Abstract
Introduction: Mastectomy skin necrosis (MSN) is a common complication occurring in up to 50% of patients. In patients with risk factors for poor wound healing such as immunosuppression, prior radiotherapy (XRT), and high body mass index (BMI > 30.0), this number is even higher. MSN can lead to infection, loss of reconstruction, poorer aesthetics, and most ominously, delay in adjuvant cancer therapy. Instead of forgoing reconstruction in these patients, adjunctive therapies to optimize wound healing are necessary. The purpose of this study is to introduce the use of cryopreserved umbilical tissue (vCUT) as an adjunct therapy for high-risk-wound-healing immediate breast reconstruction (IBR) patients. Methods: All patients who underwent breast reconstruction with vCUT as an adjunctive therapy were identified and retrospectively analyzed. Results: Seven patients who underwent breast reconstruction with vCUT placement were identified. These patients had risk factors for delayed healing, such as obesity, immunosuppression, and/or prior XRT. The mean post-operative follow-up was 252 days (range 183–287). Four out of seven patients demonstrated post-operative complications: two out of seven developed seromas, two out of seven developed wound dehiscence, two out of seven developed infection, two out of seven developed MSN, and two out of seven lost their reconstruction. Conclusion: As undergoing IBR leads to improved mental health and superior aesthetic outcomes, efforts to expand current indications for safe IBR to traditionally poorer reconstructive candidates are imperative. The results of this case series demonstrate vCUT as a promising novel adjunctive tool in the reconstructive surgeons’ armamentarium in managing the less ideal reconstructive breast candidate.
1. Introduction
Immediate breast reconstruction (IBR) has become increasingly popular among patients undergoing mastectomy, improving their quality of life and psychological well-being [1]. However, despite these advantages, IBR can result in mastectomy skin necrosis (MSN), with a reported incidence from 8.1% to 41.2% [2,3,4,5]. MSN can in turn lead to infection, additional surgery to promote wound closure, prolonged need for wound care, poor aesthetics, and most importantly, delays in the timely initiation of adjuvant oncologic treatment [3,4]. As a result, these complications can contribute to poor reconstructive outcomes, decreased patient satisfaction, and increased health care costs. Studies have linked MSN to a variety of factors, including high body mass index (BMI) (>30) [6,7], history of smoking [7,8,9], hypertension [10], diabetes [11], pre-operative XRT [11], and history of immunosuppression [6]. Many patients with these risk factors are not offered reconstruction due to increased risk of developing wound-healing complications, subsequent infection, and reconstruction loss, particularly implant based [12]. However, given the detrimental mental health effects of forgoing or delaying reconstruction in this already vulnerable population [13,14] with often unmodifiable risk factors, adjunctive therapies to optimize wound healing are necessary to safely offer them IBR.
Human placental membranes (hPM) may provide a viable adjunctive therapy for breast reconstruction patients at high risk for wound-healing complications due to their antimicrobial, analgesic, proangiogenic, and anti-inflammatory properties [15]. Studies have demonstrated tremendous regenerative benefit in traditionally recognized hostile wounds beds (i.e., vasculopathy and diabetes) in poor surgical candidates [16]. Specifically, in the diabetic foot and venous stasis ulcer (DFU and VSU, respectively) population, hPMs have shown significant improvements in healing rates [16]. They also have clinical benefits in burns, infection control, pain reduction, and thermoregulation maintenance [17,18]. Innovations in the tissue-regenerative medicine sphere have led to the development of amniotic coverings, wraps, and barriers that, when applied, serve as an adherent dressing that provides additional support and reduces post-operative inflammation, adhesion, and fibrosis [19,20,21]. Viable cryopreserved umbilical tissue (vCUT; Stravix, Osiris Therapeutics, Inc, Columbia, MD, USA) is a commercially available allograft composed of umbilical amnion and Wharton’s jelly processed using an aseptic cryopreservation technology that retains all of the native components, including the extracellular matrix rich in hyaluronic acid and growth factors [19,20]. vCUT also retains the anti-inflammatory, antioxidant, antimicrobial, anti-adhesion, and angiogenic properties [19,20,21]. Importantly, and conversely to its off-the-shelf counterparts, the cryopreservation allows the resident stem cells, thought to be most responsible for honing a pro-wound-healing milieu, to remain viable [19,20,21]. Through the retention of these properties, vCUT can be applied to reinforce wound closures to prevent and treat surgical complications [22].
Here, we introduce the use of vCUT as an adjunct therapy in a small case series of high risk for wound-healing IBR patients to illustrate its safety for this novel indication. The results of this small case series demonstrate vCUT as a promising potential adjunct therapy to decrease risks for post-operative MSN in complex reconstructive breast cancer patients.
2. Materials and Methods
Following Institutional Review Board (IRB) approval, seven patients who underwent breast reconstruction with vCUT at a large academic medical center were identified from a prospectively maintained database from 2022 to 2023. Demographic data extracted from each patient included age, race, ethnicity, BMI, history of diabetes mellitus (DM), tobacco use, immunosuppression, XRT, chemotherapy, and previous wound-healing complications. Procedure-related data extracted included type of breast reconstruction, number of vCUTs used, and vCUT dimensions. Post-operative complication rate, including MSN, seroma, hematoma, infection, dehiscence, and mean follow-up time, were evaluated. Infection was defined as erythema, signs or symptoms of systemic infection, and clinical need for antibiotics or resultant operative intervention to treat MSN or infection, as determined by the lead investigator.
Surgical Technique
The patients were taken to the operating room and placed under general anesthesia. Breast reconstructive surgery was performed with placement of a tissue expander or breast implant. Size 15 French Blake drains were placed. To reinforce the closure, inferolateral mastectomy skin was de-epithelialized and advanced under the upper mastectomy skin flap. Alternatively, Wise-pattern mastectomies were performed, where the entire lower pole was de-epithelialized and advanced on top of the prosthesis. One-to-two 3 cm × 6 cm pieces of vCUT were longitudinally split, tacked to the dermis of the de-epithelialized lower skin flap using 3-0 Monocryl sutures, and the upper skin flaps were draped over them. The pieces of vCUT were longitudinally split in order to fit into the area of de-epithelialized lower breast skin flap. The amount of vCUT (typically one to two pieces) used depended on the size of the breast, with the goal to cover the enter dermis of the de-epithelialized lower skin flap. vCUT is a soft and easy-to-maneuver, yet strong, durable, and conforming 1-to-3 mm thick material. It is easily sutured. Closed-incision negative-pressure wound therapy (ciNPT) was applied on a case-by-case basis for seven days, followed by abdominal (ABD) pad dressings and a bra.
The attending plastic surgeon (SSV) and physician-assistants assessed the conditions of all patients on regularly scheduled follow-up visits (more frequently than standard protocol if complications arose) for at least 60 days after surgery, but usually incrementally until one year post-operation and yearly thereafter.
3. Results
Patient characteristics and demographics can be found in Table 1. The mean post-operative follow-up was 252 days (range, 183–287). The average age was 52 years (range, 41–75), and all patients were female. Average BMI was 31.7 kg/m2 (range, 22.1–38.5). Of the seven patients included in this series, five patients received vCUT placement immediately during the primary reconstruction (cases 1–5), while two patients received vCUT in delayed/subsequent reconstructive procedures (cases 6 and 7). In the immediate vCUT patients, three patients (60%) experienced post-operative complications (Table 2). In the delayed vCUT patients, one patient (50%) experienced post-operative complications (Table 2). In total, four patients (57%) experienced post-operative complications (Table 2). Five patients (71.4%) had the Wise pattern, while two patients (28.6%) had inferior lateral pants over vest de-epithelization. Two patients (28.6%; one patient with immediate vCUT placement and one with delayed vCUT placement) developed post-operative seromas that were treated with aspiration. Two patients (28.6%; both in the immediate vCUT placement) developed MSN and wound dehiscence. Two patients (28.6%) (both in the immediate vCUT placement) developed infection, and one patient (14.2%) developed minor MSN. No patients had a hematoma. Two patients’ (28.6%; both in the immediate vCUT placement) complications were treated surgically, while the rest were treated conservatively. Two patients (28.6%; both in the immediate vCUT placement) had removal of reconstruction due to post-operative complications, while the rest remained healed and went on to second-stage reconstruction at the time of this publication. Post-operative complications are detailed in Table 2.

Table 1.
Patient characteristics and demographics.

Table 2.
Post-operative outcomes following breast reconstruction with vCUT.
3.1. Case 1
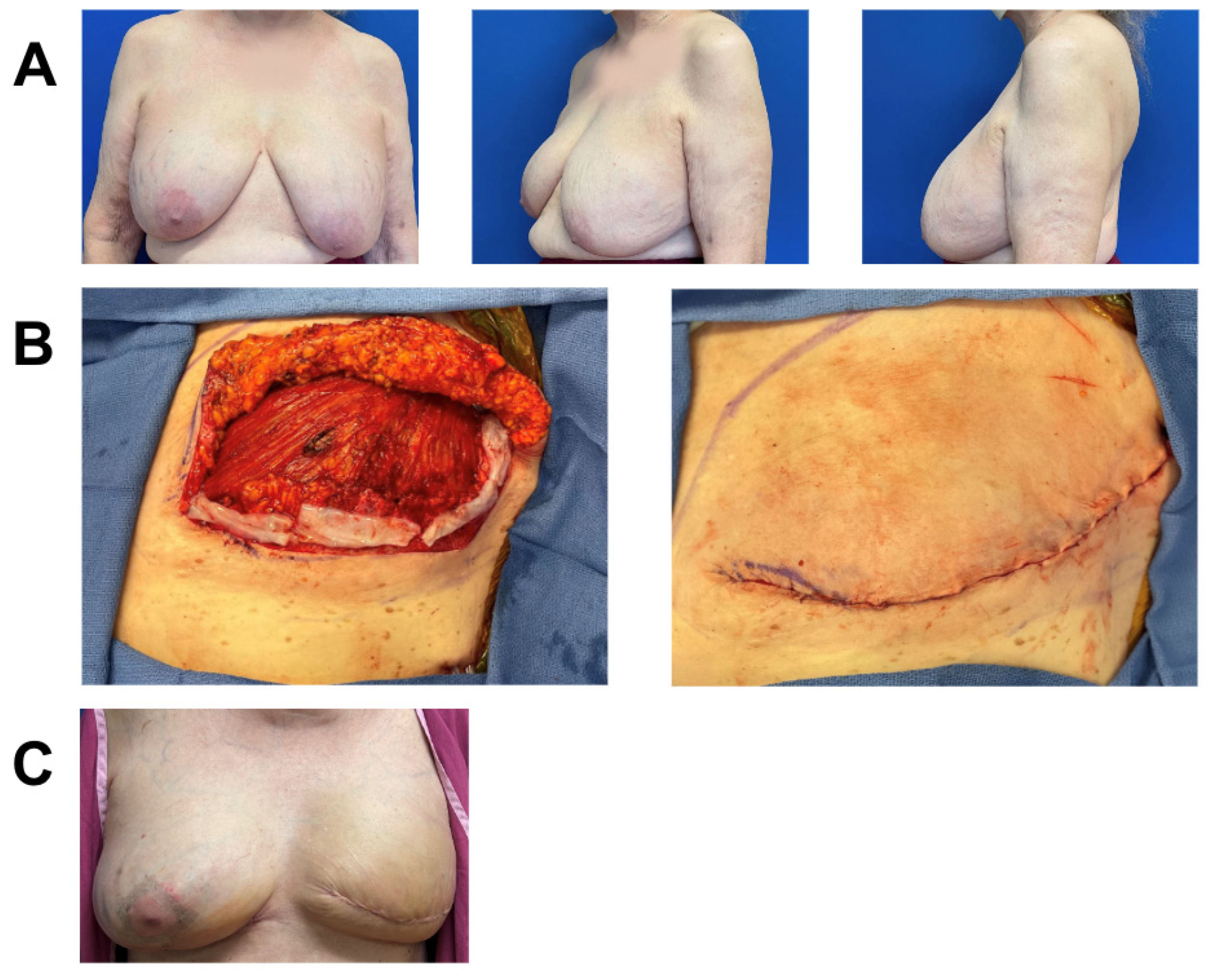
A 75-year-old female with a history of lymphangioleiomyomatosis (LAM) and a prior breast augmentation over twenty years prior and on immunosuppression medication (hydroxychloroquine and sirolimus) was diagnosed with left-breast multicentric ductal carcinoma in situ (DCIS) and treated with skin-sparing mastectomy. Given the poor quality of her skin, immunocompromised state, and a BMI of 31, the patient was deemed at high risk for infection and wound-healing complications. Thus, she underwent immediate dual-plane breast reconstruction with placement of tissue expander and inferior sling AlloDerm and application of two 3 cm × 6 cm pieces of vCUT (Figure 1). Twenty-eight days post-operation, she presented with pinpoint dehiscence on medial breast incision and seroma that was treated with fluid aspiration and a one-week course of prophylactic oral antibiotics. The seroma culture indicated no growth, and the patient resumed her tissue expansion four days after seroma aspiration. She did not experience any skin necrosis, infection, or hematoma. Seven months post-operation, she underwent second-stage breast reconstruction with exchange of her tissue expander to implant and contralateral balancing augmentation mastopexy. She remained healed, without complications, four months after second-stage surgery (Figure 1).
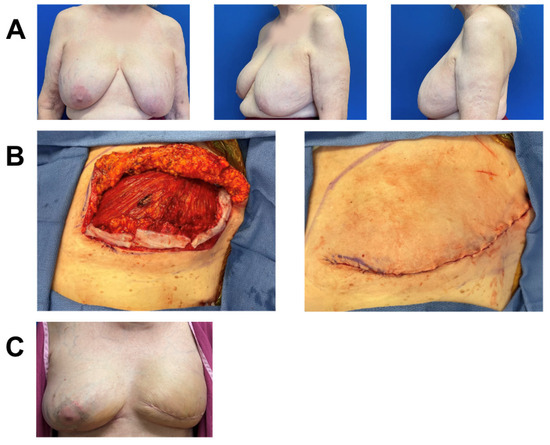
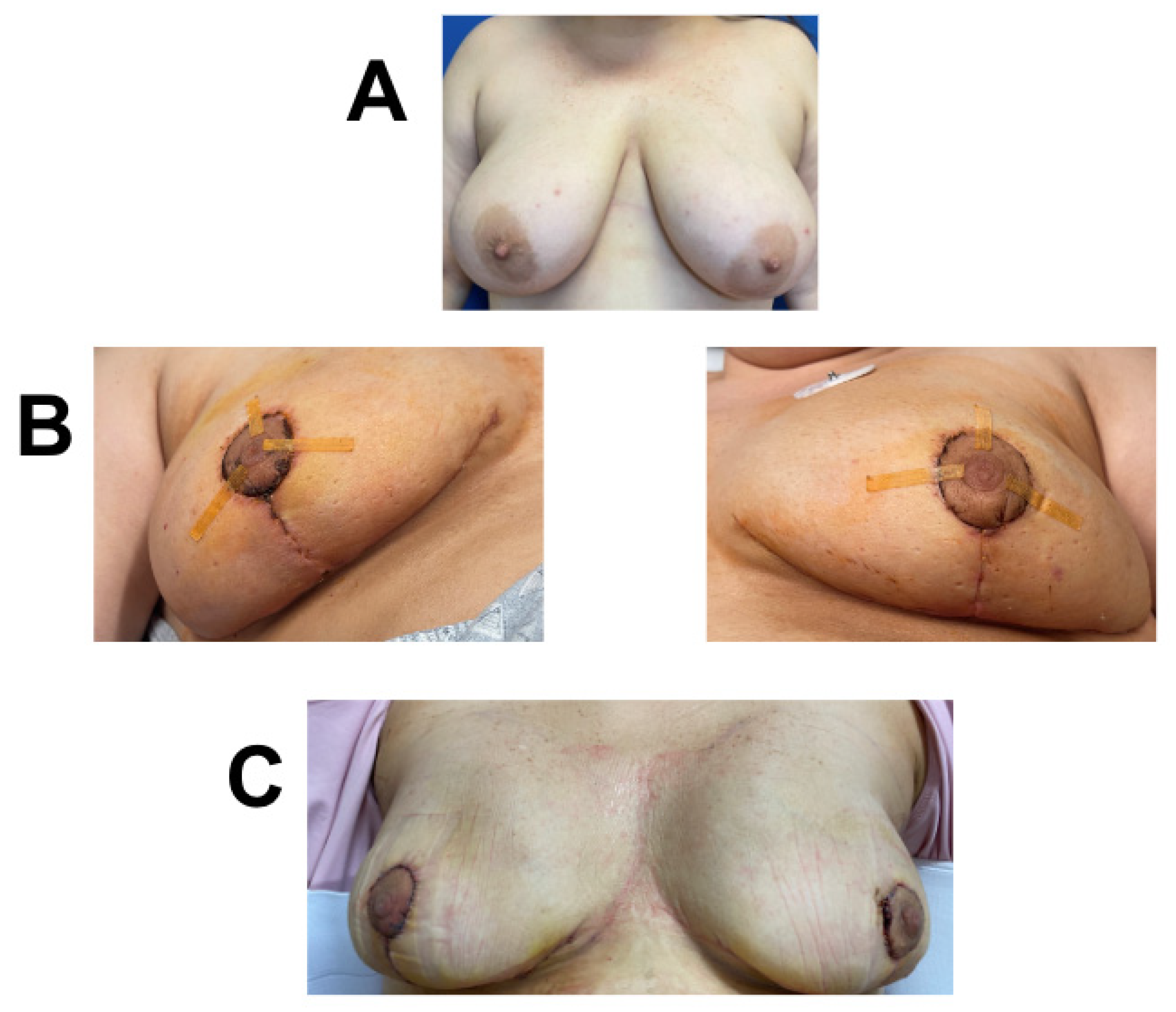
Figure 1.
Breast reconstruction with vCUT placement in this 75-year-old female patient (case 1). (A) Pre-operative. (B) Intra-operative placement of vCUT. (C) Six months post-operation.
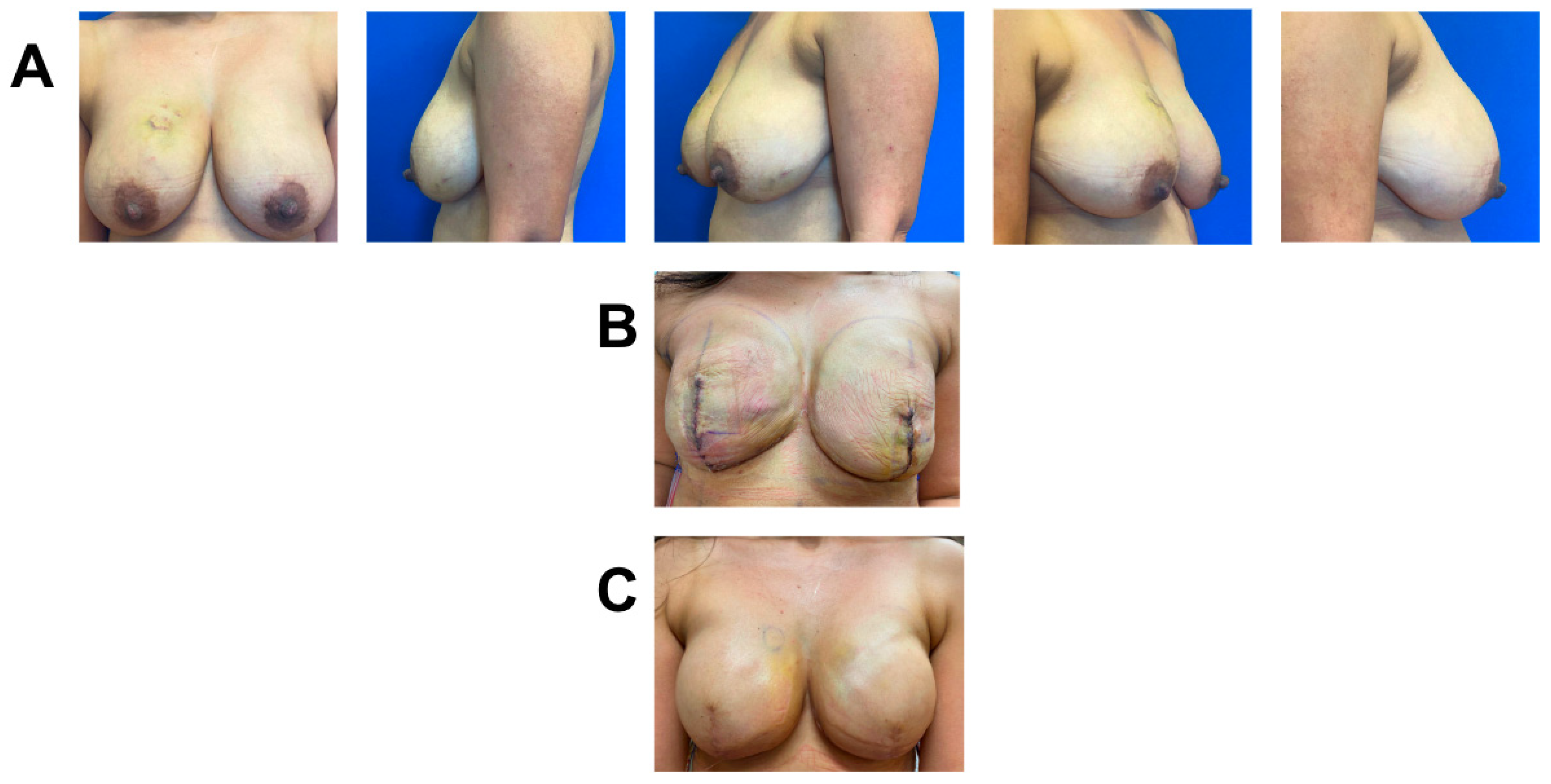
3.2. Case 2
A 45-year-old female with invasive ductal carcinoma of the left breast was treated with neoadjuvant chemotherapy and skin-sparing mastectomy. Due to her high BMI (BMI = 34.7) and having recently received neoadjuvant chemotherapy, known to increase risk for poor wound healing [23], she underwent immediate prepectoral breast reconstruction with tissue expander and application of one piece of 3 cm × 6 cm vCUT (Figure 2). A prophylactic ciNPT was also placed at the end of surgery for seven days. She did not have any infection, MSN, or wound-healing complications two months post-operation. Importantly, she started adjuvant radiation therapy on time (7 weeks post-operation), without any delays related to wound healing. The patient is ten months post-op, without any further wound-healing complications (Figure 2). Nine months post-operation, she underwent second-stage breast reconstruction with expander-to-implant exchange and balancing contralateral reduction. She currently remains healed, without complications, one month after second-stage surgery.
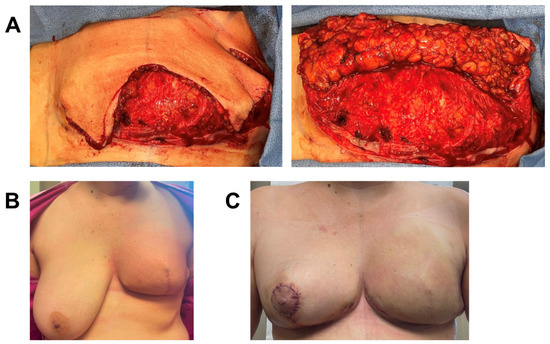
Figure 2.
Breast reconstruction with vCUT placement in this 45-year-old female patient (case 3). (A) Intra-operative placement of vCUT. (B) Two months post-operation. (C) Two days after left-implant exchange and right-balancing breast reduction.
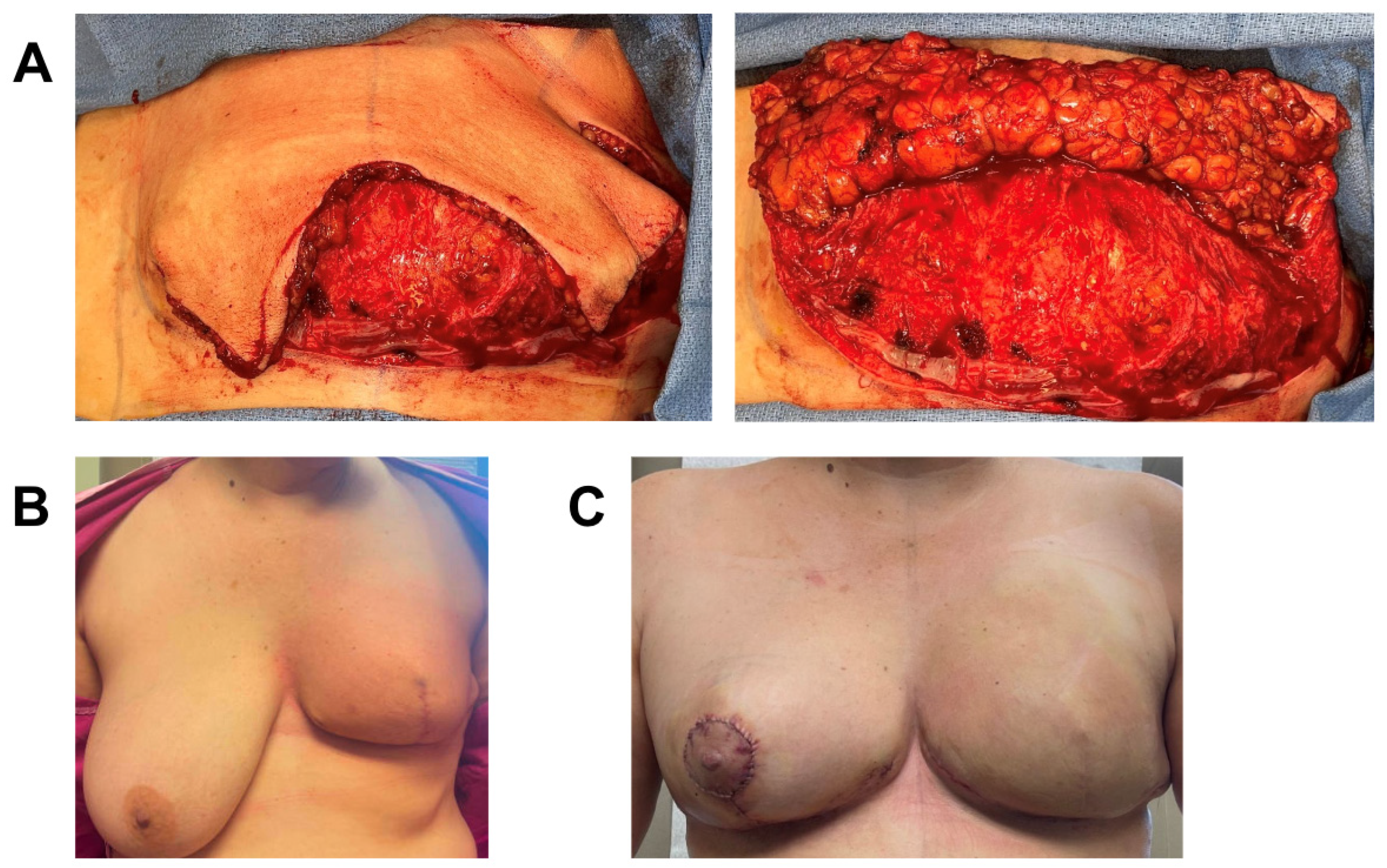
3.3. Case 3
A 48-year-old female with extensive ductal carcinoma in situ (DCIS) of the left breast was treated with skin sparing mastectomy. Given the extent of DCIS, there was a moderate pre-operative likelihood of requiring post-op radiation. Due to her high BMI (BMI = 38.5) and hypertension, she deemed high risk for wound-healing complications and subsequent infection that could delay timely initiation of future radiation therapy. She therefore underwent immediate prepectoral expander-based breast reconstruction and application of one piece of 3 cm × 6 cm vCUT (Figure 3). A prophylactic ciNPT was placed at the end of surgery for seven days. She developed superficial T-point skin necrosis 19 days post-operation that was treated with minimal excision and closure. The underlying dermoglandular flap was intact and fully viable. Unfortunately, twenty-three days post excision, she developed a periprosthetic infection with incisional dehiscence and cellulitis that was treated with intravenous (IV) antibiotics for two days, tissue-expander removal, and debridement. The patient is healed eight months post-operation and will undergo autologous reconstruction after weight reduction (Figure 3).
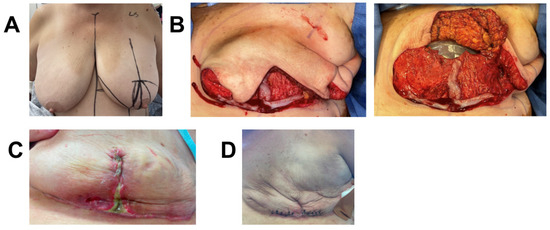
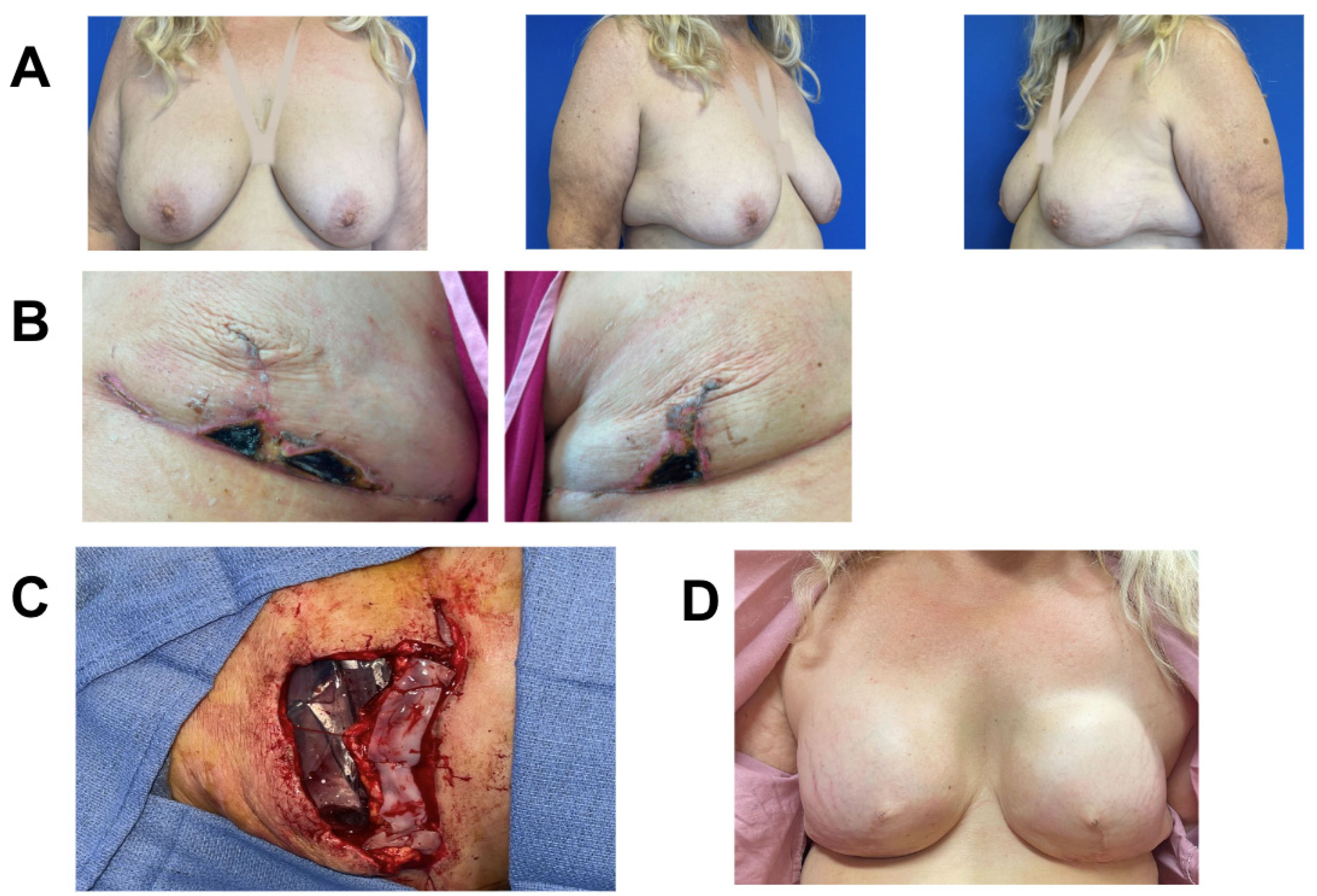
Figure 3.
Breast reconstruction with vCUT placement in this 48-year-old female patient (case 4). (A) Pre-operative. (B) Intra-operative placement of vCUT. (C) T-point necrosis 19 days post tissue expander with vCUT placement. (D) 20 days post tissue-expander explantation.
3.4. Case 4
A 44-year-old female was diagnosed with high-grade right-breast multifocal invasive lobular carcinoma. She underwent bilateral Wise-pattern mastectomy with immediate bilateral two-staged prepectoral breast reconstruction. Bilateral mastectomy for single breast cancer was performed based on the patient’s decision. Given the patient’s high pre-operative likelihood of requiring post-mastectomy radiation (PMR), one piece of 3 × 6 cm vCUT was placed on the right de-epithelialized lower skin flap to optimize healing (Figure 4). Her post-operative course was uncomplicated, and she successfully underwent implant exchange. She remains healed four months post second stage.
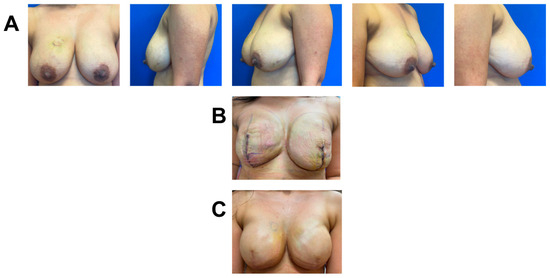
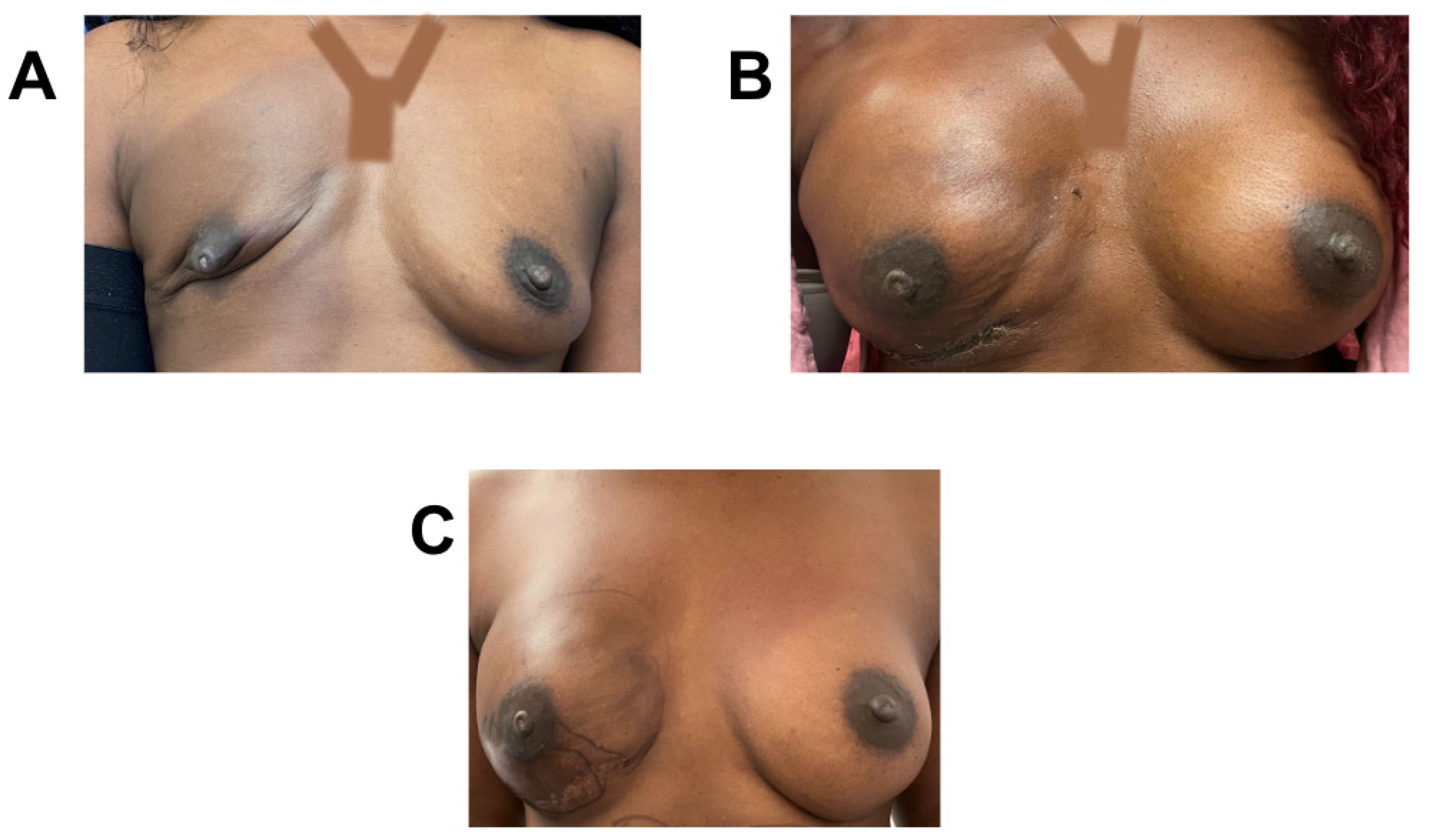
Figure 4.
Breast reconstruction with vCUT placement in this 44-year-old female patient (case 6). (A) Eighteen days pre-operation. (B) Eight days post tissue expander with placement of vCUT. (C) Seven days post implant exchange.
3.5. Case 5
A 41-year-old female was diagnosed with invasive ductal carcinoma treated with neoadjuvant chemotherapy. Due to her high BMI (34) and history of neoadjuvant chemotherapy, she was deemed to be at risk for infection and wound-healing complications. She underwent bilateral nipple sparing, Wise-pattern mastectomy, and immediate bilateral expander-based prepectoral breast reconstruction with the application of one piece of 3 cm × 6 cm vCUT over the de-epithelialized lower skin flap (Figure 5). Bilateral mastectomy for single breast cancer was performed based on patient decision. She developed no wound-healing complications. However, eighteen days post-op, she developed a tissue-expander infection that was treated with IV antibiotics and tissue-expander removal. She remains healed five months post-operation and plans on undergoing autologous reconstruction after weight reduction.
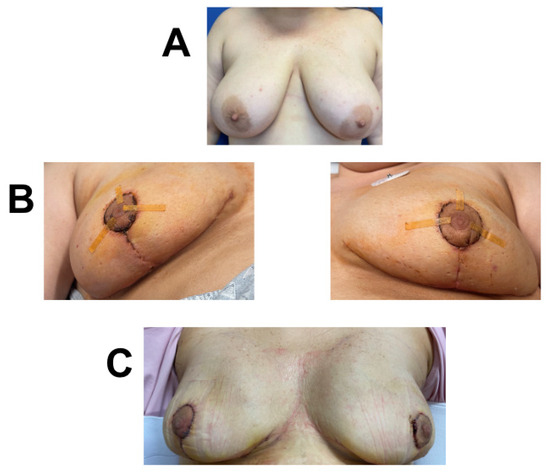
Figure 5.
Breast reconstruction with vCUT placement in this 41-year-old female patient (case 7). (A) Pre-operative. (B) Eighteen days post tissue-expander implantation with placement of vCUT. (C) Sixty-one days post-operation.
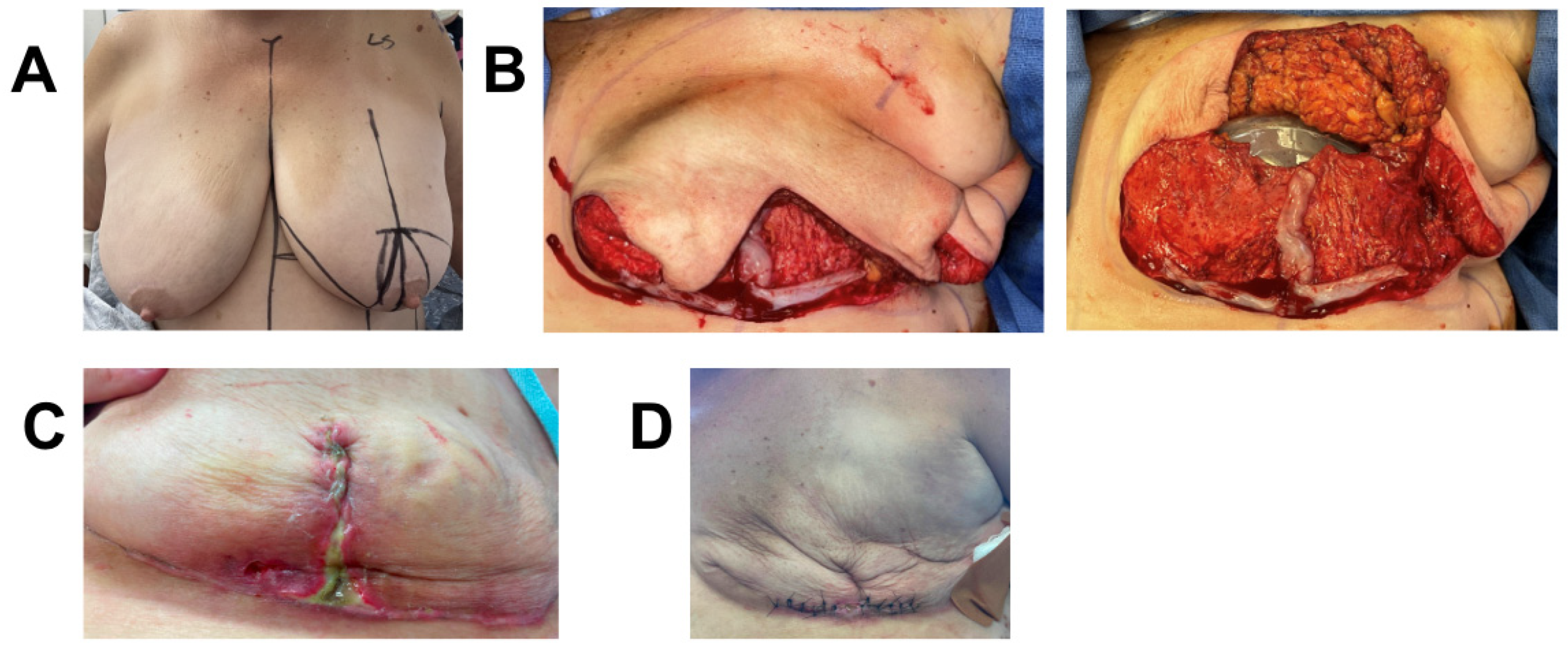
3.6. Case 6
A 63-year-old female with high-grade left-breast DCIS underwent bilateral Wise-pattern mastectomy, followed by immediate expander-based prepectoral breast reconstruction with acelluar dermal matrix (ADM). Her post-operative (post-op) course was complicated by bilateral superficial MSN measuring 1 × 1 cm on the left and 1 cm × 3 cm on the right that was diagnosed on post-op day (POD) 11. She was initially treated with topical Silvadene for 22 days. Due to risk of infection, she underwent operative debridement and prophylactic replacement of her expanders 36 days post initial operation. Due to her previous wound-healing complications and high BMI (BMI = 34.6), she was deemed high risk for further wound-healing complications. She thus also underwent the application of a 3 × 6 cm piece of vCUT only over the de-epithelialized lower pole skin, and the Wise-pattern upper skin flaps were closed on top of the secured vCUT (Figure 6). Four days post-op, she was started on a 7-day course of antibiotics due to mild erythema and delayed blanching on her right breast. Three days later, the erythema resolved, and she did not demonstrate any further signs of infection. Eight days post-operation, she developed a recurring seroma that was treated with serial aspiration. She had no further skin necrosis, infection, hematoma, or dehiscence. The patient remained healed, without any further complications, at the nine-month follow-up (Figure 6). She plans to undergo autologous breast reconstruction after BMI reduction.
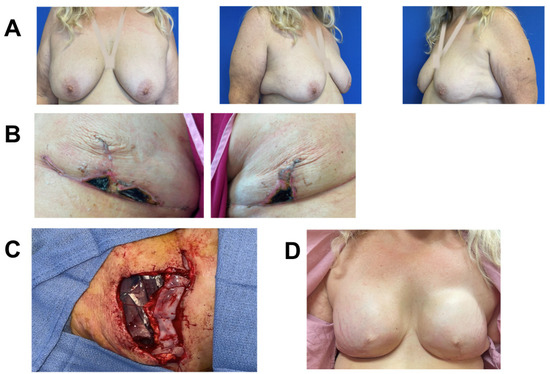
Figure 6.
Breast reconstruction with vCUT placement in this 63-year-old female patient (case 2). (A) Pre-operative. (B) Bilateral mastectomy skin necrosis 27 days post-operation. (C) Intra-operative placement of vCUT and tissue expander one month after debridement and removal of initial expander. (D) 9 months post vCUT and tissue-expander placement.
3.7. Case 7
A 48-year-old female was previously treated for right-breast invasive ductal carcinoma with right nipple-sparing mastectomy and XRT without reconstruction. One year after XRT, she elected to undergo delayed implant-based breast reconstruction with tissue expander and completed expansion and implant exchange. Approximately two months post implant exchange, she presented with wound dehiscence and an exposed right implant that was removed (Figure 7A). Due to her previous XRT, she was deemed high risk for infection and poor healing. She underwent right-implant exchange to a smaller implant and application of one piece of 3 cm × 6 cm vCUT to buttress the closure over a small area of de-epithelialized lower-mastectomy skin flap (Figure 7B). A prophylactic ciNPT was placed at the end of surgery for seven days. There were no other complications eight months post-operation with vCUT (Figure 7C).
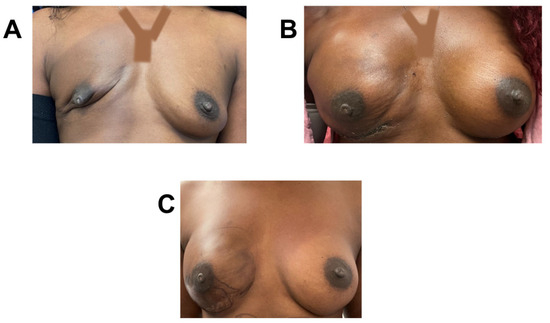
Figure 7.
Breast reconstruction with vCUT placement in this 48-year-old female patient (case 5). (A) Pre-operative. (B) Five months post-operation. (C) Eight months post-operation and after one round of fat grafting.
4. Discussion
A breast cancer diagnosis and the necessary accompanying treatment often take a grueling physical and emotional toll on affected women. Not only do patients have to prematurely face their own mortality, but the very essence of their identity as a woman is threatened. As such, delaying reconstruction places women at higher risk for poor mental health outcomes [13,14]. IBR is therefore hugely important in restoring their sense of self, body image, and quality of life [24,25,26]. Unfortunately, patients with comorbidities, such as high BMI and immunosuppression, are at higher risk for MSN and infection, potentially leading to delays in the timely initiation of adjuvant cancer therapy, poor aesthetic outcomes, reconstructive failures, and high financial and psychological costs. Importantly, many known risk factors (i.e., obesity) are not easily modifiable, especially in a time-sensitive fashion, during which mastectomy must be performed. In order to balance the many known benefits of IBR with complications that could impact adjuvant cancer care, novel and innovative therapies and approaches are necessary to expand current indications for safe IBR in traditionally poorer reconstructive candidates.
Over the last decade, vCUT and other hPMs and umbilical-cord tissues have become readily commercially available, and a growing body of literature [22,27,28,29,30] has robustly demonstrated their promising pro-wound-healing capabilities in a variety of traditionally hostile environments [27,30,31]. In a case series of 10 patients with DFUs and gas gangrene, McGinness et al. showed the use of vCUT obviated the need for flaps and higher-level amputations [27]. Brandeisky et al. similarly demonstrated the successful use of vCUT as an adjunct in Achilles tendon repair to decrease wound complications and improve post-operative recovery time [28].
Additionally, due to their low immunogenicity, hPM and vCUT can conveniently be used point-of-care, without the need for matching donors and recipients [30,32,33,34]. The anti-inflammatory, antifibrotic, and antibacterial properties of placental tissues also make them an attractive option to augment wound healing in high-risk surgical applications [21,30]. In contrast to hPMs, vCUT is more durable, suturable, and thicker (ranging from 1 mm to 3 mm in thickness), and it has superior tensile strength, making it a more suitable for surgical indications [27]. Although the safety and efficacy of various amniotic, placental, and umbilical tissues have been demonstrated in a variety of surgical and non-operative indications [27,30,31], to our knowledge, this is the first series to report on its use in breast reconstruction.
In the current study, five of seven patients (71%) at high risk for post-operative wound-healing complications and subsequent infection successfully underwent immediate breast reconstruction using vCUT as an adjunct therapy, without losing the implants. Clinically, most importantly, the patient who required post-mastectomy radiation underwent timely adjuvant therapy, without delays due to wound-healing complications. In the literature, surgical complications in high-risk women range from 30 to 70% [35]. Obesity alone is an independent positive predictor for the development of MSN and infection, two of the major complications associated with implant-based breast reconstruction necessitating reoperation [36]. In fact, risk of prosthetic loss in this patient population is reported to be as high as 25% [37]. While in the current series, two of the seven patients (28.5%) developed post-operative infections ultimately necessitating expander removal, it is noteworthy that both patients had more than one known risk factor (Patient 7, obesity and neoadjuvant chemotherapy; and Patient 4, morbid obesity and hypertension), and only one developed wound-healing complications despite the other undergoing a more ischemic operation with higher risk for T-point necrosis (Wise-pattern mastectomy). In women with multiple risk factors, especially those with high pre-operative suspicion for requiring post-mastectomy radiation therapy, delaying reconstruction should be considered.
Thus, while vCUT may not completely eliminate wound-healing complications, especially in very high risk patients, data from this series are encouraging, showing that vCUT, as an adjunct therapy, may offer high-risk patients the opportunity to undergo safe IBR. While the high cost of vCUT is certainly a drawback (ranging between USD 1836.66 and USD 2002.75 depending on size), this is likely to be offset by the even higher cost associated with wound-healing complications, including multiple unbillable office visits and even reoperations. Furthermore, unlike in chronic wounds, where numerous applications of vCUT over several months are often necessary, in the setting of IBR, a single matrix application at the index operation is usually sufficient, thereby making the cost burden more manageable.
Given the minimal inherent risk of the product itself (i.e., incidence of transmitted disease is 0.014% [38]), findings from this series suggest that vCUT might be considered as part of the breast-reconstructive surgeon’s armamentarium in less ideal reconstructive candidates.
This study was limited by a small number of patients and lack of a true control group and randomization. Almost half of the patients (three out of seven) had closed-incision negative-pressure wound therapy, which may also be a major bias factor regarding preventing wound-healing complications. This study is primarily meant to demonstrate the safety of vCUT in breast reconstruction and provide an introduction as a potential promising adjunct in this arena. Further, larger, and randomized prospective studies with a control group are underway to ascertain the true clinical benefits of vCUT in IBR.
5. Conclusions
Clinical outcomes of this case series support the safety of using vCUT in IBR for patients who are at high risk for wound-healing complications. Larger studies are necessary to determine the efficacy of using vCUT in reducing the rate of post-operative complications and thus improve outcome in high-risk breast-reconstruction patients.
Author Contributions
All authors contributed to the final approval of this manuscript. K.M.O., conceptualization, data curation, formal analysis, investigation, and writing; N.T., conceptualization, data curation, formal analysis, investigation, and writing; S.H.H., conceptualization, formal analysis, and writing—original draft; M.M., conceptualization, formal analysis, and writing—original draft; L.S., conceptualization, methodology, and writing; K.D., conceptualization, methodology, and writing; S.S.V., conceptualization, methodology, formal analysis, investigation, and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the University of Florida IRB (IRB202202140). Written informed consent was obtained by the patients in this case series and for the publication of their images. All procedures were followed in accordance with the Helsinki Declaration of 1975, as revised in 2008.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article. The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
This research did not receive any specific grant or funding from agencies in the public, commercial, or not-for-profit sectors. No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. Sorice-Virk is a consultant for Sientra, Inc.
References
- Chen, W.; Lv, X.; Xu, X.; Gao, X.; Wang, B. Meta-analysis for psychological impact of breast reconstruction in patients with breast cancer. Breast Cancer 2018, 25, 464–469. [Google Scholar] [CrossRef]
- Yun, M.H.; Yoon, E.S.; Lee, B.I.; Park, S.H. The Effect of Low-Dose Nitroglycerin Ointment on Skin Flap Necrosis in Breast Reconstruction after Skin-Sparing or Nipple-Sparing Mastectomy. Arch. Plast. Surg. 2017, 44, 509–515. [Google Scholar] [CrossRef]
- Sue, G.R.; Long, C.; Lee, G.K. Management of Mastectomy Skin Necrosis in Implant Based Breast Reconstruction. Ann. Plast. Surg. 2017, 78 (Suppl. S4), S208–S211. [Google Scholar] [CrossRef]
- Sue, G.R.; Lee, G.K. Mastectomy Skin Necrosis After Breast Reconstruction: A Comparative Analysis Between Autologous Reconstruction and Implant-Based Reconstruction. Ann. Plast. Surg. 2018, 80 (Suppl. S5), S285–S287. [Google Scholar] [CrossRef]
- Oleck, N.C.; Gu, C.; Pyfer, B.J.; Phillips, B.T. Defining Mastectomy Skin Flap Necrosis: A Systematic Review of the Literature and a Call for Standardization. Plast. Reconstr. Surg. 2022, 149, 858e–866e. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Allan, L.; Roblin, P.; Ross, D.; Farhadi, J. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast 2011, 20, 21–25. [Google Scholar] [CrossRef]
- Pinsolle, V.; Grinfeder, C.; Mathoulin-Pelissier, S.; Faucher, A. Complications analysis of 266 immediate breast reconstructions. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Reece, G.P.; Wang, B.; Robb, G.L.; Miller, M.J.; Evans, G.R.D.; Langstein, H.N.; Kroll, S.S. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast. Reconstr. Surg. 2000, 105, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Mlodinow, A.S.; Fine, N.A.; Khavanin, N.; Kim, J.Y. Risk factors for mastectomy flap necrosis following immediate tissue expander breast reconstruction. J. Plast. Surg. Hand Surg. 2014, 48, 322–326. [Google Scholar] [CrossRef]
- McCarthy, C.M.; Mehrara, B.J.; Riedel, E.; Davidge, K.; Hinson, A.; Disa, J.J.; Cordeiro, P.G.; Pusic, A.L. Predicting complications following expander/implant breast reconstruction: An outcomes analysis based on preoperative clinical risk. Plast. Reconstr. Surg. 2008, 121, 1886–1892. [Google Scholar] [CrossRef]
- Robertson, S.A.; Jeevaratnam, J.A.; Agrawal, A.; Cutress, R.I. Mastectomy skin flap necrosis: Challenges and solutions. Breast Cancer 2017, 9, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Matsen, C.B.; Mehrara, B.; Eaton, A.; Capko, D.; Berg, A.; Stempel, M.; Van Zee, K.J.; Pusic, A.; King, T.A.; Cody, H.S., III; et al. Skin Flap Necrosis after Mastectomy with Reconstruction: A Prospective Study. Ann. Surg. Oncol. 2016, 23, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazal, S.K.; Sully, L.; Fallowfield, L.; Blamey, R.W. The psychological impact of immediate rather than delayed breast reconstruction. Eur. J. Surg. Oncol. 2000, 26, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Ray, C. Psychological implications of mastectomy. Br. J. Soc. Clin. Psychol. 1977, 16, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kesting, M.R.; Wolff, K.D.; Hohlweg-Majert, B.; Steinstraesser, L. The role of allogenic amniotic membrane in burn treatment. J. Burn. Care Res. 2008, 29, 907–916. [Google Scholar] [CrossRef]
- Sorice, S.; Rustad, K.C.; Li, A.Y.; Gurtner, G.C. The Role of Stem Cell Therapeutics in Wound Healing: Current Understanding and Future Directions. Plast. Reconstr. Surg. 2016, 138 (Suppl. S3), 31s–41s. [Google Scholar] [CrossRef]
- Sawhney, C.P. Amniotic membrane as a biological dressing in the management of burns. Burns 1989, 15, 339–342. [Google Scholar] [CrossRef]
- Gajiwala, K.; Lobo Gajiwala, A. Use of banked tissue in plastic surgery. Cell Tissue Bank. 2003, 4, 141–146. [Google Scholar] [CrossRef]
- Duan-Arnold, Y.; Gyurdieva, A.; Johnson, A.; Jacobstein, D.A.; Danilkovitch, A. Soluble Factors Released by Endogenous Viable Cells Enhance the Antioxidant and Chemoattractive Activities of Cryopreserved Amniotic Membrane. Adv. Wound Care 2015, 4, 329–338. [Google Scholar] [CrossRef]
- Duan-Arnold, Y.; Gyurdieva, A.; Johnson, A.; Uveges, T.E.; Jacobstein, D.A.; Danilkovitch, A. Retention of Endogenous Viable Cells Enhances the Anti-Inflammatory Activity of Cryopreserved Amnion. Adv. Wound Care 2015, 4, 523–533. [Google Scholar] [CrossRef]
- Duan-Arnold, Y.; Uveges, T.E.; Gyurdieva, A.; Johnson, A.; Danilkovitch, A. Angiogenic Potential of Cryopreserved Amniotic Membrane Is Enhanced through Retention of All Tissue Components in Their Native State. Adv. Wound Care 2015, 4, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Sundblad, K.W.; Tassis, E.K. A quality improvement pilot assessment of the safety and associated outcomes of a viable cryopreserved umbilical tissue allograft as an adjunct surgical wrap in peroneus brevis tendon repair. Medicine 2018, 97, e13662. [Google Scholar] [CrossRef] [PubMed]
- Mitchem, J.; Herrmann, D.; Margenthaler, J.A.; Aft, R.L. Impact of neoadjuvant chemotherapy on rate of tissue expander/implant loss and progression to successful breast reconstruction following mastectomy. Am. J. Surg. 2008, 196, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Serletti, J.M.; Fosnot, J.; Nelson, J.A.; Disa, J.J.; Bucky, L.P. Breast reconstruction after breast cancer. Plast. Reconstr. Surg. 2011, 127, 124e–135e. [Google Scholar] [CrossRef] [PubMed]
- Toyserkani, N.M.; Jørgensen, M.G.; Tabatabaeifar, S.; Damsgaard, T.; Sørensen, J.A. Autologous versus implant-based breast reconstruction: A systematic review and meta-analysis of Breast-Q patient-reported outcomes. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 278–285. [Google Scholar] [CrossRef]
- Guyomard, V.; Leinster, S.; Wilkinson, M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast 2007, 16, 547–567. [Google Scholar] [CrossRef]
- McGinness, K.; Kurtz Phelan, D.H. Use of Viable Cryopreserved Umbilical Tissue for Soft Tissue Defects in Patients With Gas Gangrene: A Case Series. Wounds 2018, 30, 90–95. [Google Scholar]
- Brandeisky, J.; Kurtz Phelan, D.H. Clinical Outcome of Achilles Tendon Repair Using Viable Intact Cryopreserved Umbilical Tissue Versus Standard of Case. Wounds 2017, 29, E111–E114. [Google Scholar]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cell Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef]
- Dhall, S.; Coksaygan, T.; Hoffman, T.; Moorman, M.; Lerch, A.; Kuang, J.-Q.; Sathyamoorthy, M.; Danilkovitch, A. Viable cryopreserved umbilical tissue (vCUT) reduces post-operative adhesions in a rabbit abdominal adhesion model. Bioact. Mater. 2019, 4, 97–106. [Google Scholar] [CrossRef]
- Karon, M. Viable Umbilical Tissue Use in Laparoscopic Myomectomy: Study Design and Short-Term Post-Operative Outcomes [29Q]. Obstet. Gynecol. 2018, 131, 192S. [Google Scholar] [CrossRef]
- Kubo, M.; Sonoda, Y.; Muramatsu, R.; Usui, M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1539–1546. [Google Scholar]
- Cho, P.S.; Messina, D.J.; Hirsh, E.L.; Chi, N.; Goldman, S.N.; Lo, D.P.; Harris, I.R.; Popma, S.H.; Sachs, D.H.; Huang, C.A. Immunogenicity of umbilical cord tissue derived cells. Blood 2008, 111, 430–438. [Google Scholar] [CrossRef]
- Akle, C.A.; Adinolfi, M.; Welsh, K.I.; Leibowitz, S.; McColl, I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981, 2, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Roubaud, M.S.; Carey, J.N.; Vartanian, E.; Patel, K.M. Breast reconstruction in the high-risk population: Current review of the literature and practice guidelines. Gland Surg. 2021, 10, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Gfrerer, L.; Mattos, D.; Mastroianni, M.; Weng, Q.Y.; Ricci, J.A.; Heath, M.P.; Lin, A.; Specht, M.C.; Haynes, A.B.; Austen, W.G., Jr.; et al. Assessment of patient factors, surgeons, and surgeon teams in immediate implant-based breast reconstruction outcomes. Plast. Reconstr. Surg. 2015, 135, 245e–252e. [Google Scholar] [CrossRef] [PubMed]
- Luce, E.A.; Adams, R.L.; Chandler, R.G.; Parks, J. Tissue Expander versus Tissue Expander and Latissimus Flap in Morbidly Obese Breast Reconstruction Patients. Plast. Reconstr. Surg. Glob. Open 2015, 3, e323. [Google Scholar] [CrossRef]
- Moore, M.A.; Samsell, B.; McLean, J. Allograft Tissue Safety and Technology. Biol. Orthop. Surg. 2019, 49–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).