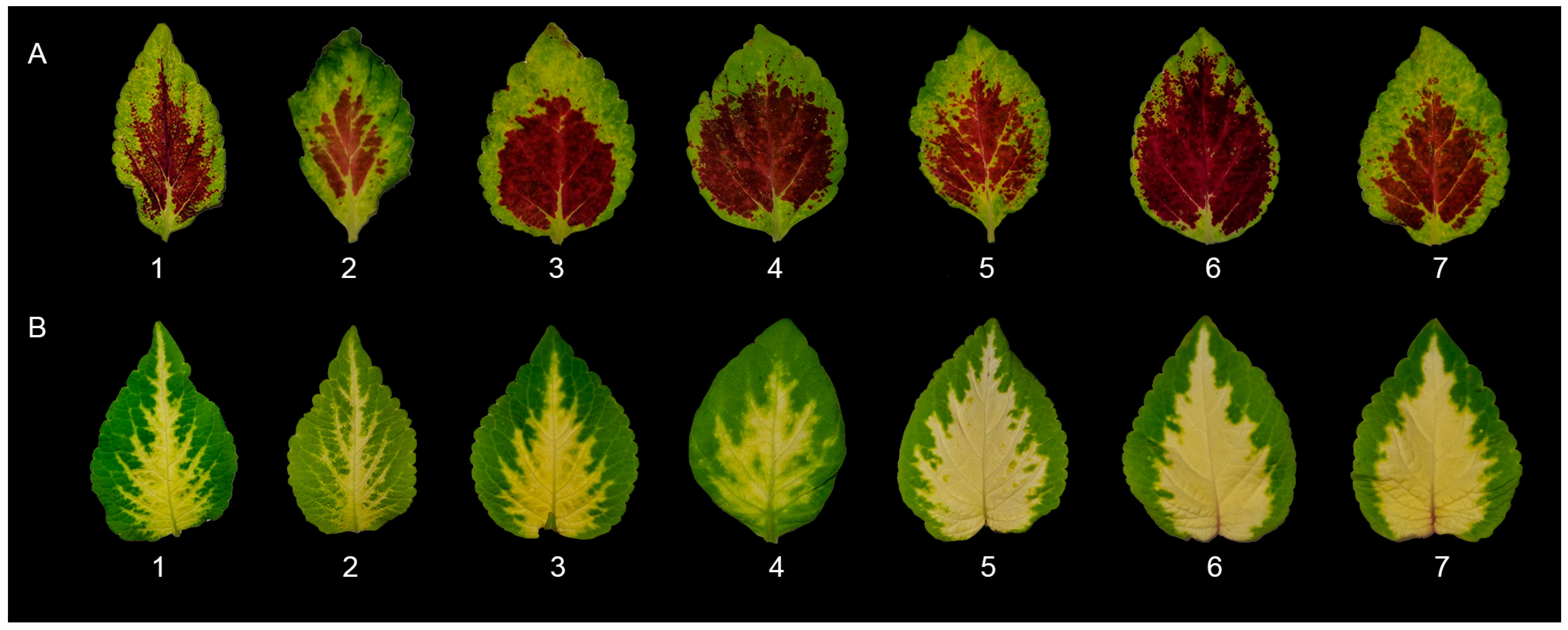
2.4.2. Leaf Color Analysis
2.4.3. Remote Sensing Vegetation Indices
2.4.4. Chlorophyll Fluorescence Analysis
2.5. Statistical Analysis
3. Results
3.1. Plant Growth Parameters
3.2. Leaf Color Analysis and Remote Sensing Vegetation Indices
3.2.1. Leaf Color Analysis
3.2.2. Remote Sensing Vegetation Indices
3.3. Chlorophyll Fluorescence Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Coneva, V.; Robbins, K.R.; Clark, D.; Chitwood, D.; Frank, M. Quantitative dissection of color patterning in the foliar ornamental Coleus. Plant Physiol. 2021, 187, 1310–1324. [Google Scholar] [CrossRef]
- Lebowitz, R.J. The genetics and breeding of Coleus. Plant Breed. Rev. 1985, 3, 343–360. [Google Scholar]
- Kavitha, C.; Rajamani, K.; Vadivel, E. Coleus forskohlii: A comprehensive review on morphology, phytochemistry and pharmacological aspects. J. Med. Plants Res. 2010, 4, 278–285. [Google Scholar]
- Malathi, R.; Cholarajan, A.; Karpagam, K.; Jaya, K.R.; Muthukumaran, P. Antimicrobial studies on selected medicinal plants (Coleus amboinicus, Phyla nodiflora and Vitex negundo). Asian J. Pharm. Technol. 2011, 1, 53–55. [Google Scholar]
- Rout, O.P.; Acharya, R.; Mishra, S.K.; Sahoo, R. Pathorchur (Coleus aromaticus): A review of the medicinal evidence for its phytochemistry and pharmacology properties. Int. J. Appl. Biol. Pharma Technol. 2012, 3, 348–355. [Google Scholar]
- Khattak, M.M.A.K.; Taher, M.; Abdulrahman, S.; Abu Bakar, I.; Damanik, R.; Yahaya, A. Anti-bacterial and anti-fungal activity of Coleus leaves consumed as breast-milk stimulant. Nutr. Food Sci. 2013, 43, 582–590. [Google Scholar] [CrossRef]
- Duke, J.A.; Godwin, M.J.; Cellier, D.U. Handbook of Medicinal Herbs, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 210–215. [Google Scholar]
- Buddhika, W.M.C.; Srikrishnah, S.; Sutharsan, S. Effects of different levels of shade on the growth and quality characters of Coleus (Plectranthus scutellarioides) var. “Chocolate Covered Cherry”. J. Agric. Sci. Crop Res. 2020, 1, 101. [Google Scholar]
- Paton, A.; Mwanyambo, M.; Culham, A. Phylogenetic study of Plectranthus, Coleus and allies (Lamiaceae): Taxonomy, distribution and medicinal use. Bot. J. Linn. Soc. 2018, 188, 355–376. [Google Scholar] [CrossRef]
- Albert, N.W.; Davies, K.M.; Lewis, D.H.; Zhang, H.; Montefiori, M.; Brendolise, C.; Boase, M.R.; Ngo, H.; Jameson, P.E.; Schwinn, K.E. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell 2014, 26, 962–980. [Google Scholar] [CrossRef]
- Fitter, A.H.; Hay, R.K. Environmental Physiology of Plants; Academic Press: London, UK, 2012; pp. 34–50. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Reg. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-emitting diodes in horticulture. Hortic. Rev. 2015, 43, 1–88. [Google Scholar]
- Lin, K.H.; Huang, M.Y.; Hsu, M.H. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green, and blue LED lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Wei, H.; Kim, S.H.; Jeong, B.R. Blue and red light-emitting diodes improve the growth and physiology of in vitro-grown carnations ‘green beauty’ and ‘purple beauty’. Hortic. Environ. Biotechnol. 2017, 58, 12–20. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of impatiens, petunia, salvia, and tomato seedlings under blue, green, and red light-emitting diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Lee, J.H.; Soh, S.Y.; Kim, H.J.; Nam, S.Y. Effects of LED light quality on the growth and leaf color of Orostachys japonica and O. boehmeri. J. Biol. Environ. Control 2022, 31, 104–113. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.Y. Vegetative propagation of six Pachyphytum species as influenced by different LED light qualities. Korean J. Hortic. Sci. Technol. 2023, 41, 237–249. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.H.; Nam, S.Y. Changes in growth, visual qualities, and photosynthetic parameters in Peperomia species and cultivars under different color temperatures of white lighting conditions. J. Agric. Life Environ. Sci. 2023, 35, 307–321. [Google Scholar]
- Lee, J.H.; Cabahug, R.A.M.; You, N.H.; Nam, S.Y. Chlorophyll fluorescence and growth evaluation of ornamental foliage plants in response to light intensity levels under continuous lighting conditions. Flower Res. J. 2021, 29, 153–164. [Google Scholar] [CrossRef]
- Nguyen, P.; Dal Cin, V. The role of light on foliage colour development in coleus (Solenostemon scutellarioides (L.) Codd). Plant Physiol. Biochem. 2009, 47, 934–945. [Google Scholar] [CrossRef]
- Halaban, R. Effects of light quality on the circadian rhythm of leaf movement of a short-day plant. Plant Physiol. 1969, 44, 973–977. [Google Scholar] [CrossRef]
- Cho, K.H.; Laux, V.Y.; Wallace-Springer, N.; Clark, D.G.; Folta, K.M.; Colquhoun, T.A. Effects of light quality on vegetative cutting and in vitro propagation of Coleus (Plectranthus scutellarioides). HortScience 2019, 54, 926–935. [Google Scholar] [CrossRef]
- Dörr, O.S.; Zimmermann, B.F.; Kögler, S.; Mibus, H. Influence of leaf temperature and blue light on the accumulation of rosmarinic acid and other phenolic compounds in Plectranthus scutellarioides (L.). Environ. Exp. Bot. 2019, 167, 103830. [Google Scholar] [CrossRef]
- Jang, I.T.; Lee, J.H.; Shin, E.J.; Nam, S.Y. Evaluation of growth, flowering, and chlorophyll fluorescence responses of Viola cornuta cv. Penny Red Wing according to spectral power distributions. J. People Plants Environ. 2023, 26, 335–349. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Gamon, J.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, C.S.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation, 1st ed.; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Cabahug, R.A.M.; Soh, S.Y.; Nam, S.Y. Effects of light intensity on the growth and anthocyanin content of Echeveria agavoides and E. marcus. Flower Res. J. 2017, 25, 262–269. [Google Scholar] [CrossRef]
- Kelly, S.A.; Panhuis, T.M.; Stoehr, A.M. Phenotypic plasticity: Molecular mechanisms and adaptive significance. Compr. Physiol. 2011, 2, 1417–1439. [Google Scholar]
- Ilyas, M.; Liu, Y.Y.; Shah, S.; Ali, A.; Khan, A.H.; Zaman, F.; Yucui, Z.; Saud, S.; Adnan, M.; Ahmed, N.; et al. Adaptation of functional traits and their plasticity of three ornamental trees growing in urban environment. Sci. Hortic. 2021, 286, 110248. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. LED lighting to produce high-quality ornamental plants. Plants 2023, 12, 1667. [Google Scholar] [CrossRef]
- Franklin, K.A.; Praekelt, U.; Stoddart, W.M.; Billingham, O.E.; Halliday, K.J.; Whitelam, G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003, 131, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Devlin, P.F.; Robson, P.R.; Patel, S.R.; Goosey, L.; Sharrock, R.A.; Whitelam, G.C. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999, 119, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Shacklock, P.; Read, N.; Trewavas, A. Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 1992, 358, 753–755. [Google Scholar] [CrossRef]
- Wang, F.F.; Lian, H.L.; Kang, C.Y.; Yang, H.Q. Phytochrome B is involved in mediating red light-induced stomatal opening in Arabidopsis thaliana. Mol. Plant 2010, 3, 246–259. [Google Scholar] [CrossRef]
- Casson, S.A.; Franklin, K.A.; Gray, J.E.; Grierson, C.S.; Whitelam, G.C.; Hetherington, A.M. Phytochrome B is required for light-mediated systemic control of stomatal development. Curr. Biol. 2009, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, S.; Oh, M. Growth and cell division of lettuce plants under various ratios of red to far-red light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 186–194. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, N.; Ebrahimi, M. Red LED light promotes biomass, flowering and secondary metabolites accumulation in hydroponically grown Hypericum perforatum L. (cv. Topas). Ind. Crops Prod. 2022, 175, 114239. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Moosavi-Nezhad, M.; Alibeigi, B.; Estaji, A.; Gruda, N.S.; Aliniaeifard, S. Growth, biomass partitioning, and photosynthetic performance of chrysanthemum cuttings in response to different light spectra. Plants 2022, 11, 3337. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Runkle, E. Growing plants with green light. GPNMag. Mich. State Univ. 2017, 20, 58. [Google Scholar]
- Kim, S.H.; Park, J.H.; Kim, E.J.; Lee, J.M.; Park, J.W.; Kim, Y.S.; Kim, G.R.; Lee, J.S.; Lee, E.P.; You, Y.H. White LED lighting increases the root productivity of Panax ginseng CA Meyer in a hydroponic cultivation system of a plant factory. Biology 2023, 12, 1052. [Google Scholar] [CrossRef]
- Nagel, K.A.; Schurr, U.; Walter, A. Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity. Plant Cell Environ. 2006, 29, 1936–1945. [Google Scholar] [CrossRef]
- Liu, C.; Guo, C.; Wang, Y.; Ouyang, F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua L. Process Biochem. 2002, 38, 581–585. [Google Scholar] [CrossRef]
- Walter, A.; Nagel, K. Root growth reacts rapidly and more pronounced than shoot growth towards increasing light intensity in tobacco seedlings. Plant Sign. Behav. 2006, 1, 225–226. [Google Scholar] [CrossRef]
- Irawati, E.B. Plant characteristics for green wall system. Proc. Int. Conf. Sci. Eng. 2020, 3, 77–80. [Google Scholar] [CrossRef]
- Muksan; Singh, D.; Wesley, C.J. Performance of ornamental plants in different media composition for outdoor vertical gardening: Experimental investigation. Int. J. Plant Soil. Sci. 2023, 35, 2204–2210. [Google Scholar]
- Fairchild, M.; Berns, R. Image color-appearance specification through extension of CIELAB. Color. Res. Appl. 1993, 18, 178–190. [Google Scholar] [CrossRef]
- Pan, L.; Li, J.; Yin, H.; Fan, Z.; Li, X. Integrated physiological and transcriptomic analyses reveal a regulatory network of anthocyanin metabolism contributing to the ornamental value in a novel hybrid cultivar of Camellia japonica. Plants 2020, 9, 1724. [Google Scholar] [CrossRef]
- Wang, D. Seasonal color matching method of ornamental plants in urban landscape construction. Open Geosci. 2021, 13, 594–605. [Google Scholar] [CrossRef]
- Sudhakar, P.; Latha, P.; Reddy, P.V. Plant pigments. In Phenotyping Crop Plants for Physiological and Biochemical Traits, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 121–127. [Google Scholar]
- Carvalho, S.D.; Folta, K.M. Green light control of anthocyanin production in microgreens. Acta Hortic. 2016, 1134, 13–18. [Google Scholar] [CrossRef]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by led on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic. 2011, 907, 179–184. [Google Scholar] [CrossRef]
- Katz, A.; Weiss, D. Light regulation of anthocyanin accumulation and chalcone synthase gene expression in Petunia flowers. Israel J. Plant Sci. 1999, 47, 225–229. [Google Scholar] [CrossRef]
- Lalusin, A.; Ohta, M.; Fujimura, T. Temporal and spatial expression of genes involved in anthocyanin biosynthesis during sweet potato (Ipomoea batatas [L.] Lam.) root development. Int. J. Plant Sci. 2006, 167, 249–256. [Google Scholar] [CrossRef]
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.; Tsuda, T.; Moriguchi, T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Biochem. 2002, 40, 955–962. [Google Scholar] [CrossRef]
- Lee, J.; Kang, W.; Park, K.; Son, J. Spectral dependence of electrical energy-based photosynthetic efficiency at single leaf and canopy levels in green- and red-leaf lettuces. Hortic. Environ. Biotechnol. 2017, 58, 111–118. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Klug, T.; Assumpção, C.; Magro, L.; Wieth, A.; Bender, R.; Flôres, S.; Rios, A. Evaluation of growth and phenolic compounds profile of purple lettuce under indoor cultivation. Brazil J. Food Res. 2021, 11, 62–76. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Ottosen, C.; Rosenqvist, E. Spectral effects of LEDs on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘Vivien’ and ‘Purple Star’. Physiol. Plant 2015, 154, 314–327. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.G.; Zhu, X. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2018, 139, 107–121. [Google Scholar] [CrossRef]
- Kondzior, P.; Tyniecki, D.; Butarewicz, A. Influence of color temperature of white LED diodes and illumination intensity on the content of photosynthetic pigments in Chlorella vulgaris algae cells. Proceedings 2019, 16, 46. [Google Scholar] [CrossRef]
- Kozukue, N.; Tsuchida, H.; Mizuno, S. Effect of light intensity, duration, and photoperiod on chlorophyll and glycoalkaloid production by potato tubers. J. Jpn. Soc. Hortic. Sci. 1993, 62, 669–673. [Google Scholar] [CrossRef][Green Version]
- Su, N.; Wu, Q.; Shen, Z.; Xia, K.; Cui, J. Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Grow. Reg. 2014, 73, 227–235. [Google Scholar] [CrossRef]
- Williams, M.; Waters, E.; Golbek, M.; Wormington, J. Effect of different shades of light on photosynthesis. J. Introd. Biol. Investig. 2015, 2. [Google Scholar]
- Pettorelli, N. The Normalized Difference Vegetation Index, 1st ed.; Oxford University Press: New York, NY, USA, 2013; pp. 70–79. [Google Scholar]
- Nagler, P.L.; Daughtry, C.S.T.; Goward, S.N. Plant litter and soil reflectance. Remote Sens. Environ. 2000, 71, 229. [Google Scholar] [CrossRef]
- Magney, T.S.; Vierling, L.A.; Eitel, J.U.; Huggins, D.R.; Garrity, S.R. Response of high frequency photochemical reflectance index (PRI) measurements to environmental conditions in wheat. Remote Sens. Environ. 2016, 73, 84–97. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Habibi, G. Effects of high light and chilling stress on photosystem II efficiency of Aloe vera L. plants probing by chlorophyll a fluorescence measurements. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 7–13. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Strasser, R.J.; Govindjee, G. Greening of peas: Parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica 1999, 37, 365–392. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Liu, W.; Gan, Y.; Wu, Y. Biomass allocation, compensatory growth and internal C/N balance of Lolium perenne in response to defoliation and light treatments. Polish J. Ecol. 2016, 64, 485–499. [Google Scholar] [CrossRef]
- Nishio, J.N. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2002, 23, 539–548. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.H.; Nam, S.Y. Evaluation of growth, vegetation indices, and photosynthesis of Cichorium intybus L. seedlings as affected by LED light qualities in a closed nursery facility. Hortic. Sci. Technol. 2024, 42, 350–364. [Google Scholar] [CrossRef]


| Light Quality | Shoot Parameters (cm) | Leaf Parameters (cm) | Root Length (cm) | Ground Cover (cm2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Height | Width | Stem Diameter | No. of Leaves | Length | Width | ||||
| C. ‘Highway Ruby’ | |||||||||
| Red | 15.0 ± 0.48 z a y | 15.7 ± 1.16 a | 0.5 ± 0.05 a | 24.8 ± 2.4 a | 7.7 ± 0.45 a | 4.4 ± 0.28 a | 14.0 ± 2.37 b | 225.6 ± 34.7 a | |
| Green | 9.1 ± 0.61 b | 10.6 ± 0.96 b | 0.3 ± 0.02 b | 17.3 ± 1.1 c | 4.6 ± 0.37 b | 2.8 ± 0.27 b | 8.8 ± 1.78 c | 120.2 ± 23.4 b | |
| Blue | 4.6 ± 0.23 d | 7.5 ± 0.35 c | 0.2 ± 0.02 b | 15.8 ± 0.5 c | 3.1 ± 0.15 c | 2.2 ± 0.13 c | 6.9 ± 0.50 c | 56.7 ± 4.9 c | |
| Purple | 6.1 ± 0.51 c | 8.9 ± 0.81 b | 0.3 ± 0.03 b | 16.8 ± 1.4 c | 3.4 ± 0.29 c | 2.5 ± 0.24 bc | 12.1 ± 2.46 b | 84.5 ± 13.9 bc | |
| 3000 K White | 6.3 ± 0.32 c | 9.2 ± 0.71 b | 0.3 ± 0.02 b | 19.6 ± 1.1 b | 3.6 ± 0.24 c | 2.6 ± 0.17 bc | 11.6 ± 1.68 b | 87.3 ± 14.8 bc | |
| 4100 K White | 6.1 ± 0.11 c | 9.8 ± 0.48 b | 0.3 ± 0.03 b | 20.8 ± 1.1 b | 3.8 ± 0.20 c | 3.0 ± 0.11 b | 17.0 ± 3.45 a | 96.7 ± 9.6 bc | |
| 6500 K White | 5.8 ± 0.21 c | 8.6 ± 0.47 bc | 0.2 ± 0.02 b | 20.0 ± 0.8 b | 3.5 ± 0.27 c | 2.6 ± 0.17 bc | 12.1 ± 2.36 b | 76.1 ± 8.1 bc | |
| Significance x | ** | ** | ** | ** | ** | ** | * | ** | |
| C. ‘Wizard Jade’ | |||||||||
| Red | 18.4 ± 1.40 a | 24.0 ± 0.57 a | 0.9 ± 0.05 a | 34.8 ± 1.4 a | 10.9 ± 0.31 a | 7.0 ± 0.40 a | 22.0 ± 0.75 c | 579.2 ± 27.6 a | |
| Green | 13.5 ± 0.69 b | 23.1 ± 0.80 a | 0.7 ± 0.04 b | 31.7 ± 3.9 b | 10.3 ± 0.39 a | 6.9 ± 0.12 a | 25.1 ± 2.23 b | 535.2 ± 37.2 a | |
| Blue | 7.0 ± 0.25 c | 15.4 ± 0.24 bc | 0.7 ± 0.02 b | 25.5 ± 1.3 c | 6.6 ± 0.17 c | 5.5 ± 0.21 b | 23.7 ± 0.68 c | 238.0 ± 7.4 bc | |
| Purple | 7.0 ± 0.53 c | 13.3 ± 0.48 d | 0.6 ± 0.05 c | 22.8 ± 1.7 d | 5.8 ± 0.48 d | 4.4 ± 0.44 c | 22.5 ± 3.10 c | 181.7 ± 26.9 c | |
| 3000 K White | 8.3 ± 0.50 c | 16.0 ± 0.25 b | 0.8 ± 0.04 b | 29.3 ± 0.7 bc | 7.1 ± 0.29 b | 5.6 ± 0.18 b | 28.8 ± 1.37 ab | 255.8 ± 8.0 b | |
| 4100 K White | 8.0 ± 0.46 c | 13.9 ± 0.31 c | 0.6 ± 0.05 c | 26.0 ± 1.8 c | 6.3 ± 0.20 c | 4.9 ± 0.16 bc | 25.0 ± 2.64 b | 196.0 ± 8.7 bc | |
| 6500 K White | 7.9 ± 0.35 c | 15.6 ± 0.48 bc | 0.7 ± 0.04 b | 32.2 ± 2.3 b | 7.0 ± 0.16 b | 5.3 ± 0.16 b | 31.8 ± 2.29 a | 243.5 ± 14.8 b | |
| Significance x | ** | ** | ** | ** | ** | ** | * | ** | |
| Light Quality | Fresh Weight (g) | Dry Weight (g) | Moisture Content (%) | ||||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | ||
| C. ‘Highway Ruby’ | |||||||
| Red | 6.2 ± 0.83 z a y | 0.09 ± 0.03 a | 0.30 ± 0.06 a | 0.03 ± 0.01 a | 95.3 ± 0.3 ab | 59.9 ± 4.0 a | |
| Green | 2.2 ± 0.39 c | 0.03 ± 0.01 c | 0.07 ± 0.02 c | 0.01 ± 0.00 c | 96.7 ± 0.4 a | 53.9 ± 6.0 a | |
| Blue | 1.1 ± 0.09 d | 0.01 ± 0.00 d | 0.06 ± 0.01 c | 0.01 ± 0.00 c | 94.8 ± 0.1 b | 40.6 ± 6.1 a | |
| Purple | 1.9 ± 0.37 c | 0.04 ± 0.01 c | 0.10 ± 0.02 bc | 0.01 ± 0.00 c | 94.8 ± 0.1 b | 50.6 ± 7.1 a | |
| 3000 K White | 1.9 ± 0.28 c | 0.04 ± 0.01 c | 0.10 ± 0.01 bc | 0.02 ± 0.00 b | 94.7 ± 0.3 b | 56.7 ± 4.7 a | |
| 4100 K White | 2.8 ± 0.42 b | 0.06 ± 0.02 b | 0.15 ± 0.02 b | 0.03 ± 0.01 a | 94.6 ± 0.1 b | 47.1 ± 5.4 a | |
| 6500 K White | 2.1 ± 0.25 c | 0.03 ± 0.01 c | 0.11 ± 0.01 bc | 0.01 ± 0.00 c | 94.9 ± 0.2 b | 48.3 ± 6.6 a | |
| Significance x | ** | ** | ** | ** | ** | NS | |
| C. ‘Wizard Jade’ | |||||||
| Red | 16.0 ± 0.70 a | 0.77 ± 0.12 a | 0.90 ± 0.18 a | 0.13 ± 0.02 a | 94.3 ± 1.0 a | 82.3 ± 1.2 a | |
| Green | 11.2 ± 0.92 b | 0.26 ± 0.06 cd | 0.63 ± 0.07 b | 0.09 ± 0.02 b | 94.4 ± 0.1 a | 61.6 ± 1.0 a | |
| Blue | 6.8 ± 0.14 c | 0.29 ± 0.07 cd | 0.38 ± 0.02 c | 0.09 ± 0.01 b | 94.4 ± 0.2 a | 65.8 ± 4.2 a | |
| Purple | 4.3 ± 0.79 d | 0.20 ± 0.07 d | 0.28 ± 0.06 d | 0.06 ± 0.01 c | 93.6 ± 0.6 ab | 55.0 ± 2.2 a | |
| 3000 K White | 8.1 ± 0.49 c | 0.50 ± 0.09 b | 0.63 ± 0.06 b | 0.12 ± 0.02 a | 92.3 ± 0.4 b | 73.8 ± 1.4 a | |
| 4100 K White | 7.3 ± 0.66 c | 0.41 ± 0.06 bc | 0.55 ± 0.06 bc | 0.10 ± 0.02 ab | 92.4 ± 0.3 b | 76.6 ± 1.4 a | |
| 6500 K White | 8.2 ± 0.61 c | 0.33 ± 0.06 c | 0.51 ± 0.06 bc | 0.10 ± 0.01 ab | 93.8 ± 0.3 ab | 58.8 ± 1.3 a | |
| Significance | ** | ** | ** | * | * | NS | |
| Light Quality | Primary Leaf Color (Green Color) | RHS Values z | Color Groups | Color Palette | Secondary Color (Red or White Color) | RHS Values | Color Groups | Color Palette | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||||||||
| C. ‘Highway Ruby’ | |||||||||||||
| Red | 57.5 ± 1.66 y a x | −10.2 ± 0.66 a | 43.0 ± 0.98 a | 144A | Yellow-green |  | 24.8 ± 1.24 b | 28.1 ± 1.61 b | 10.9 ± 1.05 c | 187B | Greyed-purple |  | |
| Green | 58.0 ± 1.54 a | −9.3 ± 0.63 a | 44.7 ± 0.85 a | 144A | Yellow-green |  | 36.8 ± 1.49 a | 29.0 ± 1.74 b | 15.3 ± 0.76 a | 178B | Greyed-red |  | |
| Blue | 52.0 ± 1.01 a | −9.9 ± 0.44 a | 44.1 ± 0.94 a | 144A | Yellow-green |  | 26.4 ± 1.33 b | 24.3 ± 1.08 c | 14.4 ± 0.81 b | 183A | Greyed-purple |  | |
| Purple | 50.6 ± 2.77 a | −10.0 ± 0.48 a | 40.7 ± 2.03 a | 144A | Yellow-green |  | 23.5 ± 0.82 b | 33.7 ± 1.88 a | 12.9 ± 0.85 b | 185A | Greyed-purple |  | |
| 3000 K White | 53.4 ± 2.91 a | −7.1 ± 2.56 a | 43.2 ± 1.88 a | 152B | Yellow-green |  | 26.0 ± 1.93 b | 33.7 ± 1.92 a | 10.5 ± 1.02 c | 185A | Greyed-purple |  | |
| 4100 K White | 55.0 ± 1.02 a | −8.5 ± 0.65 a | 44.3 ± 1.10 a | 144A | Yellow-green |  | 25.3 ± 1.87 b | 32.9 ± 1.55 a | 11.6 ± 1.34 bc | 185A | Greyed-purple |  | |
| 6500 K White | 51.5 ± 1.85 a | −8.5 ± 0.64 a | 40.7 ± 2.02 a | 144A | Yellow-green |  | 23.1 ± 2.15 b | 29.2 ± 1.96 b | 11.4 ± 1.04 bc | 187B | Greyed-purple |  | |
| Significance w | NS | NS | NS | ** | ** | ** | |||||||
| C. ‘Wizard Jade’ | |||||||||||||
| Red | 53.1 ± 1.46 ab | −12.0 ± 0.21 ab | 43.5 ± 1.31 b | 144A | Yellow-green |  | 76.0 ± 0.41 a | 2.3 ± 0.72 a | 37.1 ± 1.64 a | 161A | Greyed-yellow |  | |
| Green | 55.2 ± 0.55 a | −11.3 ± 0.15 a | 47.6 ± 0.69 a | 144A | Yellow-green |  | 77.7 ± 0.51 a | 2.4 ± 0.57 a | 29.9 ± 1.38 a | 161A | Greyed-yellow |  | |
| Blue | 43.0 ± 0.82 c | −12.1 ± 0.23 b | 32.3 ± 0.60 f | 146A | Yellow-green |  | 75.2 ± 0.63 a | 3.4 ± 0.49 a | 36.9 ± 2.47 a | 161C | Greyed-yellow |  | |
| Purple | 50.0 ± 1.36 b | −12.1 ± 0.30 b | 38.9 ± 1.40 d | 144A | Yellow-green |  | 75.3 ± 1.70 a | 2.5 ± 0.49 a | 32.7 ± 2.22 a | 162C | Greyed-yellow |  | |
| 3000 K White | 52.6 ± 0.91 b | −12.5 ± 0.14 b | 42.9 ± 0.82 b | 144A | Yellow-green |  | 75.8 ± 0.50 a | 1.3 ± 0.25 a | 32.0 ± 1.13 a | 162C | Greyed-yellow |  | |
| 4100 K White | 50.8 ± 1.16 b | −12.6 ± 0.26 b | 41.1 ± 0.54 c | 144A | Yellow-green |  | 75.8 ± 0.86 a | 1.8 ± 0.45 a | 35.1 ± 2.17 a | 162C | Greyed-yellow |  | |
| 6500 K White | 45.4 ± 1.54 c | −12.5 ± 0.46 b | 35.1 ± 0.74 e | 144A | Yellow-green |  | 77.4 ± 0.47 a | 2.4 ± 0.33 a | 35.0 ± 1.88 a | 162C | Greyed-yellow |  | |
| Significance | ** | * | ** | NS | NS | NS | |||||||
| Light Quality | Chlorophyll Content (SPAD Units) | Primary Leaf Color (Green Color) | Secondary Leaf Color (Red or White Color) | |||||
|---|---|---|---|---|---|---|---|---|
| NDVI | MCARI | PRI | NDVI | MCARI | PRI | |||
| C. ‘Highway Ruby’ | ||||||||
| Red | 9.3 ± 0.80 z b y | 0.42 ± 0.02 b | 0.93 ± 0.03 a | 0.003 ± 0.002 a | 0.56 ± 0.02 b | 0.70 ± 0.04 b | −0.18 ± 0.02 a | |
| Green | 9.5 ± 0.85 b | 0.40 ± 0.04 b | 0.79 ± 0.06 a | −0.003 ± 0.004 a | 0.41 ± 0.03 d | 0.53 ± 0.07 c | −0.22 ± 0.02 a | |
| Blue | 14.0 ± 1.38 ab | 0.46 ± 0.05 b | 0.69 ± 0.13 a | −0.012 ± 0.002 b | 0.60 ± 0.02 a | 0.82 ± 0.04 a | −0.19 ± 0.01 a | |
| Purple | 12.0 ± 1.04 ab | 0.44 ± 0.02 b | 0.98 ± 0.03 a | −0.011 ± 0.007 b | 0.46 ± 0.02 cd | 0.67 ± 0.06 b | −0.23 ± 0.01 a | |
| 3000 K White | 9.6 ± 0.79 b | 0.35 ± 0.03 c | 0.75 ± 0.09 a | −0.024 ± 0.004 c | 0.51 ± 0.02 bc | 0.68 ± 0.05 b | −0.20 ± 0.02 a | |
| 4100 K White | 12.5 ± 1.44 ab | 0.44 ± 0.03 b | 0.88 ± 0.05 a | −0.031 ± 0.004 c | 0.55 ± 0.03 b | 0.75 ± 0.06 ab | −0.16 ± 0.02 a | |
| 6500 K White | 14.5 ± 1.27 a | 0.51 ± 0.03 a | 0.98 ± 0.04 a | −0.027 ± 0.006 c | 0.56 ± 0.02 b | 0.80 ± 0.04 a | −0.18 ± 0.02 a | |
| Significance x | ** | * | NS | ** | ** | ** | NS | |
| C. ‘Wizard Jade’ | ||||||||
| Red | 20.0 ± 0.44 c | 0.58 ± 0.02 c | 0.89 ± 0.05 a | 0.02 ± 0.002 b | 0.07 ± 0.01 a | 0.15 ± 0.02 a | −0.02 ± 0.003 b | |
| Green | 15.9 ± 1.33 d | 0.56 ± 0.02 d | 0.94 ± 0.06 a | 0.01 ± 0.001 c | 0.04 ± 0.00 b | 0.09 ± 0.01 b | −0.01 ± 0.001 a | |
| Blue | 29.5 ± 0.55 a | 0.73 ± 0.00 a | 0.49 ± 0.03 d | 0.03 ± 0.003 a | 0.03 ± 0.00 c | 0.05 ± 0.01 c | −0.03 ± 0.003 c | |
| Purple | 23.3 ± 0.71 b | 0.61 ± 0.02 c | 0.77 ± 0.04 b | 0.02 ± 0.007 b | 0.02 ± 0.00 c | 0.03 ± 0.01 cd | −0.03 ± 0.002 c | |
| 3000 K White | 21.6 ± 0.66 bc | 0.56 ± 0.03 d | 0.94 ± 0.06 a | 0.01 ± 0.006 c | 0.03 ± 0.01 c | 0.04 ± 0.02 c | −0.01 ± 0.001 a | |
| 4100 K White | 23.8 ± 0.50 b | 0.58 ± 0.03 c | 0.83 ± 0.05 b | 0.01 ± 0.007 c | 0.04 ± 0.01 b | 0.07 ± 0.02 c | −0.02 ± 0.003 b | |
| 6500 K White | 26.9 ± 0.37 a | 0.67 ± 0.03 b | 0.69 ± 0.03 c | 0.02 ± 0.008 b | 0.02 ± 0.00 c | 0.01 ± 0.00 d | −0.02 ± 0.001 b | |
| Significance | ** | ** | ** | ** | ** | ** | ** | |
| Light Quality | Fv/Fm | ΦDo | ABS/RC | DIo/RC | PIABS | |
|---|---|---|---|---|---|---|
| C. ‘Highway Ruby’ | ||||||
| Red | 0.825 ± 0.005 z b y | 0.175 ± 0.005 b | 2.09 ± 0.05 a | 0.369 ± 0.02 b | 3.51 ± 0.28 d | |
| Green | 0.816 ± 0.006 c | 0.184 ± 0.006 a | 2.16 ± 0.05 a | 0.403 ± 0.02 a | 3.44 ± 0.34 d | |
| Blue | 0.846 ± 0.002 a | 0.154 ± 0.002 c | 1.62 ± 0.05 c | 0.251 ± 0.01 e | 7.45 ± 0.47 a | |
| Purple | 0.838 ± 0.003 ab | 0.162 ± 0.003 bc | 1.83 ± 0.07 b | 0.300 ± 0.02 cd | 5.80 ± 0.48 bc | |
| 3000 K White | 0.833 ± 0.004 b | 0.167 ± 0.004 b | 1.62 ± 0.06 c | 0.270 ± 0.01 d | 6.65 ± 0.50 ab | |
| 4100 K White | 0.827 ± 0.005 b | 0.173 ± 0.005 b | 1.80 ± 0.08 b | 0.317 ± 0.02 c | 5.18 ± 0.55 c | |
| 6500 K White | 0.842 ± 0.002 a | 0.158 ± 0.002 c | 1.61 ± 0.07 c | 0.255 ± 0.01 e | 7.89 ± 0.75 a | |
| Significance x | ** | ** | ** | ** | ** | |
| C. ‘Wizard Jade’ | ||||||
| Red | 0.777 ± 0.01 c | 0.223 ± 0.01 b | 2.66 ± 0.07 b | 0.601 ± 0.04 b | 1.77 ± 0.29 cd | |
| Green | 0.710 ± 0.01 d | 0.290 ± 0.01 a | 3.06 ± 0.08 a | 0.898 ± 0.06 a | 0.77 ± 0.08 e | |
| Blue | 0.819 ± 0.00 a | 0.181 ± 0.00 d | 2.02 ± 0.03 d | 0.366 ± 0.01 e | 3.63 ± 0.36 a | |
| Purple | 0.799 ± 0.01 b | 0.201 ± 0.01 c | 2.34 ± 0.03 c | 0.469 ± 0.01 d | 1.93 ± 0.19 c | |
| 3000 K White | 0.776 ± 0.01 c | 0.224 ± 0.01 b | 2.46 ± 0.05 bc | 0.557 ± 0.03 bc | 1.23 ± 0.20 d | |
| 4100 K White | 0.790 ± 0.00 b | 0.210 ± 0.00 c | 2.35 ± 0.03 c | 0.493 ± 0.02 c | 1.72 ± 0.28 cd | |
| 6500 K White | 0.806 ± 0.01 ab | 0.194 ± 0.01 cd | 2.22 ± 0.04 cd | 0.435 ± 0.02 de | 2.69 ± 0.34 b | |
| Significance | ** | ** | ** | ** | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.G.; Lee, J.H.; Shin, E.J.; Kim, E.A.; Nam, S.Y. Light Quality Influence on Growth Performance and Physiological Activity of Coleus Cultivars. Int. J. Plant Biol. 2024, 15, 807-826. https://doi.org/10.3390/ijpb15030058
Park BG, Lee JH, Shin EJ, Kim EA, Nam SY. Light Quality Influence on Growth Performance and Physiological Activity of Coleus Cultivars. International Journal of Plant Biology. 2024; 15(3):807-826. https://doi.org/10.3390/ijpb15030058
Chicago/Turabian StylePark, Byoung Gyoo, Jae Hwan Lee, Eun Ji Shin, Eun A Kim, and Sang Yong Nam. 2024. "Light Quality Influence on Growth Performance and Physiological Activity of Coleus Cultivars" International Journal of Plant Biology 15, no. 3: 807-826. https://doi.org/10.3390/ijpb15030058
APA StylePark, B. G., Lee, J. H., Shin, E. J., Kim, E. A., & Nam, S. Y. (2024). Light Quality Influence on Growth Performance and Physiological Activity of Coleus Cultivars. International Journal of Plant Biology, 15(3), 807-826. https://doi.org/10.3390/ijpb15030058








