Endocrine-Disrupting Effects of Bisphenol A on the Cardiovascular System: A Review
Abstract
1. Introduction
2. Approach to the Review
3. Exposure to BPA
4. Effects of BPA on Animal Models
4.1. In Vitro Studies
4.2. Ex Vivo Studies
4.3. In Vivo Studies
5. Effects of BPA on Humans
5.1. In Vitro Studies
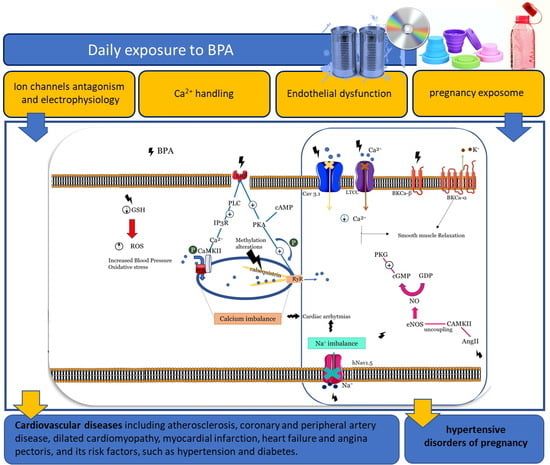
5.1.1. Effects of BPA on Ion Channels and Electrophysiology
5.1.2. Effects of BPA on Ca2+ Handling
5.1.3. Effects on Vascular Endothelium
5.1.4. Effects in Pregnancy Exposome
| Topic | Studied Mechanism | Concentration | Type of Cells | Observed Effects | References |
|---|---|---|---|---|---|
| Ion channels and electrophysiology | Nav1.5 channels | 1–100 µmol/L | HEK-transfected cell line |
| [43] |
| Nav1.5 channels | 0.0–100 µmol/L | HEK-transfected cell line |
| [42] | |
| Nav1.5 channels | 1–100 µmol/L | hiPSC-CMs |
| [41] | |
| Recombinant human R-type Ca2+ channels | 1–100 µmol/L | HEK 293 cells |
| [38] | |
| T-type Ca2+ channels | 1–100 μmol/L | HEK 293 cells |
| [39] | |
| L-type Ca2+ channels Cav1.2 | 1–100 µmol/L | hiPSC-CMs |
| [41] | |
| Maxi-K channels | 100 µmol/L | HCASMC |
| [35] | |
| Ca2+ handling | Ca2+ current channels, Ca2+ transients and contraction | 1–100 µmol/L | hiPSC-CMs |
| [41] |
| Cardiac hypertrophy by disrupting Ca2+ homeostasis | 8 ng/mL | Human embryonic stem-cell-derived cardiomyocytes |
| [86] | |
| Vascular endothelium | Endothelial dysfunction, inflammation, and angiogenesis | 0.1–1 μmol/L | HUVECs |
| [88] |
| Cell division and chromosomal segregation | 0.5–10 ng/mL | HUVECs |
| [89] | |
| Senescence | 10 ng/mL and 1 µg/mL | HUVECs |
| [90] | |
| Accelerating atherosclerosis | 0.1–10 nmol/L | HUVECs |
| [59] | |
| Pregnancy exposome | Epigenetic disruption | 35.4–56.1 ng/g | Human fetal liver samples |
| [91] |
| 0.57 and 0.78 ng/mL | Maternal urine samples and Infant cord blood |
| [92] | ||
| Pregnancy physiology | 1 nmol/L | BeWo trophoblast cell line, placental explant cultures, placental perfusions, and skin diffusion models |
| [95] | |
| 0, 0.09, 0.9, and 9.0 μmol/L | BeWo trophoblast cell line |
| [96] | ||
| 1 × 10−15 to 1 × 10−7 mol/L | Human trophoblast cells HTR-8/SVneo |
| [97] | ||
| 1 nmol/L | Human trophoblast cells HTR-8/SVneo |
| [98] |
5.2. Epidemiological Studies
5.2.1. Cardiovascular Diseases and Risk Factors
5.2.2. Pregnancy Exposure
| Topic | Studied Population | Gender | Concentration | Observed Effects | References |
|---|---|---|---|---|---|
| Cardiovascular diseases | NHANES from 2003–2004 1455 adults aged 18 through 74 years | 694 men 761 women | 4.53 ng/mL (urinary) 4.66 ng/mL (urinary) |
| [103] |
| NHANES 2003–2006 data, separately between 2003/2004 and 2005/2006 and pooled n = 1455 (2003/04) and n = 1493 (2005/06) adults aged 18–74 years | 694 men and 761 women in 2003/04 720 men and 773 women in 2005/06 | 2.49 ng/mL (urinary) 1.79 ng/mL (urinary) |
| [108] | |
| Population-based Prospective Investigation of the Vasculature in Uppsala Seniors study (1016 subjects all aged 70) | 510 women and 506 men | 3.76 ng/mL (serum) |
| [105] | |
| 758 incident CAD cases and 861 controls followed for 10.8 years from the European Prospective Investigation of Cancer—Norfolk, UK | 534 men and 327 women in control group 501 men and 257 women in CAD group | Control vs. CAD group (1.24 ng/mL vs. 1.35 ng/mL) (urinary) |
| [106] | |
| 591 patients participating in The Metabonomics and Genomics in Coronary Artery Disease study in Cambridgeshire, UK | 120 controls (62 women and 58 men) 385 patients (301 men and 84 women) 86 Intermediate diseases (43 men and 43 women) | Control vs. CAD vs. intermediate (2.13 vs. 3.82 vs. 3.31 ng/mL) (urinary) |
| [107] | |
| 745 participants in the NHANES 2003–2004 | 361 women and 392 men | 2.30 ng/mL (urinary) |
| [110] | |
| 88 DCM patients and 88 age-and gender-matched healthy controls | 59 men and 29 women with DCM 55 men and 33 women Control | DCM vs. control group (6.9 ± 2.7 vs. 3.8 ± 1.9 ng/mL) (serum) |
| [109] | |
| NHANES 2003–2014 (n = 9139, aged ≥20 years) | 4467 men and 4672 women | - |
| [104] | |
| Hypertension | 1380 subjects from NHANES 2003–2004 | 700 women and 680 men (580 with hypertension) | 1.5–4.0 ng/mL (urinary) |
| [112] |
| 560 noninstitutionalized elderly citizens from August 2008 to August 2010 in Seoul from Korean Elderly Environmental Panel Study | 521 participants were included (138 men and 383 women) | (men) and 1.3 (women) μg/g of creatinine (urinary) |
| [113] | |
| 60 noninstitutionalized elderly participants, who were aged ≥60 years between February 2014 and March 2014 | 60 participants, 56 were women and 4 male | 1.13 ± 1.76 μg/L (urinary) |
| [114] | |
| A subsample of 2558 randomly selected from the Thai National Health Examination Survey IV, 2009 | 1275 men and 1283 women | 0.35 ng/mL (men) and 0.33 ng/mL (women) (serum) |
| [115] | |
| Pregnancy exposure | 645 children at the age of 4 who were born from women who participated, midterm during their pregnancy, in a birth cohort study from August 2008 to July 2011 | 486 mother–child pairs were included in the present analysis | Maternal urinary: 0.9 μg/L (urinary) |
| [121] |
| 152 female volunteer participants in the Human Early-Life Exposome project | 152 pregnant women | 3.1 μg/g creatinine (urinary) |
| [120] | |
| 1064 mother-child pairs/childhood at a mean age of 9.7 years old | 1064 mother-child pais | 6.0 nmol/L (boys) and 7.2 nmol/L (girls) (urinary) |
| [122] | |
| 58 pregnancies, including 35 normotensive and 23 preeclamptic women | 35 normotensive pregnant women and 23 preeclamptic pregnant women | Control vs. PE (3.00 vs. 2.80 ng/mL–maternal serum; 2.17 vs. 2.23 ng/mL–fetal serum; 3.00 vs. 9.40–placental homogenate) |
| [124] | |
| A nested case-control population consisting of 130 mothers who delivered preterm and 352 who delivered term from a prospective birth cohort | 130 women and 352 controls | 7.08% change (adjusted with soluble fms-like tyrosine kinase-1) (urinary) |
| [125] | |
| A nested case-control study of preterm birth was performed in 2011 from women enrolled in a prospective birth cohort study at Women’s Hospitals in Brigham and in Boston (included 50 cases of PE) | 482 women (50 with PE) | Cases vs. control (1.56 vs. 1.38 ng/mL) (urinary) |
| [126] | |
| 1233 women excluding those without any BP measurement or with pre-existing hypertension | 1233 women | 1.65 ng/mL (urinary) |
| [127] | |
| 1 year postpartum among 199 women in Mexico City | 199 women | 1.18 ng/mL (urinary) |
| [129] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ang-II | angiotensin II |
| BP | blood pressure |
| BPA | bisphenol A |
| BPAF | bisphenol AF |
| BPC | bisphenol C |
| Ca2+ | calcium |
| CAD | coronary artery disease |
| CnAβ | calcineurin |
| CVDs | cardiovascular diseases |
| DCM | dilated cardiomyopathy |
| DRP1 | dynamin-related protein 1 |
| EC | endothelial cells |
| EDC | endocrine-disrupting compound; |
| EDHF | endothelium-derived hyperpolarizing factor |
| ER | estrogen receptors |
| ET-1 | endothelin 1 |
| GPR30 | G-protein-coupled estrogen receptor |
| HDPs | hypertensive disorders of pregnancy |
| HEK | human embryonic kidney |
| hiPSC-CMs | human-induced pluripotent stem-cell-derived cardiomyocytes |
| HRV | heart rate variability |
| HUVECs | human umbilical vein endothelial cells |
| IC50 | half-maximal inhibitory concentration |
| iPSCs | induced pluripotent stem cells |
| IR | ischemia–reperfusion |
| K+ | potassium |
| MAP | mean atrial pressures |
| MBP | 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene |
| MI | myocardial infarction |
| MOA | mechanisms of action |
| Na+ | sodium |
| NHANES | National Health and Nutrition Examination Survey |
| NO | nitric oxide |
| PAD | peripheral artery disease |
| PE | preeclampsia |
| PFOS | perfluorooctane sulfonate; |
| PGI-2 | prostacyclin |
| PLB | phospholamban |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| RIP3 | Rat Receptor-Interacting Protein 3 |
| SMC | smooth muscle cells |
| TxA2 | thromboxane |
| U.S. | United States |
| XME | xenobiotic-metabolizing enzymes |
References
- Francula-Zaninovic, S.; Nola, I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Ramadan, M.; Cooper, B.; Posnack, N.G. Bisphenols and phthalates: Plastic chemical exposures can contribute to adverse cardiovascular health outcomes. Birth Defects Res. 2020, 112, 1362–1385. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Dodds, E.C.; Lawson, W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature 1936, 137, 996. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef]
- Banerjee, O.; Singh, S.; Saha, I.; Pal, S.; Banerjee, M.; Kundu, S.; Syamal, A.K.; Maji, B.K.; Mukherjee, S. Molecular dissection of cellular response of pancreatic islet cells to Bisphenol-A (BPA): A comprehensive review. Biochem. Pharmacol. 2022, 201, 115068. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, H.-S. Impact of Bisphenol A on the Cardiovascular System—Epidemiological and Experimental Evidence and Molecular Mechanisms. Int. J. Environ. Res. Public Health 2014, 11, 8399–8413. [Google Scholar] [CrossRef] [PubMed]
- Rancière, F.; Lyons, J.G.; Loh, V.H.; Botton, J.; Galloway, T.; Wang, T.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health A Glob. Access Sci. Source 2015, 14, 46. [Google Scholar] [CrossRef]
- Vandenberg Laura, N.; Chahoud, I.; Heindel Jerrold, J.; Padmanabhan, V.; Paumgartten Francisco, J.R.; Schoenfelder, G. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Schönfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002, 110, A703–A707. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Furuta, I.; Kato, E.H.; Kataoka, S.; Usuki, Y.; Kobashi, G.; Sata, F.; Kishi, R.; Fujimoto, S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod. Toxicol. 2002, 16, 735–739. [Google Scholar] [CrossRef]
- Kuroda, N.; Kinoshita, Y.; Sun, Y.; Wada, M.; Kishikawa, N.; Nakashima, K.; Makino, T.; Nakazawa, H. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J. Pharm. Biomed. Anal. 2003, 30, 1743–1749. [Google Scholar] [CrossRef]
- Hines, E.P.; Mendola, P.; von Ehrenstein, O.S.; Ye, X.; Calafat, A.M.; Fenton, S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2015, 54, 120–128. [Google Scholar] [CrossRef]
- Tan, B.L.; Ali Mohd, M. Analysis of selected pesticides and alkylphenols in human cord blood by gas chromatograph-mass spectrometer. Talanta 2003, 61, 385–391. [Google Scholar] [CrossRef]
- Sun, Y.; Irie, M.; Kishikawa, N.; Wada, M.; Kuroda, N.; Nakashima, K. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed. Chromatogr. 2004, 18, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kuruto-Niwa, R.; Tateoka, Y.; Usuki, Y.; Nozawa, R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere 2007, 66, 1160–1164. [Google Scholar] [CrossRef]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and Kinetics of Bisphenol A in Humans at Low Doses Following Oral Administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wang, X.; Hong, H.; Benfenati, E.; Giesy, J.P.; Gini, G.C.; Kusko, R.; Zhang, X.; Yu, H.; Shi, W. Structures of Endocrine-Disrupting Chemicals Determine Binding to and Activation of the Estrogen Receptor α and Androgen Receptor. Environ. Sci. Technol. 2020, 54, 11424–11433. [Google Scholar] [CrossRef]
- Vandenberg, L.N. Non-Monotonic Dose Responses in Studies of Endocrine Disrupting Chemicals: Bisphenol a as a Case Study. Dose-Response 2014, 12, 259–276. [Google Scholar] [CrossRef]
- Cooper, B.L.; Posnack, N.G. Characteristics of Bisphenol Cardiotoxicity: Impaired Excitability, Contractility, and Relaxation. Cardiovasc. Toxicol. 2022, 22, 273–280. [Google Scholar] [CrossRef]
- Boudalia, S.; Oudir, M. Bisphenol-A: Legislation in Industrials Countries and in Algeria. Res. J. Environ. Toxicol. 2016, 10, 189–192. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, S.; Zhang, M.; Hou, J. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 254, 109275. [Google Scholar] [CrossRef]
- Posnack, N.G.; Jaimes, R., 3rd; Asfour, H.; Swift, L.M.; Wengrowski, A.M.; Sarvazyan, N.; Kay, M.W. Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ. Health Perspect. 2014, 122, 384–390. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Shan, C.; Wang, Y.; Qian, L.-L.; Jia, D.-D.; Zhang, Y.-F.; Hao, X.-D.; Xu, H.-M. Cardiovascular toxicity and mechanism of bisphenol A and emerging risk of bisphenol S. Sci. Total Environ. 2020, 723, 137952. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Araujo, J.A.; Barchowsky, A.; Belcher, S.; Berridge, B.R.; Chiamvimonvat, N.; Chiu, W.A.; Cogliano, V.J.; Elmore, S.; Farraj, A.K.; et al. Key Characteristics of Cardiovascular Toxicants. Environ. Health Perspect. 2021, 129, 95001. [Google Scholar] [CrossRef]
- Cumberland, M.J.; Riebel, L.L.; Roy, A.; O’Shea, C.; Holmes, A.P.; Denning, C.; Kirchhof, P.; Rodriguez, B.; Gehmlich, K. Basic Research Approaches to Evaluate Cardiac Arrhythmia in Heart Failure and Beyond. Front. Physiol. 2022, 13, 806366. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Marchal, G.A.; Zegers, J.G.; Kawasaki, M.; Driessen, A.H.G.; Remme, C.A.; de Groot, J.R.; Wilders, R. Patch-Clamp Recordings of Action Potentials From Human Atrial Myocytes: Optimization Through Dynamic Clamp. Front. Pharmacol. 2021, 12, 649414. [Google Scholar] [CrossRef]
- Asano, S.; Tune, J.D.; Dick, G.M. Bisphenol A activates Maxi-K (K(Ca)1.1) channels in coronary smooth muscle. Br. J. Pharmacol. 2010, 160, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Rottgen, T.S.; Fancher, I.S.; Asano, S.; Widlanski, T.S.; Dick, G.M. Bisphenol A activates BK channels through effects on α and β1 subunits. Channels 2014, 8, 249–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feiteiro, J.; Mariana, M.; Glória, S.; Cairrao, E. Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle. J. Toxicol. Sci. 2018, 43, 579–586. [Google Scholar] [CrossRef]
- Deutschmann, A.; Hans, M.; Meyer, R.; Häberlein, H.; Swandulla, D. Bisphenol A Inhibits Voltage-Activated Ca2+ Channels in Vitro: Mechanisms and Structural Requirements. Mol. Pharmacol. 2013, 83, 501. [Google Scholar] [CrossRef] [PubMed]
- Michaela, P.; Mária, K.; Silvia, H.; Ľubica, L. Bisphenol A differently inhibits CaV3.1, CaV3.2 and CaV3.3 calcium channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 153–163. [Google Scholar] [CrossRef]
- Liang, Q.; Gao, X.; Chen, Y.; Hong, K.; Wang, H.-S. Cellular Mechanism of the Nonmonotonic Dose Response of Bisphenol A in Rat Cardiac Myocytes. Environ. Health Perspect. 2014, 122, 601–608. [Google Scholar] [CrossRef]
- Hyun, S.-A.; Lee, C.Y.; Ko, M.Y.; Chon, S.-H.; Kim, Y.-J.; Seo, J.-W.; Kim, K.K.; Ka, M. Cardiac toxicity from bisphenol A exposure in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Appl. Pharmacol. 2021, 428, 115696. [Google Scholar] [CrossRef] [PubMed]
- Prudencio, T.M.; Swift, L.M.; Guerrelli, D.; Cooper, B.; Reilly, M.; Ciccarelli, N.; Sheng, J.; Jaimes, R., III; Posnack, N.G. Bisphenol S and Bisphenol F Are Less Disruptive to Cardiac Electrophysiology, as Compared with Bisphenol A. Toxicol. Sci. 2021, 183, 214–226. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.O.; Eberhardt, E.; Weidner, C.; Alzheimer, C.; Wallace, B.A.; Lampert, A. Bisphenol A Binds to the Local Anesthetic Receptor Site to Block the Human Cardiac Sodium Channel. PLoS ONE 2012, 7, e41667. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Hézső, T.; Kiss, D.; Kistamás, K.; Magyar, J.; Nánási, P.P.; Bányász, T. Late Sodium Current Inhibitors as Potential Antiarrhythmic Agents. Front. Pharmacol. 2020, 11, 413. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Dong, M.; Song, W.; Belcher, S.M.; Wang, H.-S. Bisphenol A and 17β-Estradiol Promote Arrhythmia in the Female Heart via Alteration of Calcium Handling. PLoS ONE 2011, 6, e25455. [Google Scholar] [CrossRef]
- Belcher, S.M.; Chen, Y.; Yan, S.; Wang, H.-S. Rapid Estrogen Receptor-Mediated Mechanisms Determine the Sexually Dimorphic Sensitivity of Ventricular Myocytes to 17β-Estradiol and the Environmental Endocrine Disruptor Bisphenol A. Endocrinology 2012, 153, 712–720. [Google Scholar] [CrossRef]
- Yan, S.; Song, W.; Chen, Y.; Hong, K.; Rubinstein, J.; Wang, H.S. Low-dose bisphenol A and estrogen increase ventricular arrhythmias following ischemia-reperfusion in female rat hearts. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 56, 75–80. [Google Scholar] [CrossRef]
- Gao, X.; Liang, Q.; Chen, Y.; Wang, H.-S. Molecular Mechanisms Underlying the Rapid Arrhythmogenic Action of Bisphenol A in Female Rat Hearts. Endocrinology 2013, 154, 4607–4617. [Google Scholar] [CrossRef]
- Pinto, C.; Hao, R.; Grimaldi, M.; Thrikawala, S.; Boulahtouf, A.; Aït-Aïssa, S.; Brion, F.; Gustafsson, J.; Balaguer, P.; Bondesson, M. Differential activity of BPA, BPAF and BPC on zebrafish estrogen receptors in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 380, 114709. [Google Scholar] [CrossRef]
- Ramadan, M.; Sherman, M.; Jaimes, R.; Chaluvadi, A.; Swift, L.; Posnack, N.G. Disruption of neonatal cardiomyocyte physiology following exposure to bisphenola. Sci. Rep. 2018, 8, 7356. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. Fetoplacental vasculature as a model to study human cardiovascular endocrine disruption. Mol. Aspects Med. 2021, 87, 101054. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Cheng, W.; Feng, Y.; Wang, W.; Liang, F.; Luo, F.; Yang, S.; Wang, Y. Combined effects of BPA and PFOS on fetal cardiac development: In vitro and in vivo experiments. Environ. Toxicol. Pharmacol. 2020, 80, 103434. [Google Scholar] [CrossRef] [PubMed]
- Reventun, P.; Sanchez-Esteban, S.; Cook, A.; Cuadrado, I.; Roza, C.; Moreno-Gomez-Toledano, R.; Muñoz, C.; Zaragoza, C.; Bosch, R.J.; Saura, M. Bisphenol A induces coronary endothelial cell necroptosis by activating RIP3/CamKII dependent pathway. Sci. Rep. 2020, 10, 4190. [Google Scholar] [CrossRef] [PubMed]
- Posnack, N.G.; Brooks, D.; Chandra, A.; Jaimes, R.; Sarvazyan, N.; Kay, M. Physiological response of cardiac tissue to bisphenol a: Alterations in ventricular pressure and contractility. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H267–H275. [Google Scholar] [CrossRef]
- Pant, J.; Ranjan, P.; Deshpande, S.B. Bisphenol A decreases atrial contractility involving NO-dependent G-cyclase signaling pathway. J. Appl. Toxicol. 2011, 31, 698–702. [Google Scholar] [CrossRef]
- Filice, M.; Leo, S.; Mazza, R.; Amelio, D.; Garofalo, F.; Imbrogno, S.; Cerra, M.C.; Gattuso, A. The heart of the adult goldfish Carassius auratus as a target of Bisphenol A: A multifaceted analysis. Environ. Pollut. 2021, 269, 116177. [Google Scholar] [CrossRef]
- Patel, B.B.; Raad, M.; Sebag, I.A.; Chalifour, L.E. Lifelong Exposure to Bisphenol A Alters Cardiac Structure/Function, Protein Expression, and DNA Methylation in Adult Mice. Toxicol. Sci. 2013, 133, 174–185. [Google Scholar] [CrossRef]
- Saura, M.; Marquez, S.; Reventun, P.; Olea-Herrero, N.; Arenas, M.I.; Moreno-Gómez-Toledano, R.; Gómez-Parrizas, M.; Muñóz-Moreno, C.; González-Santander, M.; Zaragoza, C.; et al. Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 2014, 28, 4719–4728. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, M.K.; Kang, G.H.; Lee, K.J.; Choi, S.H.; Lim, S.; Oh, B.-C.; Park, D.J.; Park, K.S.; Jang, H.C.; et al. Chronic Exposure to Bisphenol A can Accelerate Atherosclerosis in High-Fat-Fed Apolipoprotein E Knockout Mice. Cardiovasc. Toxicol. 2014, 14, 120–128. [Google Scholar] [CrossRef]
- Belcher, S.M.; Gear, R.B.; Kendig, E.L. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology 2015, 156, 882–895. [Google Scholar] [CrossRef]
- Aboul Ezz, H.S.; Khadrawy, Y.A.; Mourad, I.M. The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology 2015, 67, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Kasneci, A.; Bolt, A.M.; Di Lalla, V.; Di Iorio, M.R.; Raad, M.; Mann, K.K.; Chalifour, L.E. Chronic Exposure to Bisphenol A Reduces Successful Cardiac Remodeling After an Experimental Myocardial Infarction in Male C57bl/6n Mice. Toxicol. Sci. 2015, 146, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Gear, R.; Kendziorski, J.A.; Belcher, S.M. Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: A CLARITY-BPA study. Toxicol. Lett. 2017, 275, 123–135. [Google Scholar] [CrossRef]
- Hailey, J.R.; Maleeff, B.E.; Thomas, H.C.; Pearse, G.; Klapwijk, J.C.; Cristofori, P.G.; Berridge, B.; Kimbrough, C.L.; Parker, G.A.; Morton, D.; et al. A Diagnostic Approach for Rodent Progressive Cardiomyopathy and Like Lesions in Toxicology Studies up to 28 Days in the Sprague Dawley Rat (Part 1 of 2). Toxicol. Pathol. 2017, 45, 1043–1054. [Google Scholar] [CrossRef]
- Klint, H.; Lejonklou, M.H.; Karimullina, E.; Rönn, M.; Lind, L.; Lind, P.M.; Brittebo, E. Low-dose exposure to bisphenol A in combination with fructose increases expression of genes regulating angiogenesis and vascular tone in juvenile Fischer 344 rat cardiac tissue. Upsala J. Med. Sci. 2017, 122, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Park, S.-H.; Wang, F.; Zhou, C. Perinatal Bisphenol A Exposure Increases Atherosclerosis in Adult Male PXR-Humanized Mice. Endocrinology 2018, 159, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Bruno, K.A.; Mathews, J.E.; Yang, A.L.; Frisancho, J.A.; Scott, A.J.; Greyner, H.D.; Molina, F.A.; Greenaway, M.S.; Cooper, G.M.; Bucek, A.; et al. BPA Alters Estrogen Receptor Expression in the Heart After Viral Infection Activating Cardiac Mast Cells and T Cells Leading to Perimyocarditis and Fibrosis. Front. Endocrinol. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Weber, G.J.; Peterson, S.M.; Nie, L.H. Embryonic ionizing radiation exposure results in expression alterations of genes associated with cardiovascular and neurological development, function, and disease and modified cardiovascular function in zebrafish. Front. Genet. 2014, 5, 268. [Google Scholar] [CrossRef]
- Vargas, R.; Vásquez, I.C. Cardiac and somatic parameters in zebrafish: Tools for the evaluation of cardiovascular function. Fish Physiol. Biochem. 2016, 42, 569–577. [Google Scholar] [CrossRef]
- Sedmera, D.; Reckova, M.; deAlmeida, A.; Sedmerova, M.; Biermann, M.; Volejnik, J.; Sarre, A.; Raddatz, E.; McCarthy, R.A.; Gourdie, R.G.; et al. Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1152–H1160. [Google Scholar] [CrossRef]
- Murk, A.J.; Rijntjes, E.; Blaauboer, B.J.; Clewell, R.; Crofton, K.M.; Dingemans, M.M.; Furlow, J.D.; Kavlock, R.; Köhrle, J.; Opitz, R.; et al. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2013, 27, 1320–1346. [Google Scholar] [CrossRef] [PubMed]
- Menuet, A.; Pellegrini, E.; Anglade, I.; Blaise, O.; Laudet, V.; Kah, O.; Pakdel, F. Molecular Characterization of Three Estrogen Receptor Forms in Zebrafish: Binding Characteristics, Transactivation Properties, and Tissue Distributions1. Biol. Reprod. 2002, 66, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Cypher, A.D.; Ickes, J.R.; Bagatto, B. Bisphenol A alters the cardiovascular response to hypoxia in Danio rerio embryos. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 174–175, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lombó, M.; Fernández-Díez, C.; González-Rojo, S.; Navarro, C.; Robles, V.; Herráez, M.P. Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure. Environ. Pollut. 2015, 206, 667–678. [Google Scholar] [CrossRef]
- Cypher, A.D.; Fetterman, B.; Bagatto, B. Vascular parameters continue to decrease post-exposure with simultaneous, but not individual exposure to BPA and hypoxia in zebrafish larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 206–207, 11–16. [Google Scholar] [CrossRef]
- Moreman, J.; Takesono, A.; Trznadel, M.; Winter, M.J.; Perry, A.; Wood, M.E.; Rogers, N.J.; Kudoh, T.; Tyler, C.R. Estrogenic Mechanisms and Cardiac Responses Following Early Life Exposure to Bisphenol A (BPA) and Its Metabolite 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) in Zebrafish. Environ. Sci. Technol. 2018, 52, 6656–6665. [Google Scholar] [CrossRef]
- Brown, A.R.; Green, J.M.; Moreman, J.; Gunnarsson, L.M.; Mourabit, S.; Ball, J.; Winter, M.J.; Trznadel, M.; Correia, A.; Hacker, C.; et al. Cardiovascular Effects and Molecular Mechanisms of Bisphenol A and Its Metabolite MBP in Zebrafish. Environ. Sci. Technol. 2019, 53, 463–474. [Google Scholar] [CrossRef]
- Lombó, M.; González-Rojo, S.; Fernández-Díez, C.; Herráez, M.P. Cardiogenesis impairment promoted by bisphenol A exposure is successfully counteracted by epigallocatechin gallate. Environ. Pollut. 2019, 246, 1008–1019. [Google Scholar] [CrossRef]
- Lombó, M.; Herráez, M.P. Paternal Inheritance of Bisphenol A Cardiotoxic Effects: The Implications of Sperm Epigenome. Int. J. Mol. Sci. 2021, 22, 2125. [Google Scholar] [CrossRef]
- Ji, G.; Gu, J.; Guo, M.; Zhou, L.; Wang, Z.; Shi, L.; Gu, A. A systematic comparison of the developmental vascular toxicity of bisphenol A and its alternatives in vivo and in vitro. Chemosphere 2022, 291, 132936. [Google Scholar] [CrossRef]
- Schönemann, A.M.; Moreno Abril, S.I.; Diz, A.P.; Beiras, R. The bisphenol A metabolite MBP causes proteome alterations in male Cyprinodon variegatus fish characteristic of estrogenic endocrine disruption. Environ. Pollut. 2022, 300, 118936. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Soriano, S.; Ripoll, C.; Alonso-Magdalena, P.; Fuentes, E.; Quesada, I.; Nadal, A.; Martinez-Pinna, J. Effects of Bisphenol A on ion channels: Experimental evidence and molecular mechanisms. Steroids 2016, 111, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M.; Lorell, B.H. Regulation of Cardiac Contraction and Relaxation. Circulation 2000, 102, Iv-69–Iv-74. [Google Scholar] [CrossRef]
- Edwards, J.N.; Blatter, L.A. Cardiac alternans and intracellular calcium cycling. Clin. Exp. Pharmacol. Physiol. 2014, 41, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, S.; Li, X.; Liang, F.; Zhou, R.; Wang, H.; Feng, Y.; Wang, Y. Low doses of BPA induced abnormal mitochondrial fission and hypertrophy in human embryonic stem cell-derived cardiomyocytes via the calcineurin-DRP1 signaling pathway: A comparison between XX and XY cardiomyocytes. Toxicol. Appl. Pharmacol. 2020, 388, 114850. [Google Scholar] [CrossRef] [PubMed]
- Mangana, C.; Lorigo, M.; Cairrao, E. Implications of Endothelial Cell-Mediated Dysfunctions in Vasomotor Tone Regulation. Biologics 2021, 1, 231–251. [Google Scholar] [CrossRef]
- Andersson, H.; Brittebo, E. Proangiogenic effects of environmentally relevant levels of bisphenol A in human primary endothelial cells. Arch. Toxicol. 2012, 86, 465–474. [Google Scholar] [CrossRef]
- Ribeiro-Varandas, E.; Viegas, W.; Sofia Pereira, H.; Delgado, M. Bisphenol A at concentrations found in human serum induces aneugenic effects in endothelial cells. Mutat. Res. 2013, 751, 27–33. [Google Scholar] [CrossRef]
- Ribeiro-Varandas, E.; Pereira, H.S.; Monteiro, S.; Neves, E.; Brito, L.; Ferreira, R.B.; Viegas, W.; Delgado, M. Bisphenol A Disrupts Transcription and Decreases Viability in Aging Vascular Endothelial Cells. Int. J. Mol. Sci. 2014, 15, 15791–15805. [Google Scholar] [CrossRef]
- Nahar, M.S.; Kim, J.H.; Sartor, M.A.; Dolinoy, D.C. Bisphenol A-associated alterations in the expression and epigenetic regulation of genes encoding xenobiotic metabolizing enzymes in human fetal liver. Environ. Mol. Mutagen. 2014, 55, 184–195. [Google Scholar] [CrossRef]
- Montrose, L.; Padmanabhan, V.; Goodrich, J.M.; Domino, S.E.; Treadwell, M.C.; Meeker, J.D.; Watkins, D.J.; Dolinoy, D.C. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics 2018, 13, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Mikelson, C.; Richards, S.; Symes, S.; Adair, D.; Zullo, F.; Guida, M. Placental concentrations of bisphenol A and birth weight from births in the Southeastern U.S. Placenta 2014, 35, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Nahar, M.S.; Liao, C.; Kannan, K.; Harris, C.; Dolinoy, D.C. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 2015, 124, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mørck, T.J.; Sorda, G.; Bechi, N.; Rasmussen, B.S.; Nielsen, J.B.; Ietta, F.; Rytting, E.; Mathiesen, L.; Paulesu, L.; Knudsen, L.E. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010, 30, 131–137. [Google Scholar] [CrossRef]
- Ponniah, M.; Billett, E.E.; De Girolamo, L.A. Bisphenol A increases BeWo trophoblast survival in stress-induced paradigms through regulation of oxidative stress and apoptosis. Chem. Res. Toxicol. 2015, 28, 1693–1703. [Google Scholar] [CrossRef]
- Spagnoletti, A.; Paulesu, L.; Mannelli, C.; Ermini, L.; Romagnoli, R.; Cintorino, M.; Ietta, F. Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol. Cell Endocrinol. 2015, 412, 56–64. [Google Scholar] [CrossRef]
- Basak, S.; Srinivas, V.; Duttaroy, A.K. Bisphenol-A impairs cellular function and alters DNA methylation of stress pathway genes in first trimester trophoblast cells. Reprod. Toxicol. 2018, 82, 72–79. [Google Scholar] [CrossRef]
- Vrooman, L.A.; Xin, F.; Bartolomei, M.S. Morphologic and molecular changes in the placenta: What we can learn from environmental exposures. Fertil. Steril. 2016, 106, 930–940. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Schantz, S.L. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenet. 2018, 4, dvy022. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Schantz, S.L. Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives. Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 166, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Das, M.K.; Duttaroy, A.K. Plastics derived endocrine-disrupting compounds and their effects on early development. Birth Defects Res. 2020, 112, 1308–1325. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of Urinary Bisphenol A Concentration with Medical Disorders and Laboratory Abnormalities in Adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Rao, X.; Ye, J.; Ling, Y.; Mi, S.; Chen, H.; Fan, C.; Li, Y. Relationship between urinary bisphenol a levels and cardiovascular diseases in the U.S. adult population, 2003–2014. Ecotoxicol. Environ. Saf. 2020, 192, 110300. [Google Scholar] [CrossRef]
- Lind, P.M.; Lind, L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis 2011, 218, 207–213. [Google Scholar] [CrossRef]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.-T.; Wareham, N.J.; et al. Urinary Bisphenol A Concentration and Risk of Future Coronary Artery Disease in Apparently Healthy Men and Women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Gates, P.; Osborn, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Schofield, P.; Mosedale, D.; et al. Urinary Bisphenol A Concentration and Angiography-Defined Coronary Artery Stenosis. PLoS ONE 2012, 7, e43378. [Google Scholar] [CrossRef]
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of Urinary Bisphenol A Concentration with Heart Disease: Evidence from NHANES 2003/06. PLoS ONE 2010, 5, e8673. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, X.; Shen, Y.; Yu, P.; Chen, S.; Hu, J.; Yu, J.; Li, J.; Wang, H.-S.; Cheng, X.; et al. Elevated serum Bisphenol A level in patients with dilated cardiomyopathy. Int. J. Environ. Res. Public Health 2015, 12, 5329–5337. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and Peripheral Arterial Disease: Results from the NHANES. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef]
- Hu, C.; Schöttker, B.; Venisse, N.; Limousi, F.; Saulnier, P.J.; Albouy-Llaty, M.; Dupuis, A.; Brenner, H.; Migeot, V.; Hadjadj, S. Bisphenol A, Chlorinated Derivatives of Bisphenol A and Occurrence of Myocardial Infarction in Patients with Type 2 Diabetes: Nested Case-Control Studies in Two European Cohorts. Environ. Sci. Technol. 2019, 53, 9876–9883. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S. Urinary Bisphenol A and Hypertension in a Multiethnic Sample of US Adults. J. Environ. Public Health 2012, 2012, 481641. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, J.H.; Lim, Y.-H.; Park, H.Y.; Hong, Y.-C. Associations of Bisphenol A Exposure with Heart Rate Variability and Blood Pressure. Hypertension 2012, 60, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Hong, Y.C. Exposure to bisphenol A from drinking canned beverages increases blood pressure: Randomized crossover trial. Hypertension 2015, 65, 313–319. [Google Scholar] [CrossRef]
- Aekplakorn, W.; Chailurkit, L.-O.; Ongphiphadhanakul, B. Association of Serum Bisphenol A with Hypertension in Thai Population. Int. J. Hypertens. 2015, 2015, 594189. [Google Scholar] [CrossRef]
- Mehlsen, A.; Høllund, L.; Boye, H.; Frederiksen, H.; Andersson, A.-M.; Bruun, S.; Husby, S.; Jensen, T.K.; Timmermann, C.A.G. Pregnancy exposure to bisphenol A and duration of breastfeeding. Environ. Res. 2022, 206, 112471. [Google Scholar] [CrossRef]
- LaKind, J.S.; Goodman, M.; Naiman, D.Q. Use of NHANES Data to Link Chemical Exposures to Chronic Diseases: A Cautionary Tale. PLoS ONE 2012, 7, e51086. [Google Scholar] [CrossRef]
- LaKind, J.S.; Goodman, M.; Mattison, D.R. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: A systematic review of epidemiologic research. Crit. Rev. Toxicol. 2014, 44, 121–150. [Google Scholar] [CrossRef]
- Olsén, L.; Lind, L.; Lind, P.M. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol. Environ. Saf. 2012, 80, 179–183. [Google Scholar] [CrossRef]
- Warembourg, C.; Basagaña, X.; Seminati, C.; de Bont, J.; Granum, B.; Lyon-Caen, S.; Manzano-Salgado, C.B.; Pin, I.; Sakhi, A.K.; Siroux, V.; et al. Exposure to phthalate metabolites, phenols and organophosphate pesticide metabolites and blood pressure during pregnancy. Int. J. Hyg. Environ. Health 2019, 222, 446–454. [Google Scholar] [CrossRef]
- Bae, S.; Lim, Y.H.; Lee, Y.A.; Shin, C.H.; Oh, S.Y.; Hong, Y.C. Maternal Urinary Bisphenol A Concentration During Midterm Pregnancy and Children’s Blood Pressure at Age 4. Hypertension 2017, 69, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Sol, C.M.; Santos, S.; Asimakopoulos, A.G.; Martinez-Moral, M.-P.; Duijts, L.; Kannan, K.; Trasande, L.; Jaddoe, V.W.V. Associations of maternal phthalate and bisphenol urine concentrations during pregnancy with childhood blood pressure in a population-based prospective cohort study. Environ. Int. 2020, 138, 105677. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, J.; Smith, A.; Wang, A.; Hom, E.; Izano, M.; Huang, H.; Padula, A.; Woodruff, T.J. Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health. Reprod. Toxicol. 2020, 92, 14–56. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, F.; Dubois, M.F.; Aris, A. Maternal, placental and fetal exposure to bisphenol A in women with and without preeclampsia. Hypertens. Pregnancy 2014, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta 2015, 36, 699–703. [Google Scholar] [CrossRef]
- Cantonwine David, E.; Meeker John, D.; Ferguson Kelly, K.; Mukherjee, B.; Hauser, R.; McElrath Thomas, F. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ. Health Perspect. 2016, 124, 1651–1655. [Google Scholar] [CrossRef]
- Philips, E.M.; Trasande, L.; Kahn, L.G.; Gaillard, R.; Steegers, E.A.P.; Jaddoe, V.W.V. Early pregnancy bisphenol and phthalate metabolite levels, maternal hemodynamics and gestational hypertensive disorders. Hum. Reprod. 2019, 34, 365–373. [Google Scholar] [CrossRef]
- Philips, E.M.; Jaddoe, V.W.V.; Trasande, L. Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod. Toxicol. 2017, 68, 105–118. [Google Scholar] [CrossRef]
- Perng, W.; Kasper, N.M.; Watkins, D.J.; Sanchez, B.N.; Meeker, J.D.; Cantoral, A.; Solano-González, M.; Tellez-Rojo, M.M.; Peterson, K. Exposure to Endocrine-Disrupting Chemicals During Pregnancy Is Associated with Weight Change Through 1 Year Postpartum Among Women in the Early-Life Exposure in Mexico to Environmental Toxicants Project. J. Women’s Health 2020, 29, 1419–1426. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
- Hengstler, J.G.; Sjögren, A.-K.; Zink, D.; Hornberg, J.J. In Vitro prediction of organ toxicity: The challenges of scaling and secondary mechanisms of toxicity. Arch. Toxicol. 2020, 94, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, W. Which concentrations are optimal for in vitro testing? EXCLI J 2020, 19, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Vandenberg, L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 2021, 162, bqaa171. [Google Scholar] [CrossRef] [PubMed]


| Drugs | Concentration | Animals/Organs/Cells | Results | References |
|---|---|---|---|---|
| BPA and Penitrem | 10 µmol/L 1 µmol/L | Canine coronary smooth muscle cells AD 293 cells |
| [35] |
| BPA and/or 17β-estradiol(E2)- | 1 nmol/L | Ventricular myocytes and Sprague Dawley adult mice heart and ERβ knockout mice (Erβ−/−) |
| [45] |
| BPA and/or E2 | 0.001–1 nmol/L | Rat Sprague Dawley myocytes and female knockout Erβ mice. |
| [46] |
| BPA | 1–100 µmol/L | HEK293 cells transfected with Human Cardiac Sodium Channel |
| [43] |
| BPA or BPA and E2 | 1 nmol/L | Adult Sprague Dawley rats’ hearts |
| [47] |
| BPA | 1 nmol/L | Female rat ventricular myocytes |
| [48] |
| BPA | 1–100 μmol/L | Mouse cardiac myocytes |
| [38] |
| BPA membrane-impermeant BPA-monosulfate (BPA-MS) | 100 µmol/L | AD 293 cells expressing α or α + β1 subunits |
| [36] |
| BPA | 1–100 μmol/L | HEK 293 cells transfected with CaV3.1-CaV3.3 |
| [39] |
| BPA | 0.1 nmol/L−1–1 μmol/L | Female rat ventricular myocytes |
| [40] |
| BPA | 0.001–100 µmol/L | Neonatal rat cardiomyocytes |
| [54] |
| BPA | 0.001–100 µmol/L | A7R5 cells from rat aorta |
| [37] |
| BPA | 100 µmol/L | Neonatal rat cardiomyocytes |
| [50] |
| BPA | 1–100 µmol/L | Zebrafish larvae Zebrafish cell lines |
| [49] |
| BPA and/or PFOS | 25 μmol/L for 14 days | Rat cardiomyocytes |
| [52] |
| BPA | 0–10 µmol/L BPA for 24 h | Murine aortic ECs (MAECs) and H9c2 cells. |
| [53] |
| BPA | 1–100 μmol/L | hiPSC-CM |
| [41] |
| BPA Bisphenol S Bisphenol F | 0.0–100 µmol/L | hiPSC-CM |
| [42] |
| Drugs | Concentration | Animals/Organs/Cells | Results | References |
|---|---|---|---|---|
| BPA | 0.1–100 μmol/L | Adult albino rats of Charles Foster strain |
| [55] |
| BPA | 0.1–100 µmol/L | Sprague Dawley rat adult hearts |
| [30] |
| BPA | 0.001–100 µmol/L | Sprague Dawley rat hearts |
| [54] |
| BPA | 0.001–100 µmol/L | Male Wistar aorta rats |
| [37] |
| BPA | 10 µmol/L and 25 µmol/L | Goldfish (C. auratus) adults hearts |
| [56] |
| Drugs | Concentration | Animals/Organs/Cells | Results | References |
|---|---|---|---|---|
| BPA | 0.5, 5.0 and 200 µg/kg day | Rats |
| [57] |
| BPA | 50 μg/kg body weight/day–12 weeks | ApoE−/− male mice |
| [59] |
| BPA | 4 nmol/L–400 µmol/L | Mice CD1 |
| [58] |
| BPA or EE | 0.15–5000 µg/kg/day | CD1 mice |
| [60] |
| BPA | 25 mg/kg 10 mg/kg | Adult male Wistar albino rats |
| [61] |
| BPA | 25 ng/mL–5 µg BPA/kg BW/day | C57bl/6n mice |
| [62] |
| BPA | 100 and 2000 µg/L | Zebra fish |
| [74] |
| BPA and/or hipóxia | 0.25, 1 and 5 mg/L 1.0 mg O2/L | Zebra fish embryos |
| [73] |
| BPA or EE | BPA (2.5–25,000 µg/kg day) EE (0.05 or 0.5 µg/kg/day) | PND21, PND90, PND180 Sprague Dawley rat |
| [63] |
| BPA | 5, 50, and 500 μg BPA/kg bodyweight/day | Juvenile female Fischer 344 rats |
| [65] |
| BPA | 50 mg/kg | Adult PXR-Humanized Mice |
| [66] |
| BPA | 0.1 and 1.0 mg/L | Zebrafish embryos |
| [76] |
| BPA and/or hypoxia | 0.001–100 µg/L | Zebrafish larvae |
| [75] |
| BPA | 0.5, 5, 50 µg BPA/kg body weight | BALB/c Mice |
| [67] |
| BPA and/or EGCG | 2000 and 4000 µg/L BPA 50 and 100 µmol/L EGCG | Zebrafish embryos |
| [78] |
| BPA or metabolite MBP | 100 and 1000 µg/L 2.5 and 25 µg/L | Embryo—larval zebrafish |
| [77] |
| BPA | 1–100 µmol/L | Zebrafish larvae |
| [49] |
| BPA | Orally exposed to 4 × 10−5 mol/L of BPA in drinking water for 4, 8, and 16 weeks | Wild-type CD1 mice |
| [53] |
| BPA | 2 and 100 μg/L BPA | Pregnant rats |
| [52] |
| BPA EGCG | 100 and 2000 μg/L BPA 50 µmol/L EGCG | Zebrafish male |
| [79] |
| BPA | BPA (0.25–12 mg L−1) | Zebrafish embryos |
| [80] |
| BPA | 10 µmol/L and 25 µmol/L | Goldfish (C. auratus) adult hearts |
| [56] |
| BPA and metabolite MBP | 7 d exposure to 10 μg/L of BPA and MBP | Male Cyprinodon variegatus fish |
| [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, M.I.; Lorigo, M.; Cairrao, E. Endocrine-Disrupting Effects of Bisphenol A on the Cardiovascular System: A Review. J. Xenobiot. 2022, 12, 181-213. https://doi.org/10.3390/jox12030015
Fonseca MI, Lorigo M, Cairrao E. Endocrine-Disrupting Effects of Bisphenol A on the Cardiovascular System: A Review. Journal of Xenobiotics. 2022; 12(3):181-213. https://doi.org/10.3390/jox12030015
Chicago/Turabian StyleFonseca, Maria Inês, Margarida Lorigo, and Elisa Cairrao. 2022. "Endocrine-Disrupting Effects of Bisphenol A on the Cardiovascular System: A Review" Journal of Xenobiotics 12, no. 3: 181-213. https://doi.org/10.3390/jox12030015
APA StyleFonseca, M. I., Lorigo, M., & Cairrao, E. (2022). Endocrine-Disrupting Effects of Bisphenol A on the Cardiovascular System: A Review. Journal of Xenobiotics, 12(3), 181-213. https://doi.org/10.3390/jox12030015