Pyriproxyfen Contamination in Daphnia magna: Identifying Early Warning Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Daphnia magna Culture
2.2. Test Chemical and Solutions
2.3. Experimental Design
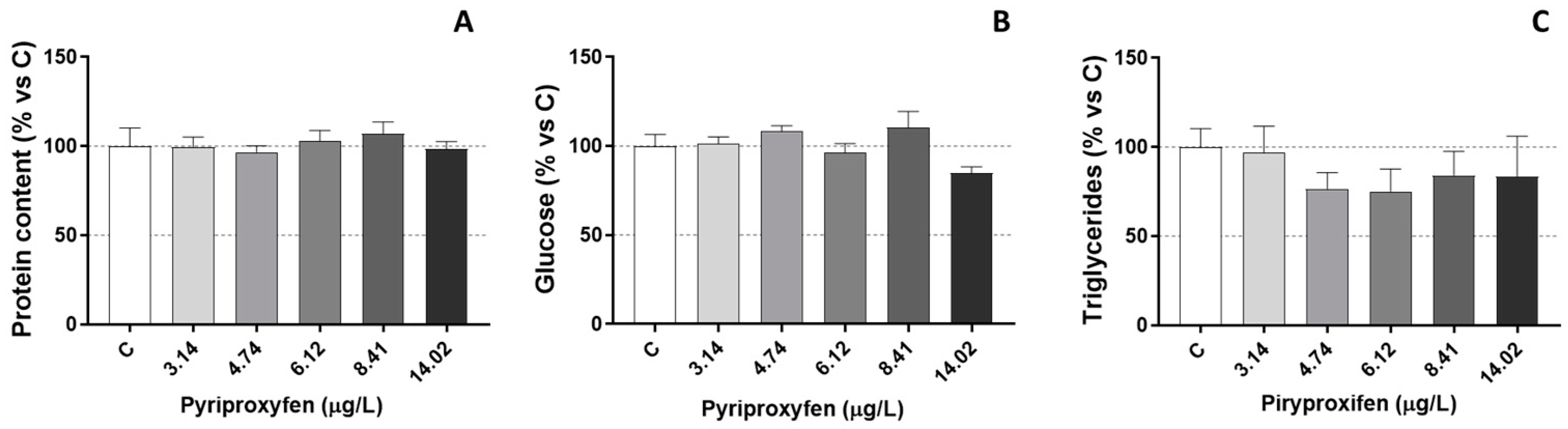
2.4. Biochemical Analysis
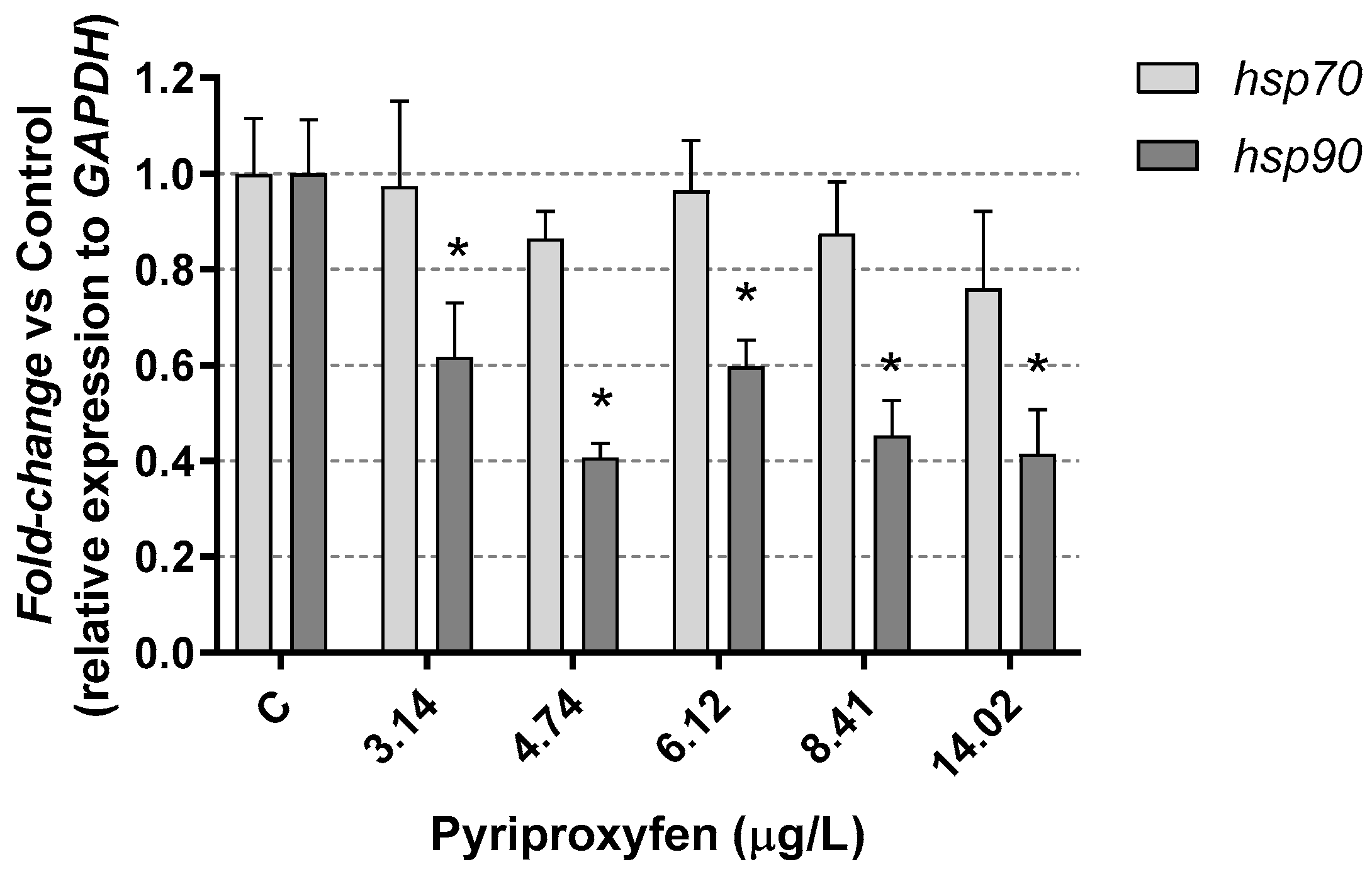
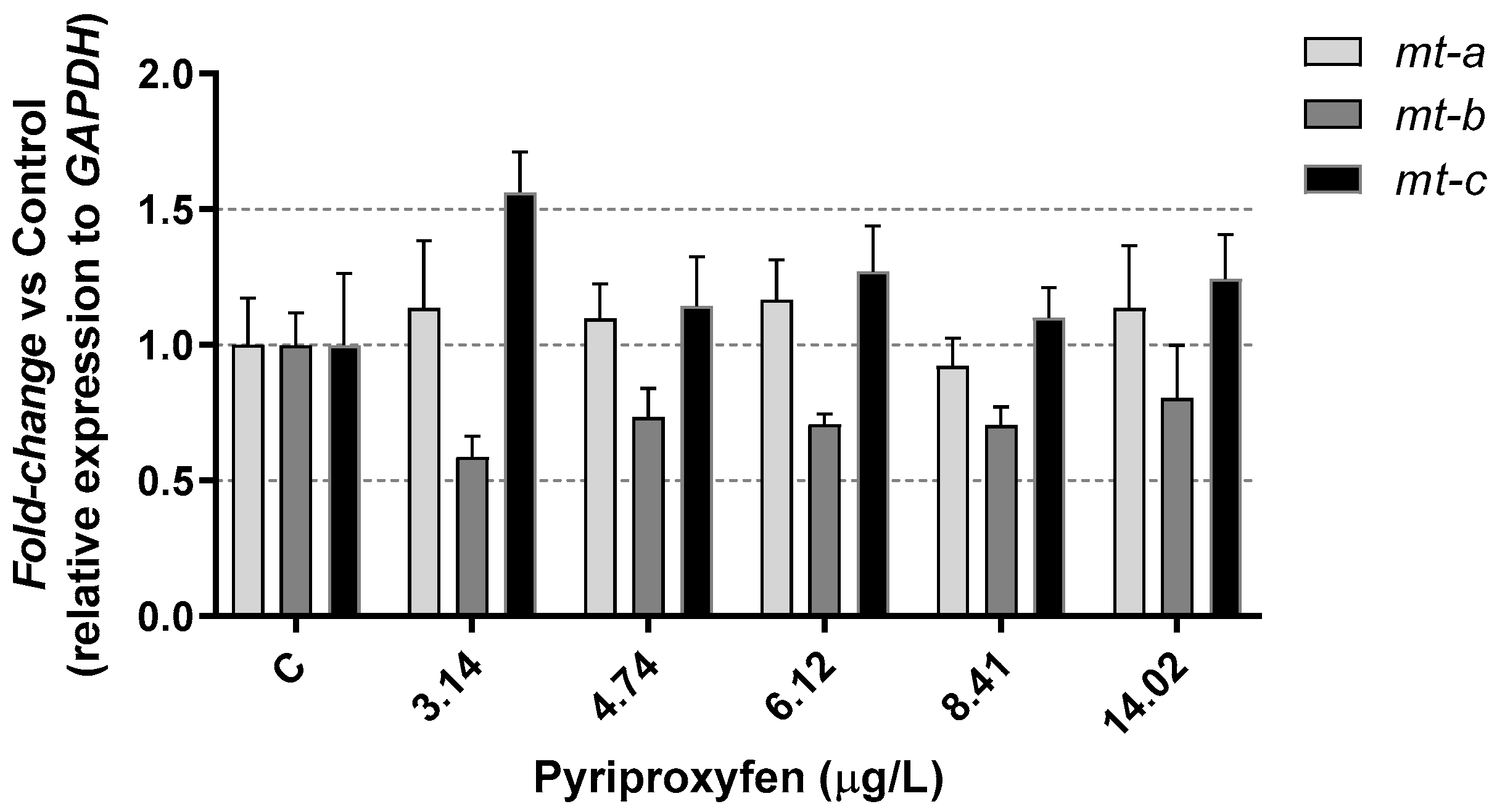
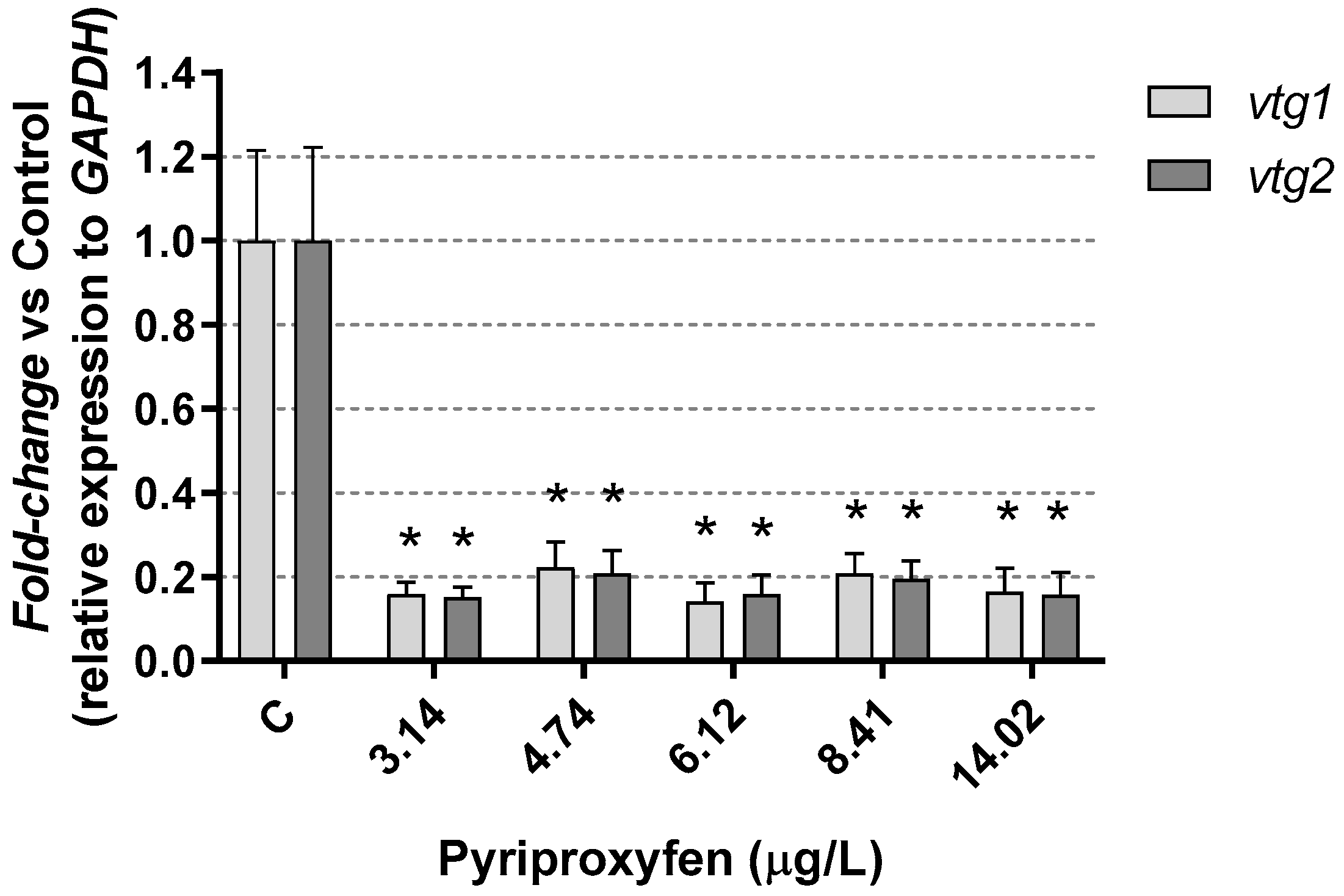
2.5. RNA Isolation and Quantitative RT-PCR
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, E.P.; Paiva, M.H.S.; de Araújo, A.P.; da Silva, E.V.G.; da Silva, U.M.; de Oliveira, L.N.; de Melo Santos, M.A.V. Insecticide resistance in Aedes aegypti populations from Ceará, Brazil. Parasit. Vectors 2011, 4, 5. [Google Scholar] [CrossRef]
- USEPA. Draft list of initial pesticide active ingredients and pesticide inerts to be considered for screening under the federal food, drug, and cosmetic act U.S. Environmental Protection Agency. In Draft List of Chemicals for Initial Tier 1 Screening; USEPA: Washington, DC, USA, 2007; p. 18. [Google Scholar]
- Meola, R.; Meier, K.; Dean, S.; Bhaskaran, G. Effect of pyriproxyfen in the blood diet of cat fleas on adult survival, egg viability, and larval development. J. Med. Entomol. 2000, 37, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Olmstead, A.W.; Li, H.; LeBlanc, G.A. The screening of chemicals for juvenoid-related endocrine activity using the water flea Daphnia magna. Aquat. Toxicol. 2005, 74, 193–204. [Google Scholar] [CrossRef]
- Ohba, S.Y.; Ohashi, K.; Pujiyati, E.; Higa, Y.; Kawada, H.; Mito, N.; Takagi, M. The effect of pyriproxyfen as a “population growth regulator” against Aedes albopictus under semi-field conditions. PLoS ONE 2013, 8, e67045. [Google Scholar] [CrossRef]
- Wang, C.Y.; Teng, H.J.; Lee, S.J.; Lin, C.; Wu, J.W.; Wu, H.S. Efficacy of various larvicides against Aedes aegypti immatures in the laboratory. Jpn. J. Infect. Dis. 2013, 66, 341–344. [Google Scholar] [CrossRef]
- Maoz, D.; Ward, T.; Samuel, M.; Müller, P.; Runge-Ranzinger, S.; Toledo, J.; Horstick, O. Community effectiveness of pyriproxyfen as a dengue vector control method: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005651. [Google Scholar] [CrossRef]
- WHO. Pyriproxyfen in drinking-water: Use for vector control in drinking-water sources and containers. In Geneva: Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Mulla, M.S.; Darwazeh, H.A.; Kennedy, B.; Dawson, D.M. Evaluation of new insect growth regulators against mosquitoes with notes on nontarget organisms. J. Am. Mosq. Control. Assoc. 1986, 2, 314–320. [Google Scholar]
- Schaefer, C.H.; Miura, T.; Dupras, E.F.; Mulligan, F.S.; Wilder, W.H. Efficacy, nontarget effects, and chemical persistence of S-31183, a promising mosquito (Diptera: Culicidae) control agent. J. Econ. Entomol. 1988, 81, 1648–1655. [Google Scholar] [CrossRef]
- Schaefer, C.H.; Dupras, E.F.J.; Mulligan, F.S. Studies on the environmental persistence of S-31183 (Pyriproxyfen): Adsorption onto organic matter and potential for leaching through soil. Ecotoxicol. Environ. Saf. 1991, 21, 207–214. [Google Scholar] [CrossRef]
- Sullivan, J.J.; Goh, K.S. Environmental fate and properties of pyriproxyfen. Pestic. Sci. 2008, 33, 339–350. [Google Scholar] [CrossRef]
- Katagi, T.; Takahashi, N. Hydrolysis of S-31183 in Buffered Aqueous Solutions; Sumitomo Chemical Company: Tokyo, Japan, 1994. [Google Scholar]
- Ccanccapa, A.; Masiá, A.; Navarro-Ortega, A.; Picó, Y.; Barceló, D. Pesticides in the Ebro River basin: Occurrence and risk assessment. Environ. Pollut. 2016, 211, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.A.S.; Souza-Santos, L.P. Environmental risk assessment (ERA) of pyriproxyfen in non-target aquatic organisms. Aquat. Toxicol. 2020, 222, 105448. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Gonnerman, G.; Simonich, M.T.; Tanguay, R.L. Assessment of the developmental and neurotoxicity of the mosquito control larvicide, pyriproxyfen, using embryonic zebrafish. Environ. Pollut. 2016, 218, 1089–1093. [Google Scholar] [CrossRef]
- Lawler, S.P. Environmental safety review of methoprene and bacterially-derived pesticides commonly used for sustained mosquito control. Ecotoxicol. Environ. Saf. 2017, 139, 335–343. [Google Scholar] [CrossRef]
- Azevedo-Linhares, M.; Souza, A.T.C.; Lenz, C.A.; Leite, N.F.; Brito, I.A.; Folle, N.M.T.; Oliveira Ribeiro, C.A. Microcystin and pyriproxyfen are toxic to early stages of development in Rhamdia quelen: An experimental and modelling study. Ecotoxicol. Environ. Saf. 2018, 166, 311–319. [Google Scholar] [CrossRef]
- Maharajan, K.; Muthulakshmi, S.; Nataraj, B.; Ramesh, M.; Kadirvelu, K. Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos (Danio rerio): A multi biomarker study. Aquat. Toxicol. 2018, 196, 132–145. [Google Scholar] [CrossRef]
- Maharajan, K.; Muthulakshmi, S.; Karthik, C.; Nataraj, B.; Nambirajan, K.; Hemalatha, D.; Ramesh, M. Pyriproxyfen induced impairment of reproductive endocrine homeostasis and gonadal histopathology in zebrafish (Danio rerio) by altered expression of hypothalamus-pituitary-gonadal (HPG) axis genes. Sci. Total Environ. 2020, 735, 139496. [Google Scholar] [CrossRef]
- Gaedke, U.; Straile, D. Daphnids: Keystone species for the pelagic food web structure and energy flow. A body size-related analysis linking seasonal changes at the population and ecosystem levels. Adv. Limnol. 1998, 53, 587–610. [Google Scholar]
- Nichols, J.; Breen, M.; Denver, R.; Distefano, J.; Edwards, J.; Hoke, R.; Volz, D.; Zhang, X. Predicting chemical impacts on vertebrate endocrine systems. Environ. Toxicol. Chem. 2011, 30, 39–51. [Google Scholar] [CrossRef]
- Chen, W.; Fu, X.; Dong, B.; Wang, Y.; Shiah, S.; Moore, D.; Huang, W. Neonatal activation of the nuclear receptor CAR results in epigenetic memory and permanent change of drug metabolism in mouse liver. Hepatology 2012, 56, 1499–1509. [Google Scholar] [CrossRef]
- Ginjupalli, G.K.; Baldwin, W.S. The time- and age-dependent effects of the juvenile hormone analog pesticide, pyriproxyfen on Daphnia magna reproduction. Chemosphere 2013, 92, 1260–1266. [Google Scholar] [CrossRef]
- Olmstead, A.W.; LeBlanc, G.A. Insecticidal juvenile hormone analogs stimulate the production of male offspring in the crustacean Daphnia magna. Environ. Health Perspect. 2003, 111, 919–924. [Google Scholar] [CrossRef]
- Sancho, E.; Villarroel, M.J.; Andreu, E.; Ferrando, M.D. Disturbances in energy metabolism of Daphnia magna after exposure to tebuconazole. Chemosphere 2009, 74, 1171–1178. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The missing biomarker link: Relationships between effects on the cellular energy allocation biomarker of toxicant-stressed Daphnia magna and corresponding population characteristics. Environ. Toxicol. Chem. 2003, 22, 1632–1641. [Google Scholar]
- Rider, C.V.; Gorr, T.A.; Olmstead, A.W.; Wasilak, B.A.; LeBlanc, G.A. Stress signaling: Coregulation of hemoglobin and male sex determination through a terpenoid signaling pathway in a crustacean. J. Exp. Biol. 2005, 208, 15–23. [Google Scholar] [CrossRef]
- Muyssen, B.T.; Janssen, C.R. Multigeneration zinc acclimation and tolerance in Daphnia magna: Implications for water-quality guidelines and ecological risk assessment. Environ. Toxicol. Chem. 2001, 20, 2053–2060. [Google Scholar]
- OECD (Organization for Economic Co-operation and Development). Test No. 211: Daphnia magna Reproduction Test, OECD Guidelines for Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2012. [Google Scholar]
- Bischoff, H.W.; Bold, H.C. Physiological studies IV. In Some Algae from Enchaded Rock and Relate Algae Species; Publication No. 6318; University of Texas: Austin, TX, USA, 1963. [Google Scholar]
- Ferrando, M.D.; Sancho, E.; Andreu-Moliner, E. Effects of lindane on Daphnia magna during chronic exposure. J. Environ. Sci. Health B 1995, 30, 815–825. [Google Scholar] [CrossRef]
- Sancho, E.; Villarroel, M.J.; Ferrando, M.D. Assessment of chronic effects of tebuconazole on survival, reproduction and growth of Daphnia magna after different exposure times. Ecotoxicol. Environ. Saf. 2016, 124, 10–17. [Google Scholar] [CrossRef]
- Ferrando, M.D.; Fernández-Casalderrey, A.; Andreu, E. Relative sensitivity of Daphnia magna and Brachionus calyciflorus to five pesticides. J. Environ. Sci. Health B 1992, 27, 511–522. [Google Scholar] [CrossRef]
- Salesa, B.; Torres-Gavilá, J.; Sancho, E.; Ferrando, M.D. Multigenerational effects of the insecticide Pyriproxyfen and recovery in Daphnia manga. Sci. Total Environ. 2023, 886, 164013. [Google Scholar] [CrossRef]
- Dom, N.; Penninck, M.; Knapen, D.; Blust, R. Discrepancies in the acute versus chronic toxicity of compounds with a designated narcotic mechanism. Chemosphere 2012, 87, 742–749. [Google Scholar] [CrossRef]
- Heckmann, L.H.; Connon, R.; Hutchinson, T.H.; Maund, S.J.; Sibly, R.M.; Callaghan, A. Expression of target and references genes in Daphnia magna exposed to ibuprofen. BMC Genom. 2006, 7, 175. [Google Scholar] [CrossRef]
- Seyoum, A.; Pradhan, A. Effect of phthalates on development, reproduction, fat metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2019, 654, 969–977. [Google Scholar] [CrossRef]
- Zeis, B.; Becher, B.; Goldmann, T.; Clark, R.; Vollmer, E.; Bölke, B.; Paul, R.J. Differential haemoglobin gene expression in the crustacean Daphnia magna exposed to different oxygen partial pressures. Biol. Chem. 2003, 384, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.S.S.; Lam, P.K.S. Glucose-6-phosphate dehydrogenase and lactate dehydrogenase in the green-lipped mussel (Perna viridis): Possible biomarkers for hypoxia in the marine environment. Water Res. 1997, 31, 2797–2801. [Google Scholar] [CrossRef]
- Van Der Thillart, G. Energy metabolism of swimming trout (Salmo gairdneri). Oxidation rates of palmitate, glucose, lactate, alanine, leucine and glutamate. J. Comp. Physiol. B 1986, 156, 511–520. [Google Scholar] [CrossRef]
- Villarroel, M.J.; Sancho, E.; Andreu-Moliner, E.; Ferrando, M.D. Biochemical stress response in tetradifon exposed Daphnia magna and its relationship to individual growth and reproduction. Sci. Total Environ. 2009, 407, 5537–5542. [Google Scholar] [CrossRef]
- Fuertes, I.; Jordao, R.; Piña, B.; Barata, C. Time-dependent transcriptomic responses of Daphnia magna exposed to metabolic disruptors that enhanced storage lipid accumulation. Environ. Pollut. 2019, 249, 99–108. [Google Scholar] [CrossRef]
- Yuan, S.; Li, H.; Dang, Y.; Liu, C. Effects of triphenyl phosphate on growth, reproduction and transcription of genes of Daphnia magna. Aquat. Toxicol. 2018, 195, 58–66. [Google Scholar] [CrossRef]
- Eytcheson, S.A.; LeBlanc, G.A. Hemoglobin levels modulate nitrite toxicity to Daphnia magna. Sci. Rep. 2018, 8, 7172. [Google Scholar] [CrossRef]
- Silva, R.; Silva, P.V.; Soares, A.; González-Alcaraz, M.; van Gestel, C.A.M.; Roelofs, D.; Moura, G.; Loureiro, S. Daphnia magna Multigeneration exposure to carbendazim: Gene transcription responses. Toxics 2023, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, M.; Yu, P.; Chen, M.; Wu, D.; Zhang, M.; Zhao, Y. Age-dependent survival, stress defense, and AMPK in Daphnia pulex after short-term exposure to a polystyrene nanoplastic. Aquat. Toxicol. 2018, 204, 1–8. [Google Scholar] [CrossRef]
- Mikulski, A.; Bernatowicz, P.; Grzesiuk, M.; Kloc, M.; Pijanowska, J. Differential levels of stress proteins (HSPs) in male and female Daphnia magna in response to thermal stress: A consequence of sex-related behavioral differences? J. Chem. Ecol. 2011, 37, 670–676. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S.; Lee, Y.M. Changes in activity and transcription of antioxidant enzymes and heat shock protein 90 in the water flea, Daphnia magna exposed to mercury. J. Toxicol. Environ. Health Sci. 2017, 9, 300–308. [Google Scholar] [CrossRef]
- Sato, M.; Kawakami, T.; Kondoh, M.; Takiguchi, M.; Kadota, Y.; Himeno, S.; Suzuki, S. Development of high-fat-diet-induced obesity in female metallothionein-null mice. FASEB 2010, 24, 2375–2384. [Google Scholar] [CrossRef]
- Rodríguez-Menéndez, S.; García, M.; Fernández, B.; Álvarez, L.; Fernández-Vega-Cueto, A.; Coca-Prados, M.; González-Iglesias, H. The zinc-metallothionein redox system reduces oxidative stress in retinal pigment epithelial cells. Nutrients 2018, 10, 1874. [Google Scholar] [CrossRef]
- Salesa, B.; Torres-Gavilá, J.; Ferrando-Rodrigo, M.D.; Sancho, E. Gene expression study alerted to possible impairment in Daphnia magna individuals as a consequence of exposure to sublethal concentrations of prochloraz. Chemosphere 2022, 308, 136040. [Google Scholar] [CrossRef]
- Seyoum, A.; Pradhan, A.; Jass, J.; Olsson, P.E. Perfluorinated alkyl substances impede growth, reproduction, lipid metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2020, 737, 139682. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Ban, Y.; Shen, C.; Shen, Q.; Chai, X.; Wei, J. Di-(2-ethylhexyl) phthalate exposure modulates antioxidant enzyme activity and gene expression in juvenile and adult Daphnia magna. Arch. Environ. Contam. Toxicol. 2018, 75, 145–156. [Google Scholar] [CrossRef]
- Aksakal, F.I. Evaluation of boscalid toxicity on Daphnia magna by using antioxidant enzyme activities, the expression of genes related to antioxidant and detoxification systems, and life-history parameters. Comp. Biochem. Physiol. Part C 2020, 237, 108830. [Google Scholar] [CrossRef]
- Flora, S.J.S. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef]
- Hannas, B.R.; Wang, Y.H.; Thomson, S.; Kwon, G.; Li, H.; Leblanc, G.A. Regulation and dysregulation of vitellogenin mRNA accumulation in daphnids (Daphnia magna). Aquat. Toxicol. 2011, 101, 351–357. [Google Scholar] [CrossRef]




| Gene | Forward | Reverse | Reference |
|---|---|---|---|
| GAPDH | TGCTGATGCCCCAATGTTTGTTGT | GCAGTTATGGCGTGGACGGTTGT | [37] |
| vtg1 | CCAGCGAATCCTACACCGTCAAG | GAGCCGCACAGACCACAGAG | [38] |
| vtg2 | CGTCCGCCACTGGTTGGGTC | GGGGCAGCCAAGACAGAGCG | |
| mt-a | GAGCGCCATGCCAAAATCCC | TCGTCGTTGTAAAATCCGGCT | |
| mt-b | TGGAACCGAATGCAAATGCG | CGGACTTGCATGGACAACTG | |
| mt-c | AAAGTGTGCCCTCGTTGTCA | CTTACAGTCGTCCCCACACG | |
| gst | TCAGGCTGGTGTTGAGTTTG | GAGCAAGCATTTGTGCATCA | |
| cat | TGGCGGAGAAAGCGGTTCAGC | GTGCGTGGTCTCTGGGCGAA | |
| hsp70 | CGACGGCGGGAGATACGCAC | CCACGGAAAAGGTCGGCGCA | |
| hsp90 | CCCTCTGTGACACTGGTATTGGCA | GCCCATGGGTTCTCCATGGTCAG | |
| fabd | GCCAACTACCTGTATCCTGAATG | GTGGAACGCTCCGCTAACT | |
| hb1 | ACAAATTGCTCTGGTTGCCG | AAGGTTTTTGAGTGCCACGT | [39] |
| hb2 | TGTTACCACCAGTGTCACCA | TATTCAGGGTGGGCCTTGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salesa, B.; Torres-Gavilá, J.; Ferrando-Rodrigo, M.D.; Sancho, E. Pyriproxyfen Contamination in Daphnia magna: Identifying Early Warning Biomarkers. J. Xenobiot. 2024, 14, 214-226. https://doi.org/10.3390/jox14010013
Salesa B, Torres-Gavilá J, Ferrando-Rodrigo MD, Sancho E. Pyriproxyfen Contamination in Daphnia magna: Identifying Early Warning Biomarkers. Journal of Xenobiotics. 2024; 14(1):214-226. https://doi.org/10.3390/jox14010013
Chicago/Turabian StyleSalesa, Beatriz, Javier Torres-Gavilá, María Dolores Ferrando-Rodrigo, and Encarnación Sancho. 2024. "Pyriproxyfen Contamination in Daphnia magna: Identifying Early Warning Biomarkers" Journal of Xenobiotics 14, no. 1: 214-226. https://doi.org/10.3390/jox14010013
APA StyleSalesa, B., Torres-Gavilá, J., Ferrando-Rodrigo, M. D., & Sancho, E. (2024). Pyriproxyfen Contamination in Daphnia magna: Identifying Early Warning Biomarkers. Journal of Xenobiotics, 14(1), 214-226. https://doi.org/10.3390/jox14010013








