Abstract
Aim: This study aims to assess the suitability of the Göttingen Mini-pig (G-MP) as a large animal model for preclinical research on articular cartilage treatment procedures. Additionally, this study compares the G-MP to the domestic pig (DP) regarding surgical anatomy, postoperative care, and the challenges associated with the follow-up period. Materials and methods: Six G-MPs and four DPs underwent a two-stage surgical procedure: first, cartilage was harvested using a superolateral approach, followed by cartilage implantation via a medial parapatellar tendon approach. Results: The superolateral approach exposed 11% (SD ± 5) of the trochlea in G-MPs and 20% in DPs. The medial parapatellar tendon approach exposed 63% (SD ± 4) of the trochlear surface and 34% (SD ± 13) of the medial femoral condyle in G-MPs, allowing for the creation of four 6 mm trochlear lesions and one medial condyle lesion in four out of six G-MPs and all DPs. Cartilage thickness was less than 1 mm in G-MPs, compared to over 2 mm in DPs. Weight gain was +4 kg/week in DPs and +0.2 kg/week in G-MPs. Conclusion: Overall, the G-MP proves to be a viable model for cartilage research, offering sufficient joint access via the dual approach, which allows for 4–5 lesions of 6 mm each. However, the thinner cartilage in G-MPs should be taken into account.
1. Introduction
Articular cartilage repair remains a hot topic in clinical and surgical fields alongside the fundamental research. However, to date, no intervention or technique to repair hyaline cartilage has demonstrated satisfying efficacy, especially for long-term results [1,2,3]. In this regard, advancements in tissue engineering and cellular therapies are amongst the most promising modalities.
The use of an optimal animal model is not only critical to improve our knowledge of newly proposed therapies but is also key prior to a clinical translation [4]. The ideal model should ultimately offer a joint anatomy and proportions similar to those of a human knee, relatively easy to do surgery on in most research facilities. This is important when considering the age and size of the animal at different stages of skeletal maturity and the healing and regenerative potentials of the studied pathology. Moreover, the animals of this model should be suitable for several months’ follow-up while being cost effective. On an equally important aspect, in accordance with the 3R principals (Replace, Reduce, Refine), the animal model needs to fulfill several requirements dictated by the purpose of the study and the capacity of the host research facility [5].
Several large animal models have been described and used in the literature for cartilage research [6,7,8,9,10]. The goat model is commonly used in cartilage grafting for its surgical accessibility, knee anatomy, and easy handling [10,11]. The equine model has been used in preclinical studies for comparison with humans due to cartilage similarities in thickness, tensile forces, and age-related cartilage elasticity [8,12]. Nevertheless, it is of a high cost for hosting and needs a large, specialized facility in terms of spaces allocated for each animal and human resources to care for them.
The domestic pig (DP), large white pig, was primary chosen for its anatomical resemblance to the human knee, large joint dimensions, and affordability in terms of financial cost and availability at local production sites [6]. Unfortunately, the heavy weight at the age of skeletal maturity (up to 200 kg) represents a major obstacle, making it a difficult task for keepers, anesthesia teams, and surgeons to handle [13,14].
Göttingen mini-pigs (G-MPs) are an interesting model because the skeletally mature adult animal has a maximum weight of 45–50 kg [15]. This limited weight gain allows for easier handling by the host facility and the possibility to increase the sample size without overloading the animal caregivers.
This study aimed to demonstrate the feasibility of a two-stage cartilage surgery using pig models. It involved cartilage harvesting followed by implantation. The first stage focused on intra-operative exposure of the trochlea and condyle for treating simulated cartilage lesions. The second stage, post-mortem, analyzed joint morphology and cartilage thickness in both models, comparing postoperative handling. The goal was to assess G-MPs as a preclinical model for cartilage surgery, hypothesizing that the G-MP is suitable and comparable to the DP in surgical anatomy, with advantages in handling.
2. Materials and Methods
The animals included in this study were originally used for a preclinical safety and efficacy study on autologous cartilage treatment for focal chondral lesions, the results of which are published separately [13]. All subjects underwent a two-stage surgery on the right distal femur, with 5 to 6 weeks of interval in between, and were sacrificed at 2-week, 3-month, or 6-month follow-ups. The animal surgeries were performed in accordance with the guidelines for the Geneva cantonal veterinary authorities, and the study protocols were approved by the authorities in February 2018 (Ge 14–18).
2.1. Animals
This study included six skeletally mature female G-MPs aged 17 months and weighing 45 kg (SD ± 2 kg) at the time of the first surgery. The DPs were chosen to have similar starting weights and were therefore 5-months-old (Table 1). All animals were weighed at arrival to the research facility, prior to each surgery, and prior to euthanasia. All animals included in this study were maintained under specific pathogen-free (SPF) conditions and were hosted in an adapted farm until the surgery, where they spent one night pre-operatively and one week post-operatively at the university animal research facility. The animals were hosted in a farm-like facility for their wellbeing throughout the entire follow-up period.

Table 1.
Comparison between G-MP and DP anatomy and surgical access. * Cartilage thickness was measured in histological cuts after staining. ** The potential harvest of cartilage is through the superolateral approach.
2.2. Anesthesia
The animals were pre-medicated in their holding box, with Azaperone (Stresnil, 0.1 mL/kg, Janssen Pharmaceutica, Beerse, Belgium) and Midazolam (Dormicum, 0.1 mL/kg, Hoffmann-La Roche AG, Basel, Switzerland) administrated subcutaneously. An intravenous access was gained through the auricular vein; then, general anesthesia was achieved with etomidate (Hypnomidate, 0.25 mL/kg, Janssen Pharmaceutica, Belgium), and a standard tracheal tube, size 6.5, was inserted.
General anesthesia was maintained with Sevoflurane® (3%, AbbVie, Copenhage, Denmark) and fentanyl (0.175 mL/kg/h, Hameln pharmaceuticals, Hamelin, Germany). Prior to the incision, the animals received prophylactic antibiotics (0.03 mL/kg, Penicillinprokain, Ceva Sante Animale, Paris, France).
2.3. Surgeries
All animals underwent a two-staged surgical protocol of the right knee using two separate approaches with minimally invasive incisions and arthrotomies. The chosen approaches are a superolateral parapatellar approach and a medial parapatellar–tendon approach, which are comparable to what is applied on the human knee in terms of open-knee surgery.
The operated zone was shaved in theater, alcoholic disinfection (Betaseptic®, Mundipharma, Hamilton/Bermuda, Succursale Basel, Switzerland) was performed three times; and then, a sterile draping was applied.
The first surgery for cartilage harvesting was performed through a superolateral parapatellar approach, incising the lateral retinaculum and the articular joint capsule by sparing the vastus lateralis. The superolateral trochlear facet was exposed, and cartilage samples were harvested. This zone was chosen to avoid cartilage harvesting from a load-bearing region while also avoiding overlapping between the harvest site and the intended lesion. This dual approach was meant to minimize the surgical morbidity by accessing the knee joint by the same approach used for the cartilage implantation.
The second surgery was performed 5–6 weeks later through a medial parapatellar-tendon approach. The maximum of the trochlear surface was exposed to create two full-thickness cartilage lesions on the medial and lateral trochlear facet each, all approximately 2 cm above the trochlear notch and on the medial femoral condyle. Great care was taken that the lesions were at least a 5 mm distance from each other. Full-thickness chondral defects were outlined using a 6 mm skin biopsy punch, and the cartilage was carefully stripped off using a curette, with caution not to damage the subchondral bone plaque. In the same surgical step, the cultured cartilage cells were implanted.
2.4. Postoperative Protocol
Postoperative protocol included weight-adapted anti-inflammatory medications orally for three consecutive days and no weight-bearing-limiting measures due to the graft requirement of the original study. The animals were kept in the research facility for a week post-operation and examined daily for limping, pain, infection signs, and wound healing.
2.5. Euthanasia and Descriptive Analysis
All the animals were euthanized at different time points to meet the needs of the original study, using terminal-dose anesthesia. The four DPs were euthanized 2 weeks post-second-surgery, while three G-MPs were euthanized at 3 months and the remaining three at 6 months.
At euthanasia, the accessible areas of the trochlea and the medial femoral condyle from both incisions were marked using an electrical cautery knife to allow for an accurate calculation of the visible articular surface. The dimensions of the entire trochlea were obtained by measuring the medial and lateral length and width at their widest. The number of possible separate lesions that can be fitted on the femoral part of the knee joint was evaluated. Cartilage thickness was measured using histological cuts of the lateral facete of trochlea from a healthy cartilage zone.
A macroscopic evaluation of the fully exposed joint, post-full-capsulotomy, was performed by the same investigators, and potential complications were looked for, such as overlap of donor site and recipient site cartilage degeneration, synovitis, and ectopic tissue growth.
For microscopic evaluation, the samples were prepared for a histology readout. After soft tissue removal, the distal femur was separated from its shaft using a bone saw. The samples were fixed in 4% formol for a week, then decalcified using 10% formic acid for 2 weeks at room temperature [16]. Then, they were cut into 5 μm serial sections using a microtome (Reichert Jung polycot, San Marcos, CA, USA) and mounted on adhesion slides (Thermo Scientific™ SuperFrost Plus™, Basel, Switzerland). Before staining, the sections were de-plasticized in dimetoxyethylacetate and rehydrated. The samples were stained with safranin-O for glycosaminoglycan and then fixed with EurKit resin (Wibatec AG, Malters, Switzerland [17].
2.6. Contralateral Knee Comparison
At euthanasia, the range of motion between the two knees was recorded with full passive flexion/extension maneuvers. Then, the left knee underwent an identical two-approach surgery without graft and then was analyzed for the anatomical features. Post-mortem comparison between the operated and non-operated knee was carried on in terms of cartilage degradation, patellar tendon shortening, and differences in the range of motion of the knee. The patellar tendon was measured from the distal edge of the patella to its insertion at the proximal tibial tubercule and compared to the contralateral side.
2.7. G-MP and DP Comparison
G-MPs and DPs were compared in terms of distal femur joint dimensions and surgical exposure and accessibility. We documented the number of possible lesions on each trochlear facet, conflict with donor site, and cartilage thickness in a post-mortem analysis. The weight gain was compared between the two models throughout the study-defined time points (on arrival, prior to each surgery, and prior to euthanasia). We also compared the cost of handling and documented unforeseen care and follow-up issues in a real-time manner as they occurred.
2.8. Graphical Generation
Graphs were generated using python to extrapolate the expected weight gain in each group. No statistical analysis was carried out due to the small sample size in each group and the descriptive nature of our study.
3. Results
3.1. Anatomy and Surgical Approaches
The hind-extremity in both groups was comparable in both groups in terms of joint anatomy and its relation to the animal body as it is high near the trunk and not to be confused with its tibiotalar articulation. The joint morphology resembled the human knee with femorotibial compartments, retro-patellar compartment, cruciate ligaments, and menisci. An important difference that was noted is the absence of a quadriceps tendon and the attachment of the quadriceps muscle directly on the superior pole of the patella.
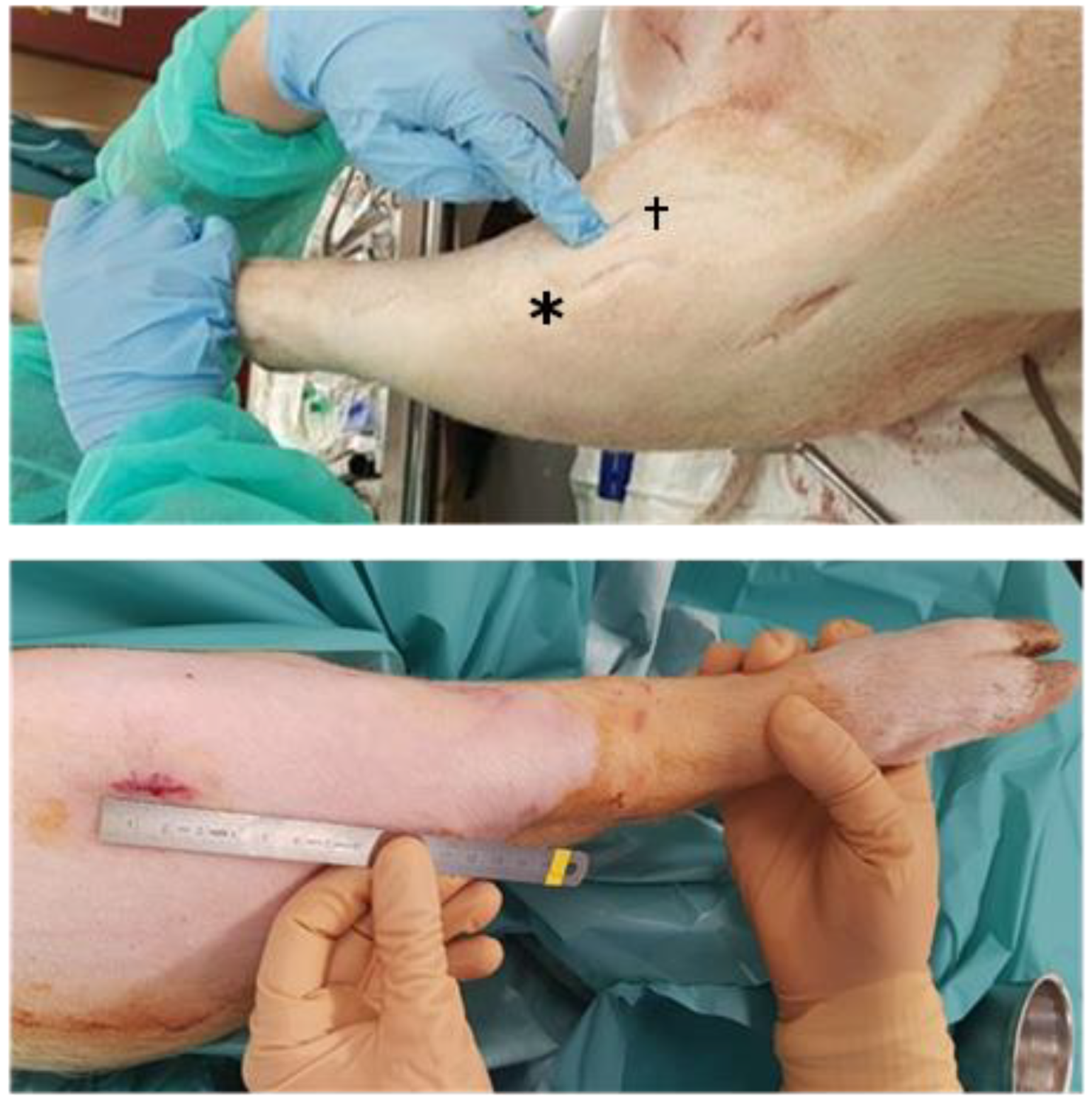
The first surgery used a 2 cm incision for the superolateral approach which was sufficient to harvest up to 70 mg of cartilage from DPs and up to 40 mg from G-MPs (Figure 1). This allowed for a proper visualization of the proximal lateral aspect of the joint surface, in both models (Table 1).
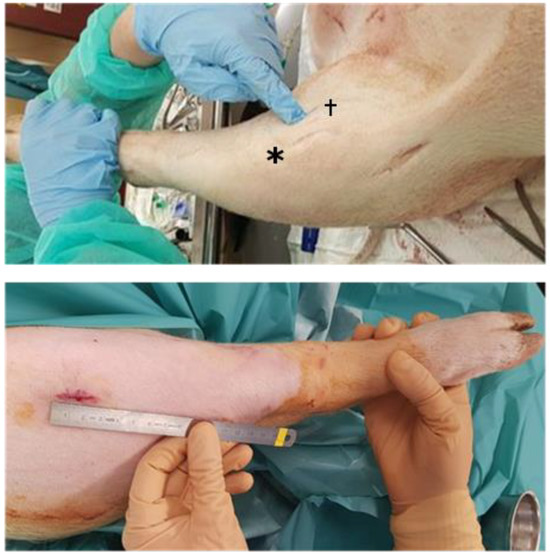
Figure 1.
Surgical approaches to the knee. (Top): Domestic pig superolateral, anteromedial (*), and medial (†) approach. Lesion creation via medial approach. (Bottom): Göttingen mini-pig superolateral approach.
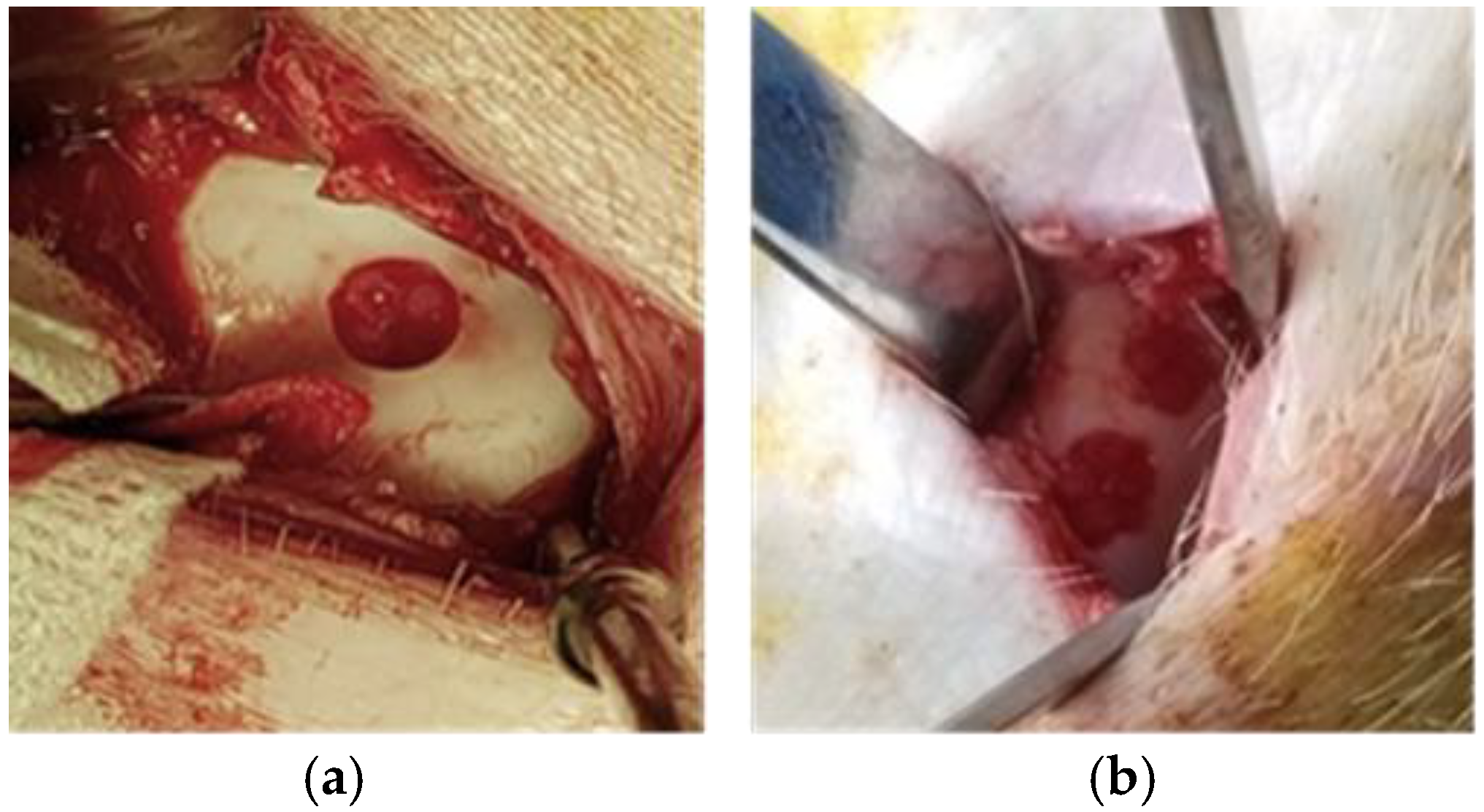
The second surgery used the medial parapatellar–tendon approach, through a slightly larger skin incision (3 cm), allowing access of the trochlea and the creation of four trochlear lesions and one medial condyle lesion (Figure 2).
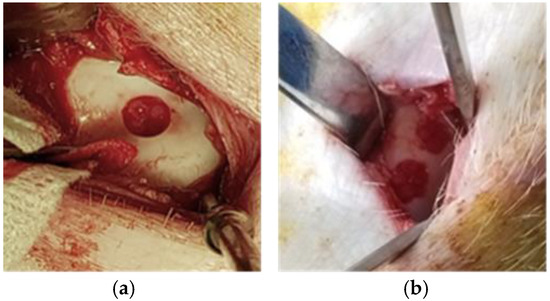
Figure 2.
Cartilage lesion creation: (a) Domestic pig showing one trochlear cartilage lesion; (b) Göttingen mini-pig two trochlear cartilage lesions.
This approach gave enough access to create the planned four trochlear lesions without over-lapping and a bridge of at least 5 mm between the lesions on the facets and the medial condyle lesion (Figure 3). A conflict with the harvest site was noted in two out of six G-MPs, leading to the loss of a planned lesion on the proximolateral trochlear facet.
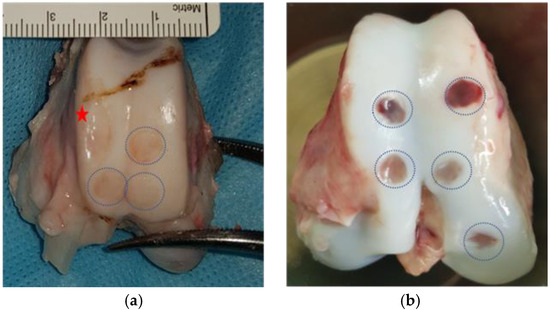
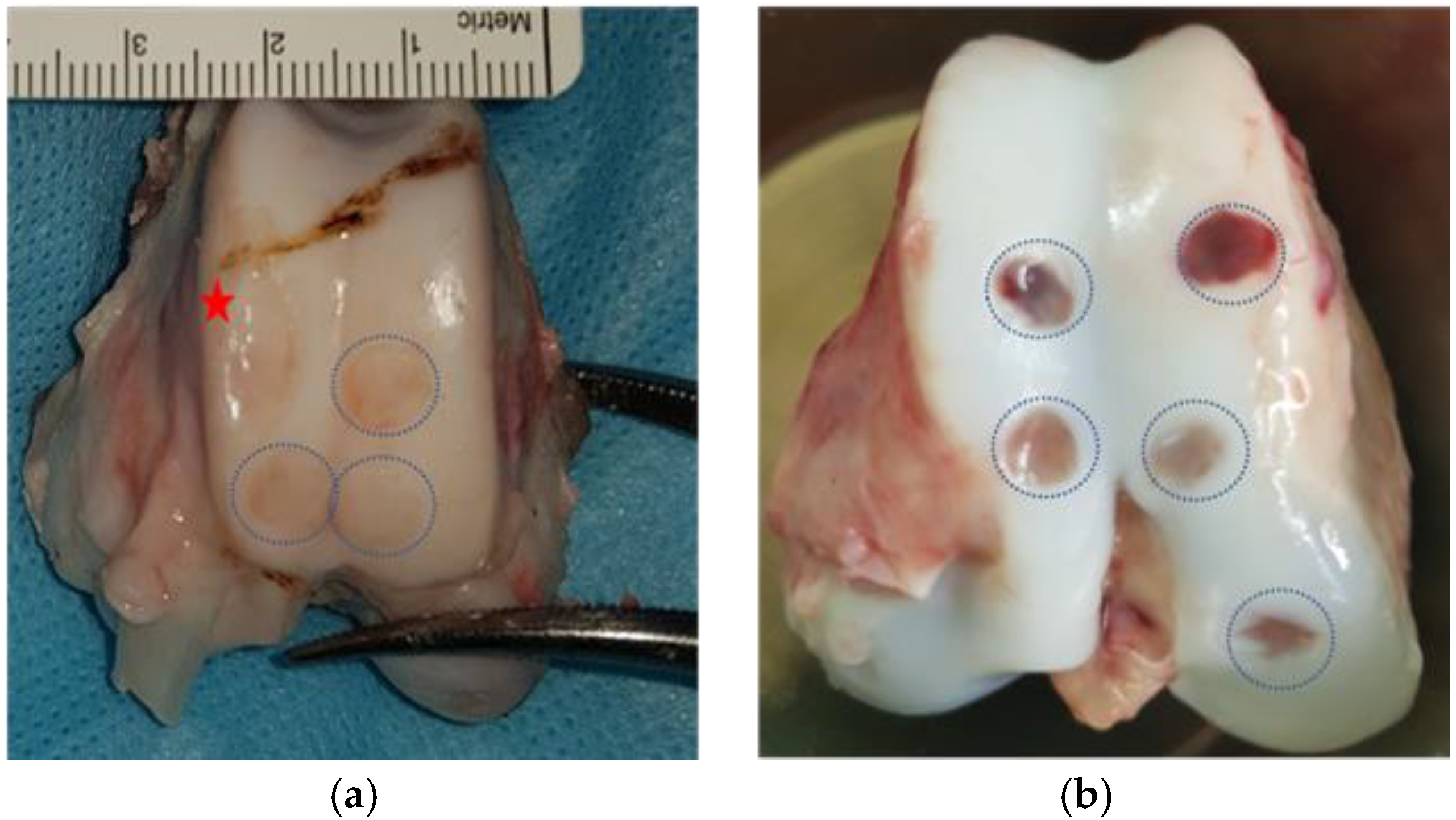
Figure 3.
Post-mortem knee extraction showing the different cartilage lesions in both models: (a) G-MP trochlear cartilage lesions: Blue dotted circles show the lesion in the trochlea, and the red star shows a donor site conflicting with an intended lesion site; (b) DP trochlear and medial condyle cartilage lesions. Blue dotted circles show the cartilage lesions.  Red star shows the harvest site.
Red star shows the harvest site.
 Red star shows the harvest site.
Red star shows the harvest site.
This approach gave access to 63% (SD ± 4.1) with knee extension and 34% (SD ± 13.5) of access to the medial condyle in knee flexion in DP (Table 1).
3.2. Complications And Morbidity
All animals recovered completely without observed complications. The animals’ mobility was back to normal without limping, and they were able to fully bear weight by day three after both surgeries. There were no wound-healing issues with any animal nor-visible joint inflammations.
In the post-mortem analysis, no degenerative process or patellar tendon shortening were noticed when comparing both sides.
3.3. Comparison
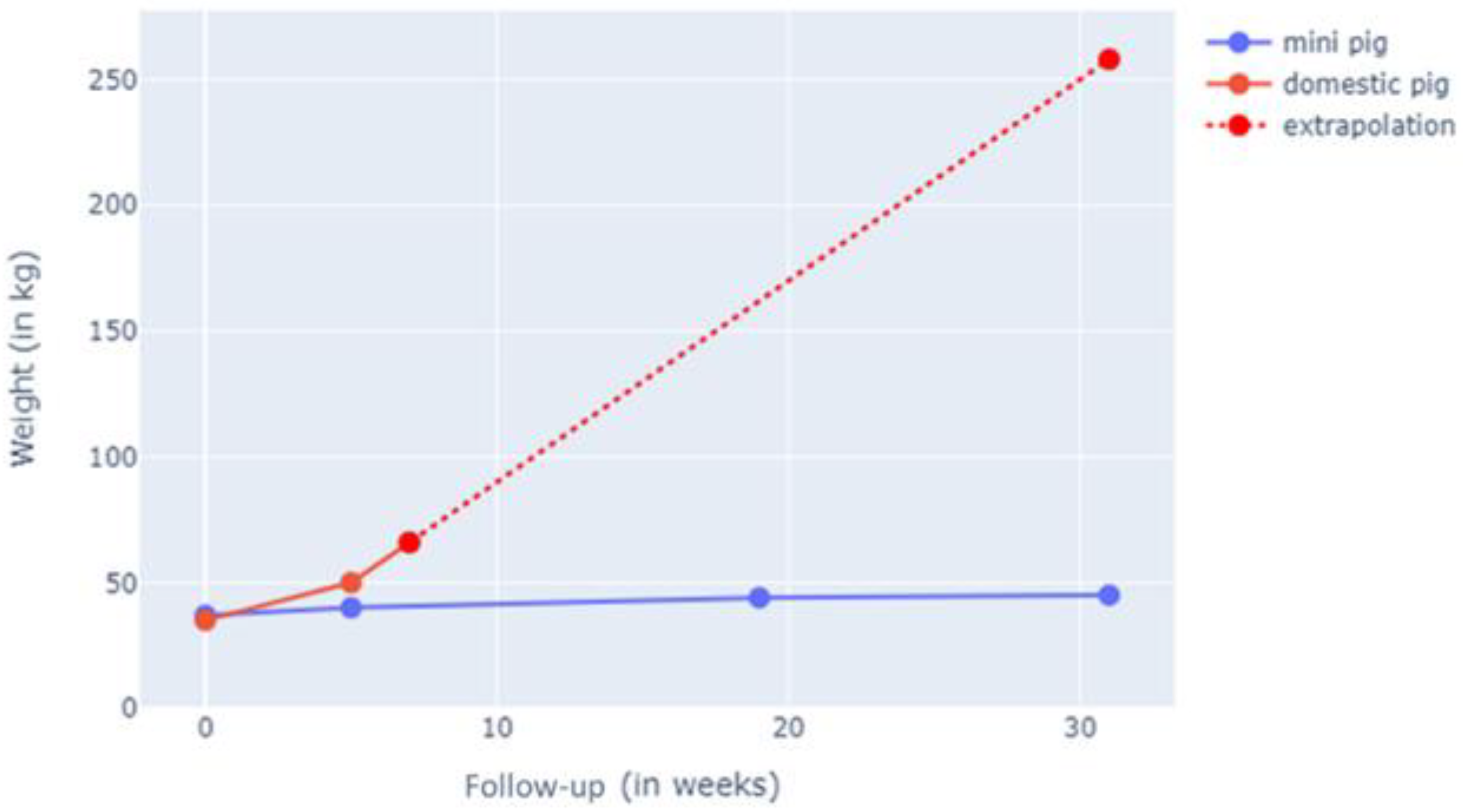
In the living animal, weight gain was documented, where both models started at 45 kg at the beginning of the study. The study animals were weighed weekly, and their weight progression was documented. We noted +0.2 kg/week for the G-MP group versus + 4 kg/week in the DP group. The G-MPs reached a maximum weight of 50 kg (SD ± 3 kg) at 6 months, whereas the DPs reached 60 kg (SD ± 2 kg) at 2 weeks of follow-up (Figure 4).
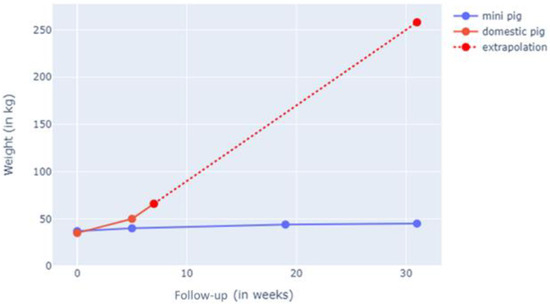
Figure 4.
Weight gain in G-MP compared to DP. The G-MP reached 50 kg (SD ± 3 kg) at 6 months, and DP reached 60 kg (SD ± 2 kg) at 2 weeks of follow-up.
The joint evaluation in peri-euthanasia showed a conserved range of motion. This was monitored by two observers (PT, HK) for all animals just prior euthanasia, and no differences in passive flexion and extension were noticed between both groups when compared to the contralateral knee. Post-mortem joint measurements showed a larger joint in the DPs compared to G-MPs (Table 1) at a comparable weight point.
Vascularized cartilage was observed in domestic pigs, which is thought to be due to their young age, but was absent in the adult G-MPs.
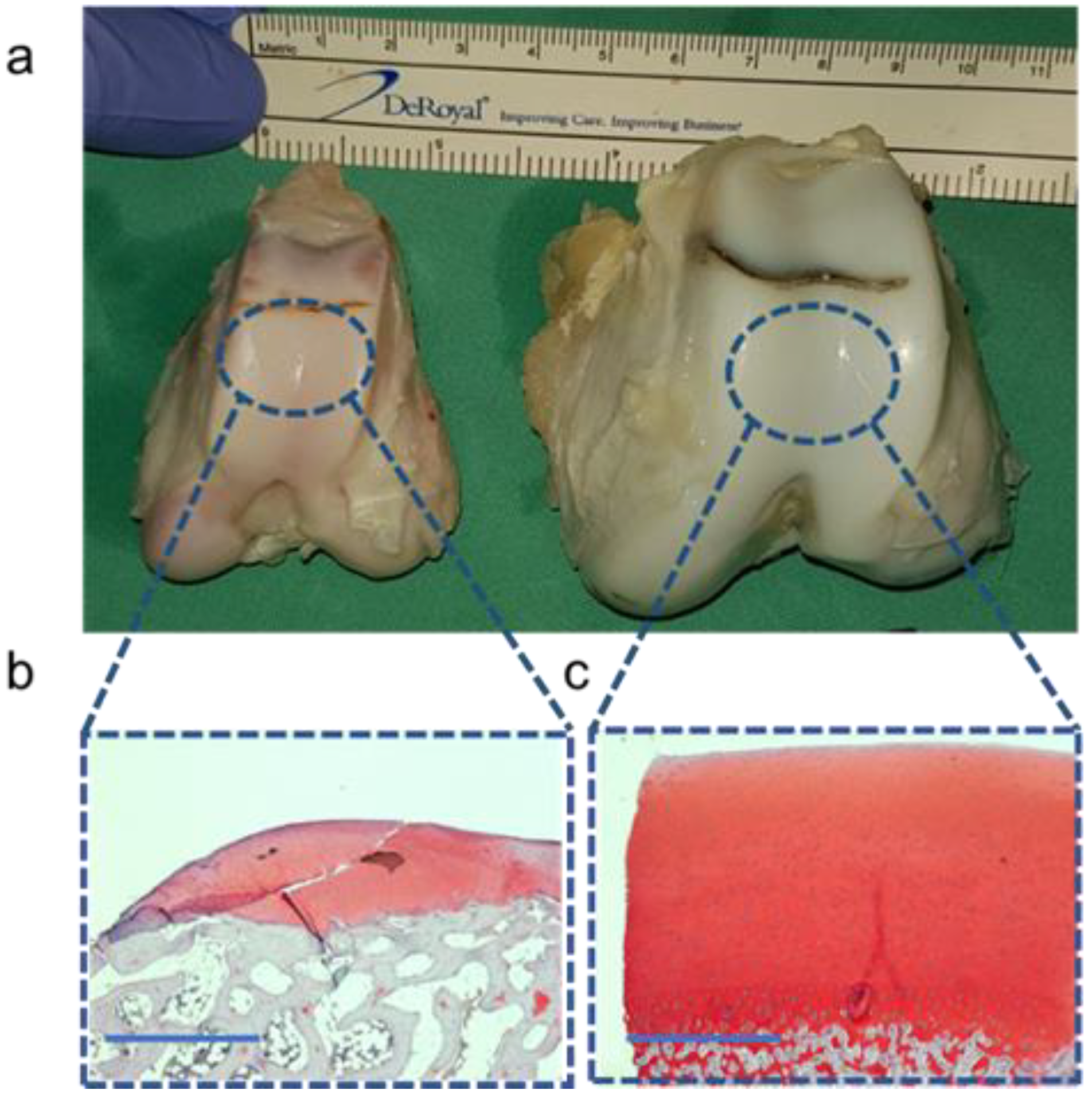
The histological analysis showed the differences in cartilage thickness between the G-MPs (<1 mm) and DPs (>2 mm) within a cross-sectional segment of the joint facet (Figure 5).
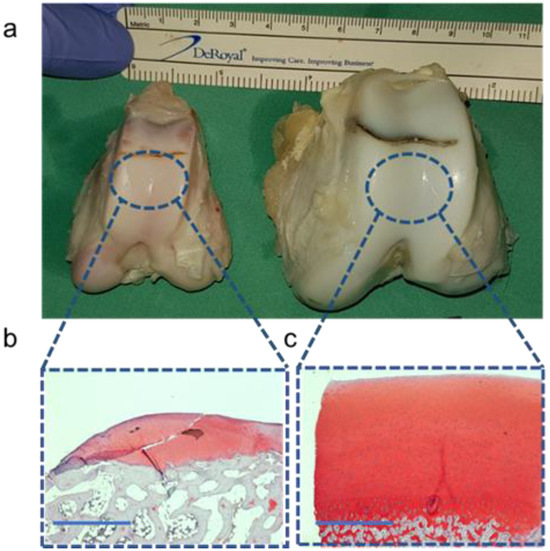
Figure 5.
Morphological and histological differences among both models: (a) Size differences in the distal femur between the G-MP (left) and DP (right). (b) Histological analysis using Safranin-O staining of the G-MP cartilage. (c) Histological analysis using Safranin-O staining of the DP cartilage. Scale bar 1 mm.
Post-mortem, joint morphology, and cartilage macroscopic evaluations showed no signs of inflammation and no changes in the rest of the joint cartilage that was not subjected to lesion creation or harvest. A narrow trochlea in the G-MPs allows for only four lesions of 6 mm diameter without overlap, while domestics permit increases in number or size of lesions (Table 2).

Table 2.
Potential post-operative complications in G-MP and DP groups: This table summarizes potential complications related to both surgeries as observed in the target joint and when compared to the contralateral, non-operated joint. * Parameters compared to the left non-operated knee. † Cartilage-lesion-scoring system.
4. Discussion
The main finding of the study is that the G-MP is an adequate and reliable large animal model for testing novel cartilage therapies. This valuable model offers some unique opportunities to explore research questions in biomechanics and biology [6,9,18,19].
In the DM group, the starting weight was tied to a very young age of the animals and cartilage immaturity, which can impact the lesion-creating and -healing potential; thus, findings cannot be generalized. The two approaches to the knee in both groups did not generate limping or alter weight-bearing for more than two days post-operatively. Scarring was minimal by the time of the second surgery, and adhesions were minimal by the time of euthanasia. The advantages of the G-MP model lies in its size and weight remaining constant in the adult animals, allowing for long-term follow-up studies. The use of adult animals means the cartilage is mature without vascularization, as noted in the young DP group [14].
The use of the G-MP model in orthopedic research is particularly valuable when compared to other animal models, like horses, dogs, and rabbits. The porcine model closely resembles human joint size and weight, which is crucial for biomechanical studies [14], unlike rabbits, whose smaller size limits the relevance of biomechanical data [20,21], or dogs, whose use osteoarticular research is widely used [22]. However, this causes important debate on the ethical aspects; pigs offer a more comparable joint structure and function and less controversy with ethical authorization. Horses are another large animal model used in orthopedic research, particularly for studying joint diseases like osteoarthritis [8,23]; however, their large size and maintenance costs make them less practical for routine studies. In contrast, the G-MP provides a balance between anatomical relevance and manageable size, making it a preferred model for cartilage research.
Our results highlight the main differences in the knee joint anatomy in G-MPs compared to DPs. In G-MPs, the joint is smaller, and the trochlea is narrower, which can lead to a conflict between the harvesting site and the defect site. In addition, in G-MPs, the articular surface was more difficult to expose, leading to additional technical challenges for the surgeons to comfortably treat and control multiple lesions. The articular cartilage thinness in G-MPs can somehow be a challenge to stabilize a graft within a limited lesion. The above-listed characteristics in the G-MPs did not prevent our group from achieving the desired results in creating and grafting multiple lesions in a study with a 6-month follow-up period [19].
The morphological and histological features of both groups showed no notable degradation of the joints after successive surgeries using two distinct approaches within 5 weeks. Comparable to what was previously reported in studies on the G-MPs, we found no degenerative changes with our five lesions per knee when compared to one or two lesions [18].
The advantages of both models are accessible knee joint by dual approach with relatively good exposure and the possibilities of realizing different types of cartilage studies.
The anatomical limitations of both models are the direct attachment of the quadriceps muscle on the proximal pole of the patella and the absence of a quadriceps tendon making the proximal trochlear surface or the patellar surface difficult to access without extensive damage to the muscle (Supplementary Figure S1). This anatomical characteristic makes these models more difficult or rather impossible to use for retropatellar lesion studies or kissing lesions without more invasive knee surgery.
A financial limitation of the G-MP model is its higher initial cost compared to the DP model, especially for larger study samples, with each G-MP costing around 2000€ versus 300€ for a DP. Additionally, G-MPs require shipping from specialized breeding facilities, which are not widely available. However, for long-term studies, the cost of care and maintenance for DP models increases significantly. This rise in cost is due to the adult DP’s large size and weight, which necessitate more space in the animal facility, larger feeding portions, and higher labor costs, as more caregivers are needed to manage the handling, transportation, and study-related procedures (MRI, anesthesia, euthanasia, etc.) of these large animals.
In summary, while the G-MP model offers significant advantages in terms of anatomical and biomechanical relevance, its limitations in cost, accessibility, and certain anatomical features must be considered when choosing an animal model for orthopedic research.
5. Conclusions
The mini-pig model allows for multiple lesions per knee and for long-term follow-up without much strain on the animals or host structure. However, it is important to note that its thin cartilage and a narrow trochlea might somehow be a challenge, depending on the tested treatment method. Domestic pig knees have a larger joint surface and thicker cartilage, making them more comparable to the human knee, but their adult weight makes them less suitable for studies needing long-term follow-ups. Both superolateral and medial approaches can be used in both groups without noticeable risk of joint degeneration or animal discomfort. Altogether, this study not only provides a methodology for mini-pig two-step surgery but also presents mini-pigs as a relevant animal model for cartilage study.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/std14020009/s1: Figure S1 Gross section of the quadriceps muscle and its direct insertion on the proximal tibial pole and the absence of a quadricepses tendon.
Author Contributions
H.K. and P.M.T. designed the experiment and executed the surgeries. V.T. designed the laboratory protocol and evaluated the results. H.K. wrote the manuscript. P.M.T. and V.T. reviewed the paper pre-submission. All authors have read and agreed to the published version of the manuscript.
Funding
Vanarix SA, provided the animals and the study cost: Centre Assal for foot and ankle surgery: provided hourly honorarium for HK, Swiss Foot and Ankle Society (SFAS): Provided a one-time grant of 10,000 Swiss francs.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Geneva Cantonal Veterinary Office (protocol code GE 14/18 and date of approval 12 February 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
All additional data and information regarding the methodology and results are available with the authors upon request.
Acknowledgments
The authors wish to thank the animal research facility and the team of Professor Walid Habre and the diagnostic facility from the Geneva University Hospitals.
Conflicts of Interest
Vannary Tieng is the developer of Cartibeads and the CEO of VANARIX SA. The development of the surgical technique described in this paper was a response to lack of published material on the domain. The authors declare no conflicts of interest.
References
- Belk, J.W.; McCarty, E. Editorial Commentary: Autologous Chondrocyte Implantation Versus Microfracture for Knee Articular Cartilage Repair: We Should Focus on the Latest Autologous Chondrocyte Implantation Techniques. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 304–306. [Google Scholar] [CrossRef]
- Hoburg, A.; Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Kolombe, T.; Fay, J.; Pietsch, S.; Kuźma, T.; Widuchowski, W.; et al. Matrix-Associated Autologous Chondrocyte Implantation with Spheroid Technology Is Superior to Arthroscopic Microfracture at 36 Months Regarding Activities of Daily Living and Sporting Activities after Treatment. CARTILAGE 2021, 13, 194760351989729. [Google Scholar] [CrossRef]
- Riboh, J.C.; Cvetanovich, G.L.; Cole, B.J.; Yanke, A.B. Comparative efficacy of cartilage repair procedures in the knee: A network meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3786–3799. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.J.; Ramesh, A.; Brama, P.A.J.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 2016, 3, 1. [Google Scholar] [CrossRef]
- Allen, M.J.; Hankenson, K.D.; Goodrich, L.; Boivin, G.P.; von Rechenberg, B. Ethical use of animal models in musculoskeletal research. J. Orthop. Res. 2017, 35, 740–751. [Google Scholar] [CrossRef]
- Gutierrez, K.; Dicks, N.; Glanzner, W.G.; Agellon, L.B.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 2015, 6, 293. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Q.; Liu, L.; Wu, H.; Zheng, L.; Zhao, J. A Novel Synthesized Sulfonamido-Based Gallate-JEZTC Blocks Cartilage Degradation on Rabbit Model of Osteoarthritis: An in Vitro and in Vivo Study. Cell. Physiol. Biochem. 2018, 49, 2304–2319. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Fortier, L.A.; Frisbie, D.D.; Nixon, A.J. Equine Models of Articular Cartilage Repair. CARTILAGE 2011, 2, 317–326. [Google Scholar] [CrossRef]
- Pfeifer, C.G.; Fisher, M.B.; Saxena, V.; Kim, M.; Henning, E.A.; Steinberg, D.A.; Dodge, G.R.; Mauck, R.L. Age-Dependent Subchondral Bone Remodeling and Cartilage Repair in a Minipig Defect Model. Tissue Eng. Part C: Methods 2017, 23, 745–753. [Google Scholar] [CrossRef]
- Xia, D.; Jin, D.; Wang, Q.; Gao, M.; Zhang, J.; Zhang, H.; Bai, J.; Feng, B.; Chen, M.; Huang, Y.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair in a goat model. J. Tissue Eng. Regen. Med. 2019, 13, 694–703. [Google Scholar] [CrossRef]
- Bekkers, J.E.J.; Creemers, L.B.; Tsuchida, A.I.; van Rijen, M.H.P.; Custers, R.J.H.; Dhert, W.J.A.; Saris, D.B.F. One-stage focal cartilage defect treatment with bone marrow mononuclear cells and chondrocytes leads to better macroscopic cartilage regeneration compared to microfracture in goats. Osteoarthr. Cartil. 2013, 21, 950–956. [Google Scholar] [CrossRef]
- Cone, S.G.; Warren, P.B.; Fisher, M.B. Rise of the Pigs: Utilization of the Porcine Model to Study Musculoskeletal Biomechanics and Tissue Engineering During Skeletal Growth. Tissue Eng. Part C Methods 2017, 23, 763–780. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Shen, B.-Y.; Wang, Y.-H.; Lin, B.; Lee, H.-M.; Hsieh, M.-F. Healing of Osteochondral Defects Implanted with Biomimetic Scaffolds of Poly(ε-Caprolactone)/Hydroxyapatite and Glycidyl-Methacrylate-Modified Hyaluronic Acid in a Minipig. Int. J. Mol. Sci. 2018, 19, 1125. [Google Scholar] [CrossRef] [PubMed]
- Rieppo, J.; Hyttinen, M.M.; Halmesmaki, E.; Ruotsalainen, H.; Vasara, A.; Kiviranta, I.; Jurvelin, J.S.; Helminen, H.J. Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarthr. Cartil. 2009, 17, 448–455. [Google Scholar] [CrossRef]
- Ganderup, N.C.; Harvey, W.; Mortensen, J.T.; Harrouk, W. The Minipig as Nonrodent Species in Toxicology—Where Are We Now? Int. J. Toxicol. 2012, 31, 507–528. [Google Scholar] [CrossRef]
- Kang, Q.; LaBreck, J.; Gruber, H.; An, Y. Histological Techniques for Decalcified Bone and Cartilage. In Handbook of Histology Methods for Bone and Cartilage; Springer: Berlin/Heidelberg, Germany, 2003; pp. 209–219. ISBN 978-1-61737-277-3. [Google Scholar]
- Schmitz, N.; Laverty, S.; Kraus, V.B.; Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 2010, 18, S113–S116. [Google Scholar] [CrossRef]
- Christensen, B.B.; Foldager, C.B.; Olesen, M.L.; Vingtoft, L.; Rölfing, J.H.D.; Ringgaard, S.; Lind, M. Experimental articular cartilage repair in the Göttingen minipig: The influence of multiple defects per knee. J. Exp. Orthop. 2015, 2, 13. [Google Scholar] [CrossRef]
- Kutaish, H.; Tscholl, P.M.; Cosset, E.; Bengtsson, L.; Braunersreuther, V.; Mor, F.M.; Laedermann, J.; Furfaro, I.; Stafylakis, D.; Hannouche, D.; et al. Articular Cartilage Repair After Implantation of Hyaline Cartilage Beads Engineered from Adult Dedifferentiated Chondrocytes: Cartibeads Preclinical Efficacy Study in a Large Animal Model. Am. J. Sports Med. 2023, 51, 237–249. [Google Scholar] [CrossRef]
- Guo, X.; Park, H.; Young, S.; Kretlow, J.D.; Van den Beucken, J.J.; Baggett, L.S.; Tabata, Y.; Kasper, F.K.; Mikos, A.G.; Jansen, J.A. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010, 6, 39–47. [Google Scholar]
- Schafrum Macedo, A.; Cezaretti Feitosa, C.; Yoiti Kitamura Kawamoto, F.; Vinicius Tertuliano Marinho, P.; dos Santos Dal-Bó, Í.; Fiuza Monteiro, B.; Prado, L.; Bregadioli, T.; Antonio Covino Diamante, G.; Ricardo Auada Ferrigno, C. Animal modeling in bone research—Should we follow the White Rabbit? Anim. Models Exp. Med. 2019, 2, 162–168. [Google Scholar] [CrossRef]
- Franklin, S.P.; Stoker, A.M.; Lin, A.S.P.; Pownder, S.L.; Burke, E.E.; Bozynski, C.C.; Kuroki, K.; Guldberg, R.E.; Cook, J.L.; Holmes, S.P. T1ρ, T2 mapping, and EPIC-µCT Imaging in a Canine Model of Knee Osteochondral Injury. J. Orthop. Res. 2020, 38, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Canonici, F.; Cocumelli, C.; Cersini, A.; Marcoccia, D.; Zepparoni, A.; Altigeri, A.; Caciolo, D.; Roncoroni, C.; Monteleone, V.; Innocenzi, E.; et al. Articular Cartilage Regeneration by Hyaline Chondrocytes: A Case Study in Equine Model and Outcomes. Biomedicines 2023, 11, 1602. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).