Synergism or Antagonism: Do Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Work Together to Benefit Plants?
Abstract
:1. Introduction
2. Plant Growth-Promoting Rhizobacteria (PGPR)
2.1. Mechanisms of Mitigating Plant Drought Stress
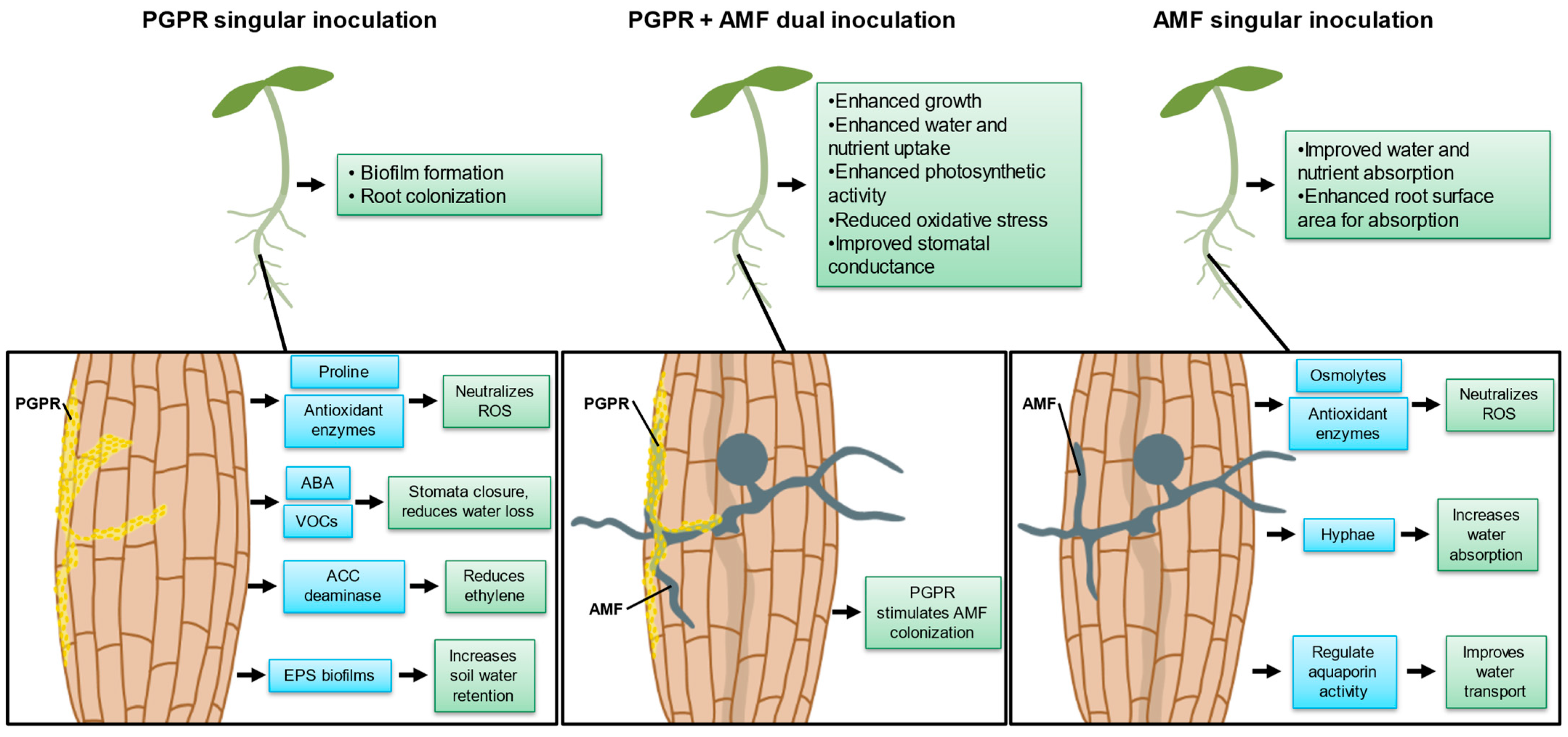
2.1.1. Increased Proline Production
2.1.2. Antioxidant Enzyme Production
2.1.3. ABA Production, Regulating Stomatal Closure
2.1.4. Reduced Ethylene Overproduction
2.1.5. Volatile Organic Compound (VOC) Production
2.1.6. Extracellular Polymeric Substance (EPS) Production
2.1.7. Summary
2.2. PGPR and Plant Defense Response
2.3. PGPR Mitigating Plant Drought and Pathogen Stress
3. Arbuscular Mycorrhizal Fungi
3.1. AMF Mechanisms of Mitigating Plant Drought Stress
3.1.1. Hyphae Improve Plant Water Uptake
3.1.2. Increased Reactive Oxygen Species (Hydrogen Peroxide) Efflux
3.1.3. Increased Osmolyte Production
3.1.4. Increased Antioxidant Enzyme Production
3.1.5. Regulation of Aquaporins to Control Water Movement
3.1.6. Summary
3.2. AMF and Plant Defense Response
3.3. AMFs Improve Drought and Pathogen Resistance
4. AMF and PGPR Dual Inoculation
4.1. Interactions of AMF and PGPR during Dual Inoculation
4.2. Dual AMF and PGPR Inoculation Mitigates Plant Drought Stress
4.3. AMF and PGPR Dual Inoculation Improves Pathogen Resistance
5. Commercial AMF and PGPR Inoculums in the Agricultural Sector
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, S.; Newton, A.C. Climate Change, Plant Diseases and Food Security: An Overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Nwosu, N.J.; Li, Y. Abiotic Stress in Agricultural Crops Under Climatic Conditions. In Sustainable Agriculture, Forest and Environmental Management; Jhariya, M.K., Banerjee, A., Meena, R.S., Yadav, D.K., Eds.; Springer: Singapore, 2019; pp. 71–100. ISBN 978-981-13-6830-1. [Google Scholar]
- Usta, C. Microorganisms in Biological Pest Control—A Review (Bacterial Toxin Application and Effect of Environmental Factors). In Current Progress in Biological Research; Silva-Opps, M., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1097-2. [Google Scholar]
- de Faria, M.R.; Costa, L.S.A.S.; Chiaramonte, J.B.; Bettiol, W.; Mendes, R. The Rhizosphere Microbiome: Functions, Dynamics, and Role in Plant Protection. Trop. Plant Pathol. 2021, 46, 13–25. [Google Scholar] [CrossRef]
- Vidal, C.; González, F.; Santander, C.; Pérez, R.; Gallardo, V.; Santos, C.; Aponte, H.; Ruiz, A.; Cornejo, P. Management of Rhizosphere Microbiota and Plant Production under Drought Stress: A Comprehensive Review. Plants 2022, 11, 2437. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Khaliq, A.; Sattar, A.; Shukla, R.S.; Anwar, M.; Dharni, S. Synergistic Effect of Arbuscular Mycorrhizal Fungi and Bacillus Subtilis on the Biomass and Essential Oil Yield of Rose-Scented Geranium (Pelargonium Graveolens). Arch. Agron. Soil Sci. 2011, 57, 889–898. [Google Scholar] [CrossRef]
- Toro, M.; Azcon, R.; Barea, J. Improvement of Arbuscular Mycorrhiza Development by Inoculation of Soil with Phosphate-Solubilizing Rhizobacteria To Improve Rock Phosphate Bioavailability ((Sup32)P) and Nutrient Cycling. Appl. Environ. Microbiol. 1997, 63, 4408–4412. [Google Scholar] [CrossRef]
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G. Species-Specific Interactions of Bacillus Innocula and Arbuscular Mycorrhizal Fungi Symbiosis with Winter Wheat. Microorganisms 2020, 8, 1795. [Google Scholar] [CrossRef]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as Strategies to Improve Photosynthetic Apparatus, Growth, and Drought Stress Tolerance in the Date Palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-Inoculation of Arbuscular Mycorrhizal Fungi and the Plant Growth-Promoting Rhizobacteria Improve Growth and Photosynthesis in Tobacco Under Drought Stress by Up-Regulating Antioxidant and Mineral Nutrition Metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef]
- Marulanda, A.; Barea, J.M.; Azcón, R. An Indigenous Drought-Tolerant Strain of Glomus Intraradices Associated with a Native Bacterium Improves Water Transport and Root Development in Retama Sphaerocarpa. Microb. Ecol. 2006, 52, 670–678. [Google Scholar] [CrossRef]
- Nanjundappa, A.; Bagyaraj, D.J.; Saxena, A.K.; Kumar, M.; Chakdar, H. Interaction between Arbuscular Mycorrhizal Fungi and Bacillus spp. in Soil Enhancing Growth of Crop Plants. Fungal Biol. Biotechnol. 2019, 6, 23. [Google Scholar] [CrossRef]
- Vivas, A.; Marulanda, A.; Ruiz-Lozano, J.M.; Barea, J.M.; Azcón, R. Influence of a Bacillus sp. on Physiological Activities of Two Arbuscular Mycorrhizal Fungi and on Plant Responses to PEG-Induced Drought Stress. Mycorrhiza 2003, 13, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Chandrasekaran, M.; Shagol, C.; Kim, K.-Y.; Sa, T.-M. Spore Associated Bacteria (SAB) of Arbuscular Mycorrhizal Fungi (AMF) and Plant Growth Promoting Rhizobacteria (PGPR) Increase Nutrient Uptake and Plant Growth Under Stress Conditions. Korean J. Soil Sci. Fertil. 2012, 45, 582–592. [Google Scholar] [CrossRef]
- Primieri, S.; Magnoli, S.M.; Koffel, T.; Stürmer, S.L.; Bever, J.D. Perennial, but Not Annual Legumes Synergistically Benefit from Infection with Arbuscular Mycorrhizal Fungi and Rhizobia: A Meta-analysis. New Phytol. 2022, 233, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, D. Plant Growth Promoting Rhizobacteria-an Efficient Tool for Agriculture Promotion. Adv. Plants Agric. Res. 2016, 4, 426–434. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schroth, M.N. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the Station de Pathologie, 4th International Conference on Plant Pathogenic Bacteria, Tours, France, 27 August–2 September 1978; Végétale et Phyto-Bactériologie, Ed.; pp. 879–882. [Google Scholar]
- Borriss, R. Use of Plant-Associated Bacillus Strains as Biofertilizers and Biocontrol Agents in Agriculture. In Bacteria in Agrobiology: Plant Growth Responses; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 41–76. ISBN 978-3-642-20331-2. [Google Scholar]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent Advances in PGPR and Molecular Mechanisms Involved in Drought Stress Resistance. J. Soil Sci. Plant Nutr. 2023, 23, 106–124. [Google Scholar] [CrossRef]
- Wang, C.-J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.-D.; Liu, H.-X.; Wang, Y.-P.; Guo, J.-H. Induction of Drought Tolerance in Cucumber Plants by a Consortium of Three Plant Growth-Promoting Rhizobacterium Strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-Tolerant Plant Growth Promoting Bacillus spp.: Effect on Growth, Osmolytes, and Antioxidant Status of Maize under Drought Stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Armada, E.; Barea, J.-M.; Castillo, P.; Roldán, A.; Azcón, R. Characterization and Management of Autochthonous Bacterial Strains from Semiarid Soils of Spain and Their Interactions with Fermented Agrowastes to Improve Drought Tolerance in Native Shrub Species. Appl. Soil Ecol. 2015, 96, 306–318. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-Tolerant Bacillus Megaterium Isolated from Semi-Arid Conditions Induces Systemic Tolerance of Wheat under Drought Conditions. Plant Cell Rep. 2022, 41, 549–569. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Thangappan, S.; Uthandi, S. Plant Growth-Promoting Bacillus sp. Cahoots Moisture Stress Alleviation in Rice Genotypes by Triggering Antioxidant Defense System. Microbiol. Res. 2020, 239, 126518. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. Guard Cell Abscisic Acid Signalling and Engineering Drought Hardiness in Plants. Nature 2001, 410, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum Brasilense Ameliorates the Response of Arabidopsis Thaliana to Drought Mainly via Enhancement of ABA Levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.V.; Bottini, R.; De Souza Filho, G.A.; Cohen, A.C.; Moreno, D.; Gil, M.; Piccoli, P. Bacteria Isolated from Roots and Rhizosphere of Vitis Vinifera Retard Water Losses, Induce Abscisic Acid Accumulation and Synthesis of Defense-related Terpenes in in Vitro Cultured Grapevine. Physiol. Plant. 2014, 151, 359–374. [Google Scholar] [CrossRef]
- Vandana, U.K.; Singha, B.; Gulzar, A.B.M.; Mazumder, P.B. Molecular Mechanisms in Plant Growth Promoting Bacteria (PGPR) to Resist Environmental Stress in Plants. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–233. ISBN 978-0-12-818469-1. [Google Scholar]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. Microbial Modulation of Plant Ethylene Signaling: Ecological and Evolutionary Consequences. Microbiome 2018, 6, 52. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kim, S.-D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus Licheniformis K11 in Pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-Ul-Hye, M.; Hussain, S.; Riaz, M.; Qayyum, M.F. Mitigation of Drought Stress in Maize through Inoculation with Drought Tolerant ACC Deaminase Containing PGPR under Axenic Conditions. Pak. J. Bot. 2020, 52, 49–60. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R,3R-Butanediol, a Bacterial Volatile Produced by Pseudomonas Chlororaphis O6, Is Involved in Induction of Systemic Tolerance to Drought in Arabidopsis Thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef]
- Cho, S.-M.; Kim, Y.H.; Anderson, A.J.; Kim, Y.C. Nitric Oxide and Hydrogen Peroxide Production Are Involved in Systemic Drought Tolerance Induced by 2R,3R-Butanediol in Arabidopsis Thaliana. Plant Pathol. J. 2013, 29, 427–434. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial Volatiles Promote Growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Roberson, E.B.; Firestone, M.K. Relationship between Desiccation and Exopolysaccharide Production in a Soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R.; et al. Improved Plant Resistance to Drought Is Promoted by the Root-associated Microbiome as a Water Stress-dependent Trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, R.; Manzanera, M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, F.M.; Romero, D.; Pérez-García, A.; Lugtenberg, B.J.J.; Vicente, A.D.; Bloemberg, G. Isolation and Characterization of Antagonistic Bacillus Subtilis Strains from the Avocado Rhizoplane Displaying Biocontrol Activity: Characterization of Antagonistic Bacillus. J. Appl. Microbiol. 2007, 103, 1950–1959. [Google Scholar] [CrossRef]
- Lowe, A.; Rafferty-McArdle, S.M.; Cassells, A.C. Effects of AMF- and PGPR-Root Inoculation and a Foliar Chitosan Spray in Single and Combined Treatments on Powdery Mildew Disease in Strawberry. Agric. Food Sci. 2012, 21, 28–38. [Google Scholar] [CrossRef]
- Medeiros, F.H.V.; Souza, R.M.; Medeiros, F.C.L.; Zhang, H.; Wheeler, T.; Payton, P.; Ferro, H.M.; Paré, P.W. Transcriptional Profiling in Cotton Associated with Bacillus Subtilis (UFLA285) Induced Biotic-Stress Tolerance. Plant Soil 2011, 347, 327–337. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The Role of Mycorrhizae and Plant Growth Promoting Rhizobacteria (PGPR) in Improving Crop Productivity under Stressful Environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Pertot, I.; Puopolo, G.; Hosni, T.; Pedrotti, L.; Jourdan, E.; Ongena, M. Limited Impact of Abiotic Stress on Surfactin Production in Planta and on Disease Resistance Induced by Bacillus Amyloliquefaciens S499 in Tomato and Bean. FEMS Microbiol. Ecol. 2013, 86, 505–519. [Google Scholar] [CrossRef]
- Prathuangwong, S.; Chuaboon, W.; Chatnaparat, T.; Kladsuwan, L.; Shoorin, M.; Kasem, S. Induction of Disease and Drought Resistance in Rice by Pseudomonas Fluorescens SP007s. CMU J. Nat. Sci. Spec. Issue Agric. Nat. Res. 2012, 11, 45–55. [Google Scholar]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dames, J.F.; Gupta, A.; Sharma, S.; Gilbert, J.A.; Ahmad, P. Current Developments in Arbuscular Mycorrhizal Fungi Research and Its Role in Salinity Stress Alleviation: A Biotechnological Perspective. Crit. Rev. Biotechnol. 2015, 35, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Hage-Ahmed, K.; Rosner, K.; Steinkellner, S. Arbuscular Mycorrhizal Fungi and Their Response to Pesticides. Pest Manag. Sci. 2019, 75, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.C. Drought Stress and Mycorrhizal Plant. In Use of Microbes for the Alleviation of Soil Stresses, Volume 1; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; pp. 97–110. ISBN 978-1-4614-9465-2. [Google Scholar]
- Zhang, F.; Zou, Y.-N.; Wu, Q.-S. Quantitative Estimation of Water Uptake by Mycorrhizal Extraradical Hyphae in Citrus under Drought Stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Gong, M.; Tang, M.; Chen, H.; Zhang, Q.; Feng, X. Effects of Two Glomus Species on the Growth and Physiological Performance of Sophora Davidii Seedlings under Water Stress. New For. 2013, 44, 399–408. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Zou, Y.-N.; Wu, Q.-S. Alleviation of Drought Stress by Mycorrhizas Is Related to Increased Root H2O2 Efflux in Trifoliate Orange. Sci. Rep. 2017, 7, 42335. [Google Scholar] [CrossRef]
- Zou, Y.-N.; Huang, Y.-M.; Wu, Q.-S.; He, X.-H. Mycorrhiza-Induced Lower Oxidative Burst Is Related with Higher Antioxidant Enzyme Activities, Net H2O2 Effluxes, and Ca2+ Influxes in Trifoliate Orange Roots under Drought Stress. Mycorrhiza 2015, 25, 143–152. [Google Scholar] [CrossRef]
- Al-Arjani, A.-B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular Mycorrhizal Fungi Modulates Dynamics Tolerance Expression to Mitigate Drought Stress in Ephedra Foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Abbaspour, H.; Saeidi-Sar, S.; Afshari, H.; Abdel-Wahhab, M.A. Tolerance of Mycorrhiza Infected Pistachio (Pistacia vera L.) Seedling to Drought Stress under Glasshouse Conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef]
- Sepahvand, T.; Etemad, V.; Matinizade, M.; Shirvany, A. Symbiosis of AMF with Growth Modulation and Antioxidant Capacity of Caucasian Hackberry (Celtis caucasica L.) Seedlings under Drought Stress. Cent. Asian J. Environ. Sci. Technol. Innov. 2021, 2, 20–35. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New Insights into the Regulation of Aquaporins by the Arbuscular Mycorrhizal Symbiosis in Maize Plants Under Drought Stress and Possible Implications for Plant Performance. Mol. Plant Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, Y.; Hao, Z.; Li, H.; Wang, Y.; Chen, B. First Cloning and Characterization of Two Functional Aquaporin Genes from an Arbuscular Mycorrhizal Fungus Glomus Intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.M.P.; Prabina, B.J. Protection of Tomato, Lycopersicon Esculentum from Wilt Pathogen, Fusarium Oxysporum f.sp. Lycopersici by Arbuscular Mycorrhizal Fungi, Glomus sp. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1368–1378. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Hou, H.; Lei, H.; Zhu, X.; Li, X.; He, X.; Tian, C. Arbuscular Mycorrhizal Fungi-Enhanced Resistance against Phytophthora Sojae Infection on Soybean Leaves Is Mediated by a Network Involving Hydrogen Peroxide, Jasmonic Acid, and the Metabolism of Carbon and Nitrogen. Acta Physiol. Plant 2013, 35, 3465–3475. [Google Scholar] [CrossRef]
- Khoshkhatti, N.; Eini, O.; Koolivand, D.; Pogiatzis, A.; Klironomos, J.N.; Pakpour, S. Differential Response of Mycorrhizal Plants to Tomato Bushy Stunt Virus and Tomato Mosaic Virus Infection. Microorganisms 2020, 8, 2038. [Google Scholar] [CrossRef] [PubMed]
- Math, S.; Arya, S.; Sonawane, H.; Patil, V.; Chaskar, M. Arbuscular Mycorrhizal (Glomus fasciculatum) Fungi as a Plant Immunity Booster against Fungal Pathogen. Curr. Agri. Res. J. 2019, 7, 99–107. [Google Scholar] [CrossRef]
- Oyewole, B.O.; Olawuyi, O.J.; Odebode, A.C.; Abiala, M.A. Influence of Arbuscular Mycorrhiza Fungi (AMF) on Drought Tolerance and Charcoal Rot Disease of Cowpea. Biotechnol. Rep. 2017, 14, 8–15. [Google Scholar] [CrossRef]
- Meddich, A.; Ait El Mokhtar, M.; Bourzik, W.; Mitsui, T.; Baslam, M.; Hafidi, M. Optimizing Growth and Tolerance of Date Palm (Phoenix dactylifera L.) to Drought, Salinity, and Vascular Fusarium-Induced Wilt (Fusarium oxysporum) by Application of Arbuscular Mycorrhizal Fungi (AMF). In Root Biology; Giri, B., Prasad, R., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 52, pp. 239–258. ISBN 978-3-319-75909-8. [Google Scholar]
- Garmendia, I.; Goicoechea, N.; Aguirreolea, J. Moderate Drought Influences the Effect of Arbuscular Mycorrhizal Fungi as Biocontrol Agents against Verticillium-Induced Wilt in Pepper. Mycorrhiza 2005, 15, 345–356. [Google Scholar] [CrossRef]
- Mohamed, I.; Eid, K.E.; Abbas, M.H.H.; Salem, A.A.; Ahmed, N.; Ali, M.; Shah, G.M.; Fang, C. Use of Plant Growth Promoting Rhizobacteria (PGPR) and Mycorrhizae to Improve the Growth and Nutrient Utilization of Common Bean in a Soil Infected with White Rot Fungi. Ecotoxicol. Environ. Saf. 2019, 171, 539–548. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Roldán, A. Interactions between a Plant Growth-Promoting Rhizobacterium, an AM Fungus and a Phosphate-Solubilising Fungus in the Rhizosphere of Lactuca sativa. Appl. Soil. Ecol. 2007, 35, 480–487. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Basiru, S.; Hijri, M. The Potential Applications of Commercial Arbuscular Mycorrhizal Fungal Inoculants and Their Ecological Consequences. Microorganisms 2022, 10, 1897. [Google Scholar] [CrossRef] [PubMed]


| PGPR | Plant | Response | Reference |
|---|---|---|---|
| Bacillus cereus AR156, Bacillus subtilis SM21, and Serratia sp. XY21 | Cucumber | Increased leaf proline and chlorophyll content, decreased wilt symptoms | [21] |
| Bacillus spp. | Maize | Increased proline content, increased water and nutrient uptake, increased biomass | [22] |
| Enterobacter sp., Bacillus thuringiensis, Bacillus sp., and Bacillus megaterium | Shrub species | Improved nutrition, improved morphological traits, increased proline production, increased ACC deaminase production | [23] |
| Bacillus megaterium | Wheat | Increased proline content, relative water content, protein content, and chlorophyll a, b, carotenoids, and antioxidant enzyme activity | [24] |
| Bacillus altitudinis FD48 and Bacillus methylotrophicus RABA6 | Rice | Increased antioxidant enzyme activity, mitigated drought stress | [25] |
| Azospirillum brasilense Sp 245 | Arabidopsis | Increased ABA production, plant biomass, and seed yield | [27] |
| Bacillus licheniformis Rt4M10 and Pseudomonas fluorescens Rt6M10 | Grapevine | Increased ABA production, water content, and turgidity | [28] |
| Bacillus licheniformis | Pepper | Increased ACC deaminase production and plant growth | [31] |
| Pseudomonas aeruginosa, Enterobacter cloacae, Achromobacter xylosoxidans, Leclercia adecarboxylata | Maize | Increased ACC deaminase production, shoot and root length | [32] |
| Bacillus subtilis GB03, Bacillus amyloliquefaciens IN937a | Arabidopsis | VOC 2,3-butanediol and acetoin production, increased plant growth | [35] |
| Pseudomonas chlororaphis | Arabidopsis | VOC 2,3-butanediol production, improved drought tolerance | [33] |
| Pseudomonas sp. S1, Acinetobacter sp. S2, Pseudomonas sp. S3, Bacillus sp. S4, Delftia sp. S5 and Sphingobacterium sp. S6 | Grapevine | Increased plant growth, mitigated drought stress | [38] |
| AMF | Plant | Response | Reference |
|---|---|---|---|
| Funneliformis mosseae, Paraglomus occultum | Trifoliate orange | Increased water absorption rate via AMF hyphae | [50] |
| Funneliformis mosseae, Glomus constrictum | Sophora davidii | Increased plan biomass, root length, and water use efficiency via AMF hyphae | [51] |
| Funneliformis mosseae | Trifoliate orange | Increased hydrogen peroxide root efflux, biomass, and plant growth promotion | [52,53] |
| Glomus etunicatum, Rhizophagus irregularis, Funneliformis mosseae | Shrubby horsetail | Increased osmolyte production, antioxidant enzyme activity, and plant growth | [54] |
| Glomus etunicatum | Pistachio | Increased proline content, antioxidant enzyme production, and plant growth | [55] |
| Rhizophagus intraradices, Funneliformis mosseae | Caucasian hackberry | Increased antioxidant enzyme activity and plant growth | [56] |
| Rhizophagus irregularis | Maize | Increased expression of aquaporin genes, improved water transport | [58] |
| Rhizophagus intraradices | Maize | Improved aquaporin regulation, increased plant growth | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savastano, N.; Bais, H. Synergism or Antagonism: Do Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Work Together to Benefit Plants? Int. J. Plant Biol. 2024, 15, 944-958. https://doi.org/10.3390/ijpb15040067
Savastano N, Bais H. Synergism or Antagonism: Do Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Work Together to Benefit Plants? International Journal of Plant Biology. 2024; 15(4):944-958. https://doi.org/10.3390/ijpb15040067
Chicago/Turabian StyleSavastano, Noah, and Harsh Bais. 2024. "Synergism or Antagonism: Do Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Work Together to Benefit Plants?" International Journal of Plant Biology 15, no. 4: 944-958. https://doi.org/10.3390/ijpb15040067






