Nitrate Reductase and Glutamine Synthetase Enzyme Activities and Chlorophyll in Sorghum Leaves (Sorghum bicolor) in Response to Organic Fertilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Determination of Nitrate Reductase Activity (NR)
2.3. Determination of Glutamine Synthetase Activity (GS)
2.4. Determination of Chlorophyll a and b
2.5. Statistical Analysis
3. Results
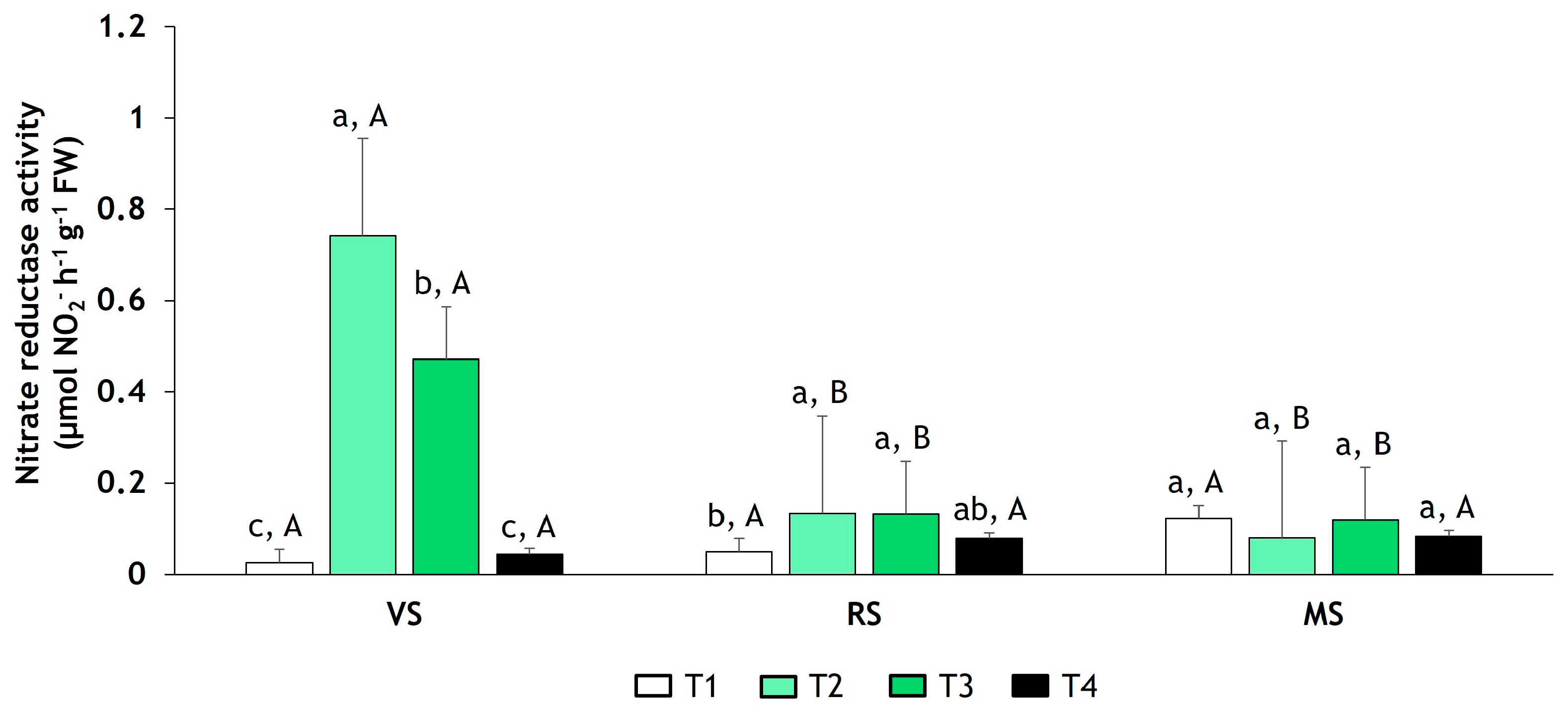
3.1. Determination of NR Activity
3.2. Determination of GS Activity
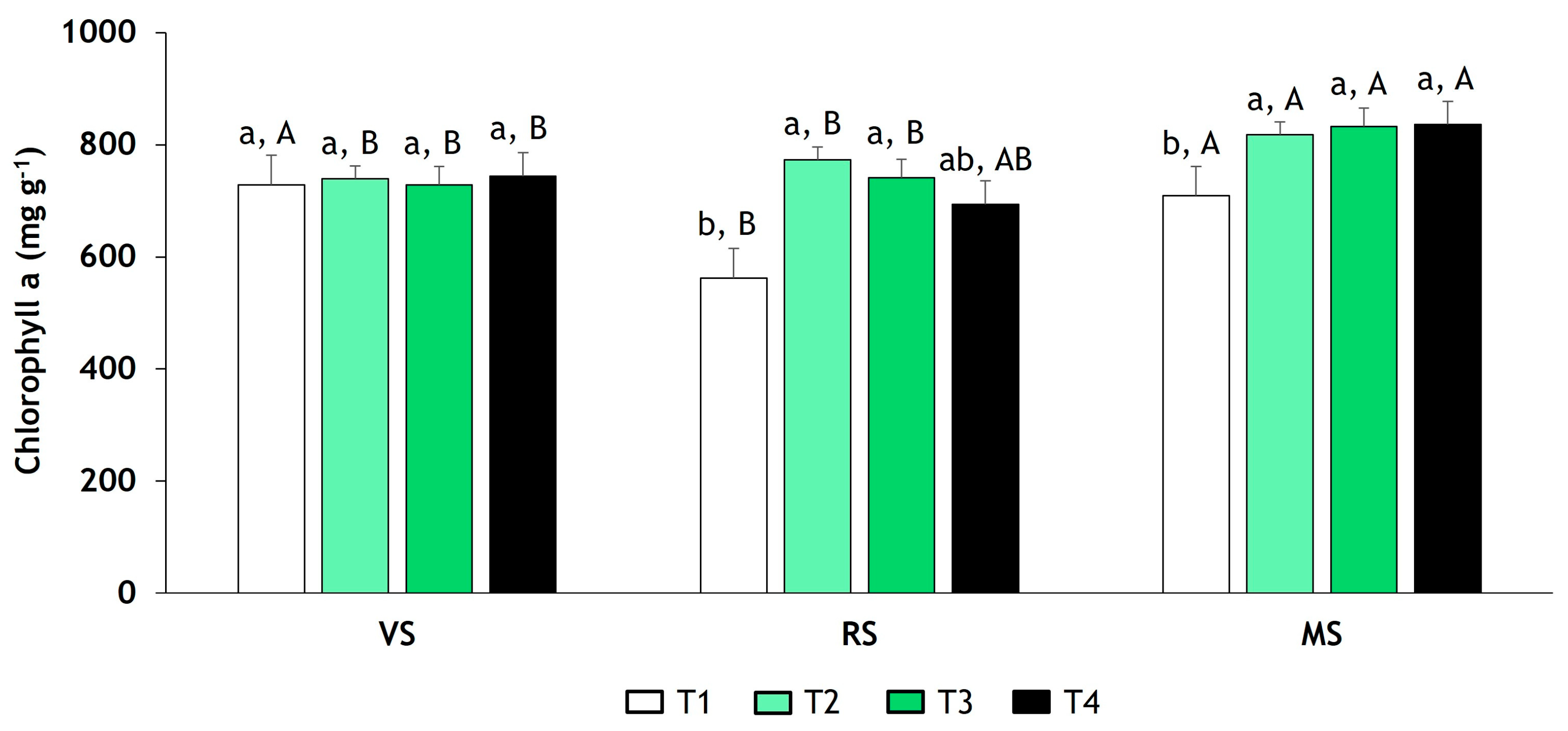
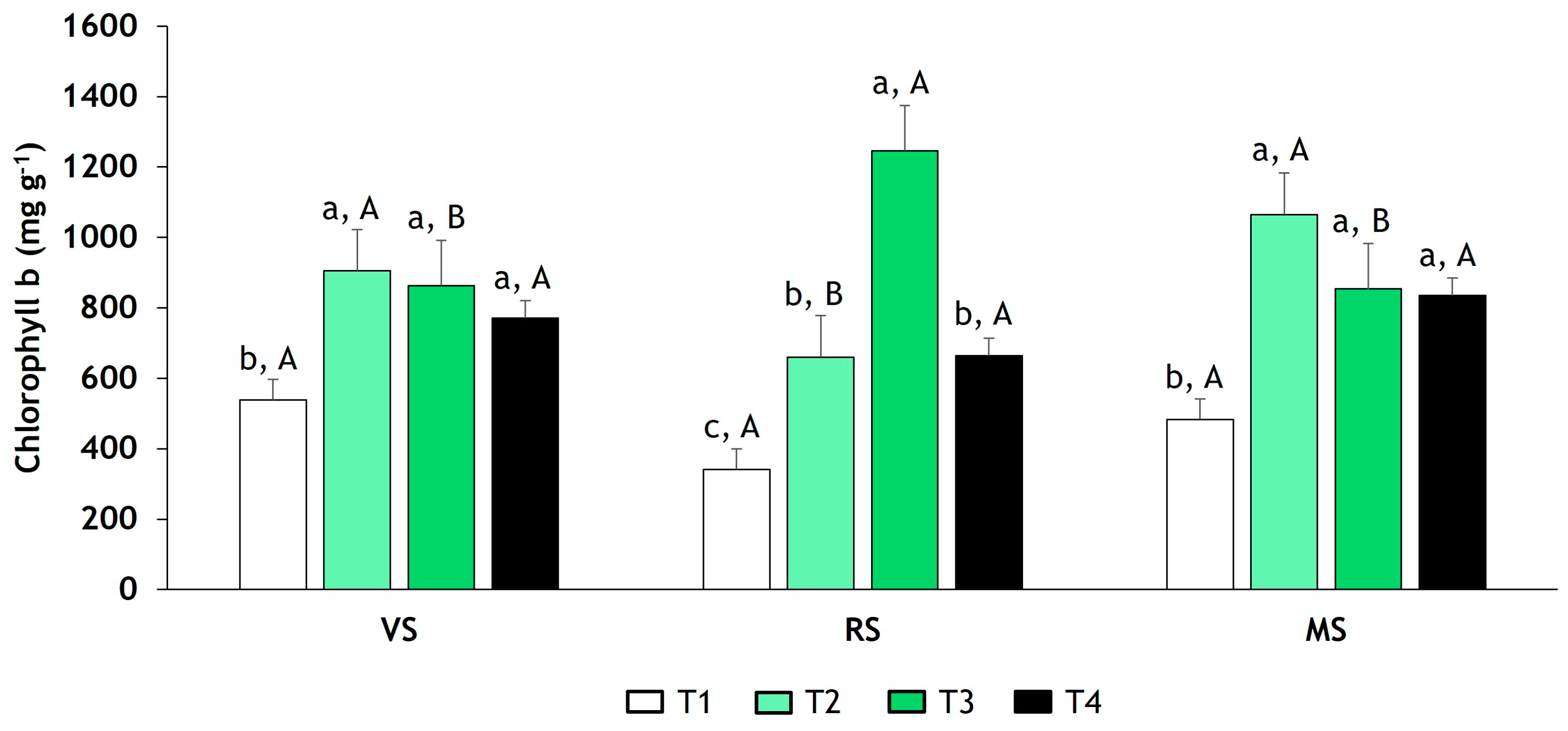
3.3. Determination of Chlorophyll a and b
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikuze, E.; Cromwell, S.; Ayayee, P.; Louis, J. Influence of microbes in mediating sorghum resistance to sugarcane aphids. Diversity 2024, 16, 85. [Google Scholar] [CrossRef]
- Liang, G.; Hua, Y.; Chen, H.; Luo, J.; Xiang, H.; Song, H.; Zhang, Z. Increased nitrogen use efficiency via amino acid remobilization from source to sink organs in Brassica napus. Crop J. 2023, 11, 119–131. [Google Scholar] [CrossRef]
- Carrasco, N.; Zamora, M.; Melin, A. Manual de Sorgo, 1st ed.; Instituto Nacional de Tecnología Agropecuaria, Ediciones INTA: Buenos Aires, Argentina, 2011; p. 110. [Google Scholar]
- Hussain, S.; Khaliq, A.; Noor, M.; Tanveer, M.; Hussain, H.; Hussain, S.; Shah, T.; Mehmood, T. Metal toxicity and nitrogen metabolism in plants: An Overview. In Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R., Pathan, S., Cecchereni, M., Eds.; Springer: Singapore, 2019; pp. 221–248. [Google Scholar] [CrossRef]
- Mandal, R.; Dutta, G. From photosynthesis to biosensing: Chlorophyll proves to be a versatile molecule. Sens. Int. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainable, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Rodríguez-Espinosa, T.; Papamichael, I.; Voukkali, I.; Pérez, A.; Almendro, M.; Navarro-Pedreño, J.; Zorpas, A.; Gómez, I. Nitrogen management in farming systems under the use of agricultural wastes and circular economy. Sci. Total Environ. 2023, 876, 162666. [Google Scholar] [CrossRef]
- Sedlar, O.; Balík, J.; Cerný, J.; Kulhánek, M.; Smatanová, M. Long-term application of organic fertilizers in relation to soil organic matter quality. Agronomy 2023, 13, 175. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S.; Sainger, M.; Sainger, P.; Barnawal, D. Eco-friendly nitrogen fertilizers for sustainable agriculture. In Adaptative Soil Management: From Theory to Practices; Rakshit, A., Abhilash, P., Singh, H., Ghosh, S., Eds.; Springer: Singapore, 2017; pp. 227–246. [Google Scholar] [CrossRef]
- Shi, W.; Zhao, H.; Chen, Y.; Wang, J.; Han, B.; Li, C.; Lu, J.; Zhang, L. Organic manure rather than phosphorus fertilization primarily determined asymbiotic nitrogen fixation rate and the stability of diazotrophic community in an upland red soil. Agric. Ecosyst. Environ. 2021, 319, 107535. [Google Scholar] [CrossRef]
- Ikyo, B.; Enenche, D.; Omotosho, S.; Ofeozo, M.; Rotimi, T. Spectroscopic analysis of the effect of organic and inorganic fertilizers on the chlorophylls pigment in amaranth and jute mallow vegetables. Nig. Ann. Pure Appl. Sci. 2020, 3, 115–121. [Google Scholar] [CrossRef]
- Cevheri, C.; Sakin, E.; Ramazanoglu, E. Effects of different fertilizers on some soil enzymes activity and chlorophyll contents of two cotton (G. hirsutum L.) varieties grown in a saline and non-saline soil. J. Plant Nutr. 2021, 45, 95–106. [Google Scholar] [CrossRef]
- Jaworski, E. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- O’neal, D.; Joy, K. Glutamine synthetase of pea leaves I. purification, stabilization, and pH optima. Arch. Biochem. Biophys. 1973, 159, 113–122. [Google Scholar] [CrossRef]
- Purbajanti, E.; Slamet, W.; Fuskhah, E. Nitrate reductase, chlorophyll content and antioxidant in okra (Abelmoschus esculentus Moench) under organic fertilizer. J. Appl. Hortic. 2019, 21, 213–217. [Google Scholar] [CrossRef]
- Purbajanti, E.; Slamet, W.; Fuskhah, E.; Rosyida, R. Effects of organic and inorganic fertilizers on growth, activity of nitrate reductase and chlorophyll contents of peanuts (Arachis hypogaea L.). IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012048. [Google Scholar] [CrossRef]
- Reis, A.; Favarin, J.; Gallo, L.; Malavolta, E.; Moraes, M.; Junior, J. Nitrate reductase and glutamine synthetase activity in coffee leaves during fruit development. Rev. Bras. Cienc. Solo 2009, 33, 315–324. [Google Scholar] [CrossRef]
- Kaur, A.; Bedi, S.; Kumar, M. Physiological basis of nitrogen use efficiency at variable nitrogen application rates in maize. Agric. Res. J. 2019, 56, 40–48. [Google Scholar] [CrossRef]
- Xiong, H.; Ma, H.; Hu, B.; Zhao, H.; Wang, J.; Rennenberg, H.; Shi, X. Nitrogen fertilization stimulates nitrogen assimilation and modifies nitrogen partitioning in the spring shoot leaves of citrus (Citrus reticulata Blanco) trees. J. Plant Physiol. 2021, 267, 153556. [Google Scholar] [CrossRef]
- Chen, H.; Huang, L. Effect of nitrogen fertilizer application rate on nitrate reductase activity in maize. Appl. Ecol. Env. Res. 2020, 18, 2879–2894. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Z.; Chen, G.; Zou, G.; Song, X.; Liu, F. Runoff nitrogen (N) losses and related metabolism enzyme activities in paddy field under different nitrogen fertilizer levels. Environ. Sci. Pollut. Res. 2018, 25, 27583–27593. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Lobato, A.; Costa, R.; Maia, W.; Santos, B.; Alves, G.; Brinez, B.; Neves, H.; Santos, M.; Cruz, F. Nitrogen compounds and enzyme activities in sorghum induced to water deficit during three stages. Plant Soil Environ. 2009, 55, 238–244. [Google Scholar] [CrossRef]
- Da Silva, A.; Borges, V.; Guimaraes, L.; De Almeida, D.; Martins, M.; Zuffo, C.; Rodrigues, S.; Lucena, I. Nitrate reductase activity in the different phenophases of “palmer” mango cultivated in the semiarid. J. Appl. Bot. Food Qual. 2021, 94, 192–198. [Google Scholar] [CrossRef]
- Argentel, L.; Garatuza, J.; Arredondo, T.; Yépez, E. Effects of experimental warming on peroxide, nitrate reductase and glutamine synthetase activities in wheat. Agron. Res. 2019, 17, 22–32. [Google Scholar] [CrossRef]
- Rehman, M.; Yang, M.; Fahad, S.; Salem, M.; Liu, L.; Liu, F.; Deng, G. Morpho-physiological traits, antioxidant capacity and nitrogen metabolism in Boehmeria nivea L. under nitrogen fertilizer. Agron. J. 2020, 112, 2988–2997. [Google Scholar] [CrossRef]
- Kirova, E.; Geneva, M.; Kostadinov, K.; Filipov, S. Improving yield and quality-related physiological characteristics of lettuce by integrated inorganic and organic fertilizers management. Saudi J. Biol. Sci. 2022, 27, 797–804. [Google Scholar] [CrossRef]
- Iqbal, A.; He, L.; Ali, I.; Ullah, S.; Khan, A.; Akhtar, K.; Wei, S.; Fahad, S.; Khan, R.; Jiang, L. Co-incorporation of manure and inorganic fertilizer improves leaf physiological traits, rice production and soil functionality in paddy field. Sci. Rep. 2021, 11, 10048. [Google Scholar] [CrossRef]
- Singh, P.; Tomar, R.; Kumar, K.; Kumar, B.; Rakshit, S.; Singh, I. Morpho-physiological and biochemical characterization of maize genotypes under nitrogen stress conditions. Indian J. Genet. Pl. Br. 2021, 81, 255–265. [Google Scholar] [CrossRef]
- Neto, A.; Favarin, J.; dos Reis, A.; Tezotto, T.; Munhoz, E.; Lavres, J.; Gallo, L. Nitrogen metabolism in coffee plants in response to nitrogen supply by fertigation. Theor. Exp. Plant Phys. 2015, 27, 41–50. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef]
- Guo, N.; Gu, M.; Hu, J.; Qu, H.; Xu, G. Rice OsLHT1 functions in leaf-to-panicle nitrogen allocation for grain yield and quality. Front. Plant Sci. 2020, 11, 1150. [Google Scholar] [CrossRef]
- Mendoza-Tafolla, R.; Juarez-Lopez, P.; Ontiveros-Capurata, R.; Sandoval-Villa, M.; Alia-Tejacal, I.; Alejo-Santiago, G. Estimating nitrogen and chlorophyll status of romaine lettuce using SPAD and at LEAF readings. Not. Bot. Horti Agrobo. Cluj-Napoca 2019, 47, 751–756. [Google Scholar] [CrossRef]
- Chamle, D.; Raut, D. Evaluation of organic and inorganic fertilizers on chlorophyll content, leaf area and yield of maize. EPRA J. Agric. Rural. Econ. Res. 2021, 10, 15–17. [Google Scholar]
- Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Ibrahim, M.; Salih, G. Effect of N on growth, antioxidant capacity, and chlorophyll content of sorghum. Agronomy 2022, 12, 501. [Google Scholar] [CrossRef]
- Biswal, A.; Pattanayak, G.; Pandey, S.; Leelavathi, S.; Reddy, V.; Govindjee, G.; Tripathy, B. Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant Physiol. 2012, 159, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Cevahir, G.; Yentür, S.; Yazgan, M.; Ünal, M.; Yilmazer, N. Peroxidase activity in relation to anthocyanin and chlorophyll content in juvenile and adult leaves of “MINI-STAR” Gazania splendens. Pak. J. Bot. 2004, 36, 603–609. [Google Scholar]
- Tranaviciene, T.; Siksnianiene, J.; Urbonaviciute, A.; Vaguseviciene, I.; Samuoliene, G.; Duchovskis, P.; Sliesaravicius, A. Effects of nitrogen fertilizers on wheat photosynthetic pigment and carbohydrate contents. Biologija 2007, 53, 80–84. [Google Scholar] [CrossRef]
- Padilla, F.; Peña-Fleitas, M.; Gallardo, M.; Giménez, C.; Thompson, B. Derivation of sufficiency values of chlorophyll meter to estimate cucumber nitrogen status and yield. Comput. Electron. Agric. 2017, 141, 54–64. [Google Scholar] [CrossRef]
- Souza, C.; Brant, C.; Soares, R. Chlorophyll index (SPAD) and macronutrients relation and productive performance of sorghum hybrids in different sowing dates. Aust. J. Crop Sci. 2016, 10, 546–555. [Google Scholar] [CrossRef]
- Argenta, G.; Regis, P.; Sangoi, L. Leaf relative chlorophyll content as an indicator parameter to predict nitrogen fertilization in maize. Cienc. Rural. 2004, 34, 1379–1387. [Google Scholar] [CrossRef]
- Kraj, W. Chlorophyll degradation and the activity of chlorophyllase and Mg-dechelatase during leaf senescence in Fagus sylvatica. Dendrobiology 2015, 74, 43–57. [Google Scholar] [CrossRef]

| Treatments | Description |
|---|---|
| T1 | Soil (4.4% organic matter; nitrogen 100 ppm; phosphorus 0.80 ppm; potassium 5.50 ppm; and pH 7.4) |
| T2 | 100 kg ha−1 N (chicken manure) + soil |
| T3 | 200 kg ha−1 N (chicken manure) + soil |
| T4 | 100 kg ha−1 N (ammonium sulfate) + soil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieves-Silva, E.; Sandoval-Castro, E.; Delgado-Alvarado, A.; Castañeda-Antonio, M.D.; Huerta-De la Peña, A. Nitrate Reductase and Glutamine Synthetase Enzyme Activities and Chlorophyll in Sorghum Leaves (Sorghum bicolor) in Response to Organic Fertilization. Int. J. Plant Biol. 2024, 15, 827-836. https://doi.org/10.3390/ijpb15030059
Nieves-Silva E, Sandoval-Castro E, Delgado-Alvarado A, Castañeda-Antonio MD, Huerta-De la Peña A. Nitrate Reductase and Glutamine Synthetase Enzyme Activities and Chlorophyll in Sorghum Leaves (Sorghum bicolor) in Response to Organic Fertilization. International Journal of Plant Biology. 2024; 15(3):827-836. https://doi.org/10.3390/ijpb15030059
Chicago/Turabian StyleNieves-Silva, Ericka, Engelberto Sandoval-Castro, Adriana Delgado-Alvarado, María D. Castañeda-Antonio, and Arturo Huerta-De la Peña. 2024. "Nitrate Reductase and Glutamine Synthetase Enzyme Activities and Chlorophyll in Sorghum Leaves (Sorghum bicolor) in Response to Organic Fertilization" International Journal of Plant Biology 15, no. 3: 827-836. https://doi.org/10.3390/ijpb15030059








