Abstract
Biochar (BC), also referred to as “black gold”, is a carbon heterogeneous material rich in aromatic systems and minerals, preparable by the thermal decomposition of vegetable and animal biomasses in controlled conditions and with clean technology. Due to its adsorption ability and presence of persistent free radicals (PFRs), BC has demonstrated, among other uses, great potential in the removal of environmental organic and inorganic xenobiotics. Bamboo is an evergreen perennial flowering plant characterized by a short five-year growth period, fast harvesting, and large production in many tropical and subtropical countries worldwide, thus representing an attractive, low-cost, eco-friendly, and renewable bioresource for producing BC. Due to their large surface area and increased porosity, the pyrolyzed derivatives of bamboo, including bamboo biochar (BBC) or activated BBC (ABBC), are considered great bio-adsorbent materials for removing heavy metals, as well as organic and inorganic contaminants from wastewater and soil, thus improving plant growth and production yield. Nowadays, the increasing technological applications of BBC and ABBC also include their employment as energy sources, to catalyze chemical reactions, to develop thermoelectrical devices, as 3D solar vapor-generation devices for water desalination, and as efficient photothermal-conversion devices. Anyway, although it has great potential as an alternative biomass to wood to produce BC, thus paving the way for new bio- and circular economy solutions, the study of bamboo-derived biomasses is still in its infancy. In this context, the main scope of this review was to support an increasing production of BBC and ABBC and to stimulate further studies about their possible applications, thus enlarging the current knowledge about these materials and allowing their more rational, safer, and optimized application. To this end, after having provided background concerning BC, its production methods, and its main applications, we have reviewed and discussed the main studies on BBC and ABBC and their applications reported in recent years.
1. Introduction: Biochar (BC)
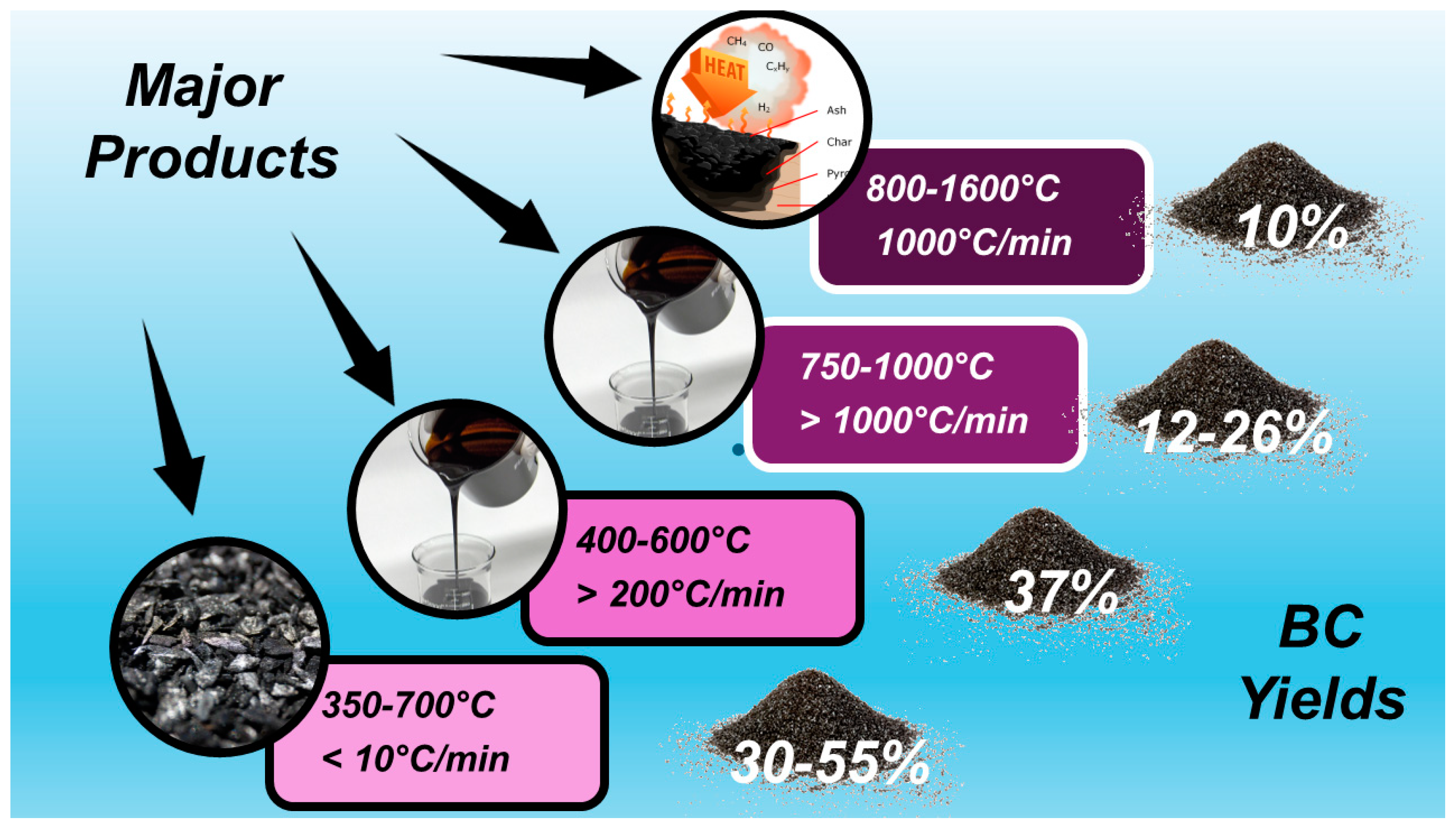
According to the definition provided by the International Biochar Initiative (IBI), biochar (BC) is “the solid material obtained from the thermochemical conversion of biomass in an oxygen-limited environment” [1]. Practically, BC is the lightweight black residue made mainly of carbon and ashes but also rich in aromatic systems and minerals produced by the thermal decomposition of organic material biomass, including wood, manure, leaves, waste, etc., under limited supply or in absence of oxygen (O2). Such a thermal decomposition process is referred to as pyrolysis. The most commonly used pyrolysis temperature is in the range of 400–600 °C [2], but a larger range of 200–1000 °C is reported [3]. When the thermal decomposition temperature is over 1000 °C and up to1600 °C, the process is named gasification. Pyrolysis comprises slow pyrolysis, fast pyrolysis, and flash pyrolysis, depending on the working temperature, the heating rate, and the residence time of the organic matter at a fixed temperature [4]. Upon the pyrolysis of biomass, three types of products with different physical states can form simultaneously in different concentrations. The solid residue comprises BC, which could contain ash and soot, bio-oils represent the liquid product, and the gaseous ones consist of syngas [4]. The relative yields of such products depend mainly on the pyrolysis temperature and heating rate, as shown in Figure 1.

Figure 1.
Influence of temperature of thermal degradation process on BC yield and that of by-products.
In conditions of slow pyrolysis (350–700 °C, heating rate < 10 °C/min), the major compound is BC; bio-oils are instead the most abundant products in conditions of flash and fast pyrolysis (400–600 °C, heating > 200 °C/min and 750–1000 °C, heating > 1000 °C/min, respectively), and syngas becomes the major product when gasification conditions are applied (800–1600 °C, heating 1000 °C/min) [5,6,7,8,9]. Syngas consists of a gas mixture whose composition can vary significantly depending on the feedstock type and gasification condition. However, typically, syngas is 30–60% carbon monoxide (CO), 25–30% hydrogen (H2), 0–5% methane (CH4), and 5–15% carbon dioxide (CO2), plus a lesser or greater amount of water vapor, smaller amounts of sulfur compounds such as hydrogen sulfide (H2S) and carbonyl sulfide (COS), and finally, some ammonia and other trace contaminants [10]. As for bio-oils, their production could be an appealing strategy for generating fuel from biomass, allowing a reduction in the global dependence on fossil fuels to produce energy [11]. Bio-oil has a higher heating value when compared to its original feedstock, and when burned, it can potentially generate a lower amount of greenhouse gas when compared to fossil fuels [12]. Consequently, even if its most studied application is still as a burning fuel, other applications are emerging due to its composition, as in the case of foams and resins [13]. Moreover, the latter applications emphasize the potential of bio-oil as a source of value-added chemicals [11].
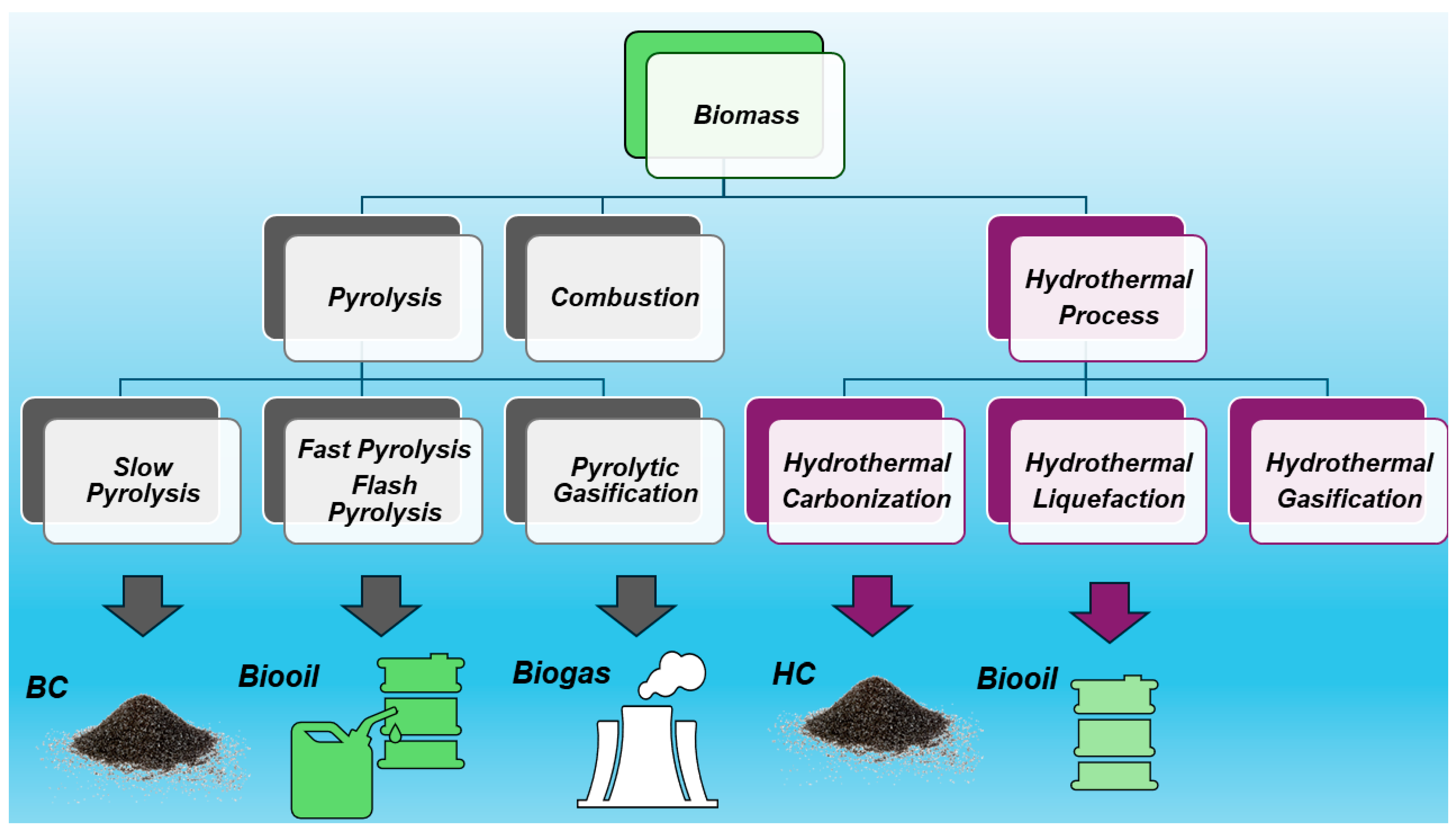
The treatment of biomass at 200–300 °C heating at <50 °C/min is named torrefaction, leading to a torrefied biomass with a high content of BC (69–80%), while hydrothermal carbonization (HTC) in the presence of oxygen, carried out at 400–1000 °C and a very low heating rate (<1 °C/min), leads to the obtainment of hydro char (HC) (50–70% BC), also referred to as HTC material [3]. HC is a solid material distinct from BC due to its production process and properties [14]. Typically, HC has higher H/C ratios and lower aromatic systems than BC, as well as little or no fused aromatic ring structures. The pyrolytic process leading to BC echoes that which yields charcoal, which is the most ancient industrial technology developed by humankind. Anyway, although very similar and of analogous origin, while charcoal is used mainly as a fuel, the primary application of BC was for soil amendment, aiming at improving soil characteristics and functions, as well as at preventing the natural degradation of biomass to greenhouse gases by sequestrating carbon, thus reducing gas emissions in the atmosphere and mitigating climate change [4]. As mentioned above, BC could contain other solids such as soot and ash. While soot is a secondary pyrogenic carbonaceous material (PCM) identified as a separate component resulting from gas condensation processes, ash typically includes inorganic oxides and carbonates not containing organic carbon. Figure 2 shows the main possible thermal treatment of biomass and the related main products of decomposition.
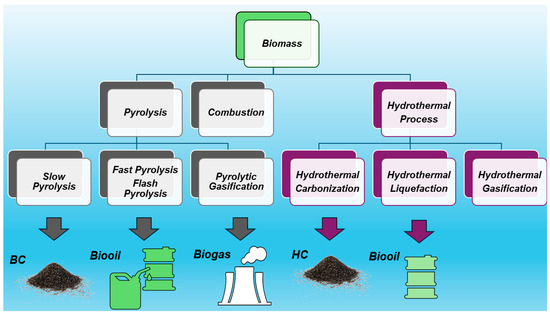
Figure 2.
Main possible thermal treatment of biomass and the related products of decomposition.
An alternative method to produce BC consists of thermocatalytic depolymerization (TCD), which utilizes microwaves. It has been used to efficiently convert organic matter to BC on an industrial scale, producing ≈ 50% char [15,16].
2. What Can Biochar Do?
Biochar (BC) is a sustainable, cost-effective, eco-friendly material that is also endowed with reusability, which is increasingly gaining the attention of many researchers [4]. In addition to other possible applications, simply by its production, BC allows for the reduction of agricultural and other types of waste. According to Table 1, among the biomass waste materials, crop residues from agriculture, forestry, municipal solid waste, and food and animal manures have a high potential and are appropriate for BC production [17,18,19,20,21,22].

Table 1.
Main sources and general applications of BCs.
BC possesses several properties, including large surface area, high porosity, presence of functional groups, high cation exchange capacity (CEC), high water-holding capacity (WHC), strong stability, etc., which make it suitable for various applications, as reported and summarized in Table 1 [4]. Particularly, in the first column of Table 1 (source biomass), we have reported the biomasses that are commonly used to produce BC along with related references (column two), while in the third column (applications), the most common uses of BCs, which can be derived from all biomasses reported in column one, have been included along with related references (column four).
More specifically, Table 2 reports the environmental applications of BC and the related mechanisms.

Table 2.
Main environmental applications of BC and related mechanisms.
By using BC, it is possible to fight global warming by reducing greenhouse gas emissions, such as CO2, NO2, and CH4, and sequestering carbon [31]. Interestingly, BC has been estimated to be capable of tackling 12% of the current anthropogenic carbon emissions [4]. Concurrently, the process of pyrolysis manages to balance fossil fuel consumption by producing clean and renewable energy (bioenergy) [49]. Due to its high content of carbon, BC can work as a soil conditioner, mainly by improving the physicochemical and biological properties of soils, increasing its WHC by ~18%, reducing nutrient leaching, and neutralizing acidic soils, thus enhancing plant productivity, seed germination, plant growth, and crop yields [25]. Especially when wet BC is applied, soil desiccation is prevented [4]. In some BC systems, all these objectives can be met, while in others, a combination of two or more objectives will be obtained. Interestingly, BC has been used in animal feed for centuries. Doug Pow, a Western Australian farmer, who won the Australian Government Innovation in Agriculture Land Management Award at the 2019 Western Australian Landcare Awards for his studies and innovations, explored the use of BC mixed with molasses as stock fodder for ruminants [50]. The study demonstrated that BC-assisted digestion was improved, and methane production was reduced. On-farm evidence indicated that the fodder led to improvements of liveweight gain in Angus-cross cattle [50]. Additionally, the production of BC by solid waste can help in solving the problem of waste management [48]. The production of the cement known as ordinary Portland cement (OPC), which is an essential component of concrete mix, is energy- and emissions-intensive and accounts for around 8% of global CO2 emissions. To limit this issue, the concrete industry has increasingly moved toward the use of supplementary cementitious materials (SCMs) and additives to both reduce the volume of OPC in the concrete mix and maintain or improve concrete properties [51,52,53]. BC has been shown to be an effective SCM, reducing concrete production emissions while maintaining the required strength and ductility properties [28,29]. Studies have been carried out demonstrating that a 1–2% wt. concentration of BC is excellent for use in concrete mixes, both in terms of low cost and good strength [28]. Moreover, the addition of a 2% wt. BC solution in the concrete mix resulted in an increased concrete flexural strength by 15% in a three-point bending test conducted after 7 days, compared to the traditional OPC-based concrete [29]. BC-based concrete also showed higher temperature resistance and reduced permeability [28]. Compared to other SCMs from industrial waste streams (such as fly ash and silica fume), BC also demonstrated decreased toxicity. Additionally, BC has been demonstrated to be capable of catalyzing chemical reactions and removing hazardous organic and inorganic pollutants from water soil and atmosphere, including antibiotics, thus reducing the emergence of microbial resistance [4]. Batch and column sorption experiments have shown that certain types of BCs have good sorption performance for heavy metals, dyes, or phosphate from aqueous solution and are being investigated as cost-effective, promising, and eco-friendly alternative materials for producing adsorbent materials [54,55,56,57,58,59,60].
2.1. BC as Climate and Soil Improver
As mentioned above, by its carbon sequestration action, BC performs climate remediation.
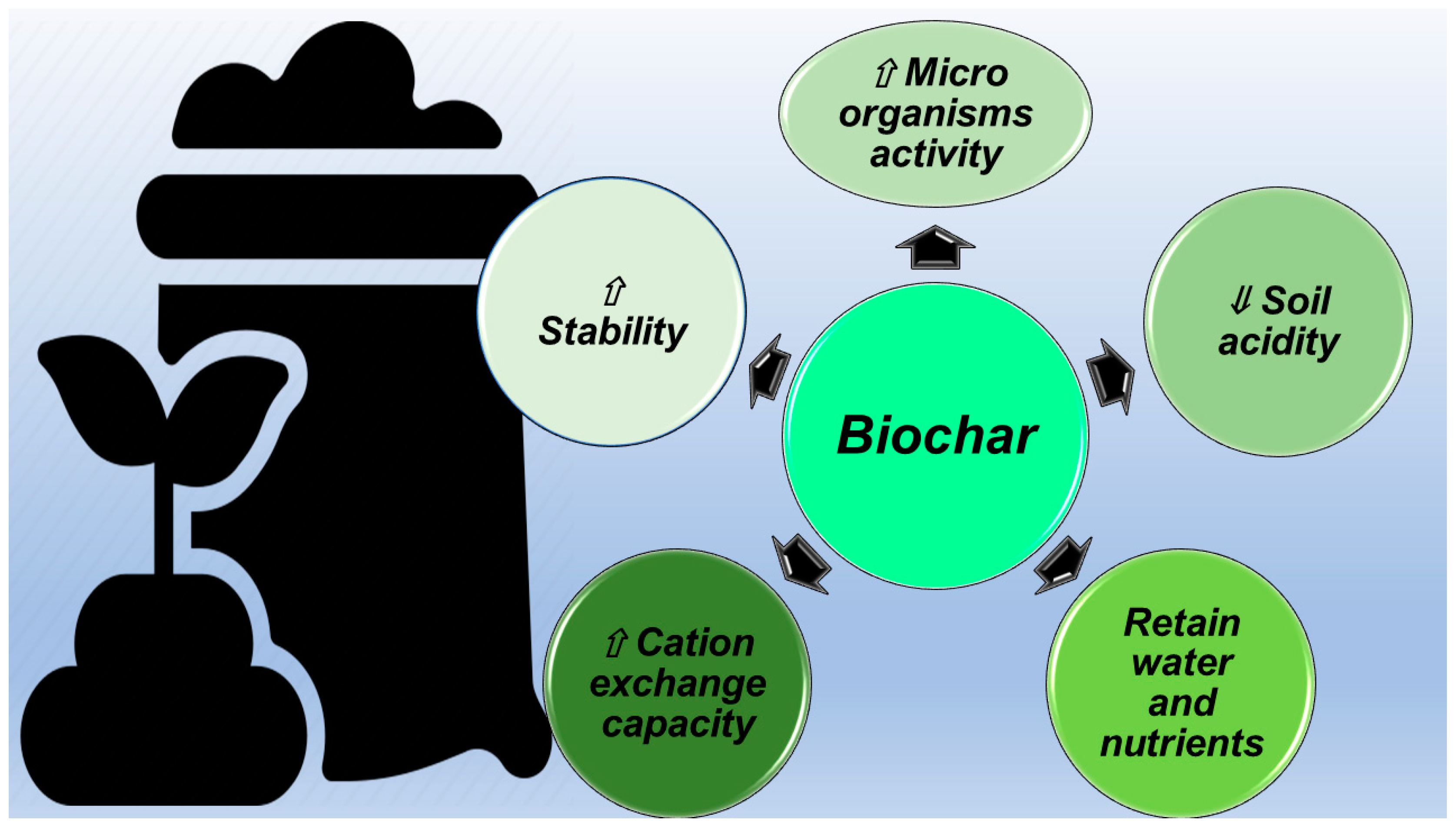
The refractory stability of BC leads to the concept of pyrogenic carbon capture and storage (PyCCS) [61], or rather, the sequestration of carbon in the form of BC [16]. Due to this potential, BC may be a means of mitigating climate change [62,63]. Due to its alkalinity, BC can increase the soil fertility of acidic soils and improve agricultural productivity [4]. When used for soil application, BC has been demonstrated to improve soil nutrient availability, aeration in soil, and soil water filtration. A large body of peer-reviewed literature exists assessing and describing the crop yield benefits of BC-amended soil [25]. Several field trials using BC conducted in the tropics have shown positive results on crop yields when BC was applied to field soils, and nutrients were managed appropriately. The most well-known example of the beneficial action of BC application to soil is the fertile Terra Preta soils in Brazil, but Japan also has a long tradition of using charcoal in soil [64,65]. Such a tradition is being revived and has been exported over the past 20 years to countries such as Costa Rica. The Brazilian and Japanese traditions together provide long-term evidence of positive BC impact on soils. Figure 3 summarizes the ways by which BC can improve soil quality.

Figure 3.
Ways by which BC acts as a soil improver. ⇓ low, lower, reduced, minor; ⇑ high, higher, increased, major.
Particularly, BC stimulates the activity of a variety of agriculturally important soil microorganisms by providing them with a suitable habitat and by protecting them from predation and drying while addressing many of their diverse carbon (C), energy, and mineral nutrient needs [66]. BC reduces soil acidity by means of the negative charges present on its surface and attracts and holds soil nutrients by the same mechanism [66]. BC has a high CEC, by which it retains positively charged ions, such as calcium (Ca2+), potassium (K+), and magnesium (Mg2+), thus improving soil fertility and exchanging them with plant roots. BC mineralizes in soils much more slowly than its uncharred precursor material (feedstock). Moreover, although most BC has a small labile (easily decomposed) fraction of carbon, typically a much larger recalcitrant (stable) fraction composes it [25]. Scientists have shown that the mean residence time (the estimated amount of time that biochar carbon will persist in soils) of this recalcitrant fraction ranges from decades to millennia [25].
The long persistence of BC when incorporated into soils is of paramount importance because it determines how long BC can provide benefits to soil and water quality.
Critical Considerations
While the larger questions concerning overall BC benefits to soils and climate have received affirmative responses, significant questions remain to be answered, including the need for a better understanding of some of the details of BC production and characterization. Although BC is widely viewed as an environmentally positive material for soil, it is crucial to also take into account the potential adverse effects of BC, including disturbing soil pH levels or introducing harmful chemical characteristics that cause problems at the micro dimensions. Therefore, caution should be exercised when considering the extensive application of BC, as the research exploring its positive and negative effects is currently ongoing.
Dust is certainly a concern associated with BC application, but best practices require that BC application be done during periods of low wind to prevent the blowing of fine particulates. Agricultural techniques already exist for applying powdered fertilizers and other amendments. Several techniques are available to help keep wind losses to a minimum, including that BC can be pelleted, prilled, mixed into a slurry with water or other liquids, mixed with manure and/or compost, or banded in rows. The optimization of BC application to soil is important, and the farm technology and methods are available to do the job.
2.2. Xenobiotics Removal by Biochar (BC)
BC is a material possessing a large surface area and high porosity based on the conditions of the pyrolysis process and the physicochemical characteristics of the original biomass [4] that allow it to interact with beneficial molecules, including water and water nutrients, as well as hazardous inorganic metal cations and organic pollutants [4]. Due to its enriched porous structure, high surface area, functional groups, and the possibility of interlacing π–π interactions and mineral components, BC is an excellent material for absorbing solutes from aqueous and organic solutions. Moreover, its naturally acquired adsorption capacity (3.6–6.3 g/g for BC prepared at a temperature range of 300–700 °C) [67] can further be enhanced by modifying its physicochemical properties through acid, alkali, or oxidizing treatments, and its surface area can be improved using acid treatments [68,69,70]. BC can absorb inorganic and organic contaminants, such as polycyclic aromatic hydrocarbons (PAHs), phenols and phthalate derivatives, and antibiotics and other drugs, and can aid in improving the treatment of sewage wastewater containing organic contaminants [71]. Table 3 reports the main mechanisms by which BC captures inorganic or organic pollutants.

Table 3.
Main mechanisms by which BC can capture inorganic or organic contaminants (reproduced by our recent paper [4]).
BCs produced at higher temperatures exhibited higher adsorption capacity in the remediation of both organic contaminants and metal cations from soil and water, due to their higher porosity and surface area. Additionally, the adsorption capacity of organic pollutants by BC is superior to that of inorganic ones, which strongly depends on the ionic radius of metals.
Xenobiotics Removal by Degradative Oxidation
The mechanism recognized for years by which BC removes toxic heavy metals and organic xenobiotics from wastewater and contaminated soil is adsorption, whose efficiency mainly depends on the type and number of functional groups, surface area, CEC, etc. Nevertheless, more research studies have evidenced on the surface or inside the BC particles the presence of free radicals, known as permanent free radicals (PFRs) due to their very long lifetime, which may be generated during the pyrolysis of biomass [72]. PFRs can exist in the air for months or even years. PFR lifetime depends mainly on the type of PFR, their possible complexation with metals or metal oxides, and their carbonaceous structure, as well as their concentration [72]. As recently reported, PFRs’ nature depends strongly on pyrolysis conditions, while their formation and characteristics differ based on feedstock types [4]. PFRs are classified into three categories, including oxygen-centered PFRs (OCPFRs), carbon-centered PFRs (CCPFRs), and oxygenated carbon-centered radicals (CCPFRs-O) [4], including, in turn, semiquinone radicals (oxygen-centered), phenoxy radicals (oxygenated carbon-centered radicals), and cyclopentadienyls (carbon-centered radicals) [4]. Several recent studies have mainly investigated the possible contribution of BC-bound PFRs in the removal of several organic xenobiotics through oxidative degradation by electron transfer-dependent production of reactive oxygen species (ROS) [73,74,75,76,77,78,79,80,81,82,83]. The degradation of hazardous cations, including As (III) and Cr (VI), by PFRs via electron transfer has also been extensively reported [84,85,86,87,88], as well as that of biological samples, including hormones and extracellular DNA (eDNA) [89,90,91,92,93]. Odinga et al. reviewed the application of BC-derived PFRs in the remediation of several environmental pollutants [94], while Fang et al. explored the reactivity of PFRs in BC and their catalytic ability to activate persulfate to degrade pollutants [95]. On the other hand, Odinga et al. also considered and commented on the possible ROS-mediated environmental risks of PFRs from BCs, mainly associated with the generation of oxidative stress, which represent the shadows associated with these chemicals and indicate they need further study, more knowledge, and regulation before their extensive application [94].
3. Bamboo-Derived Biochar (BBC)
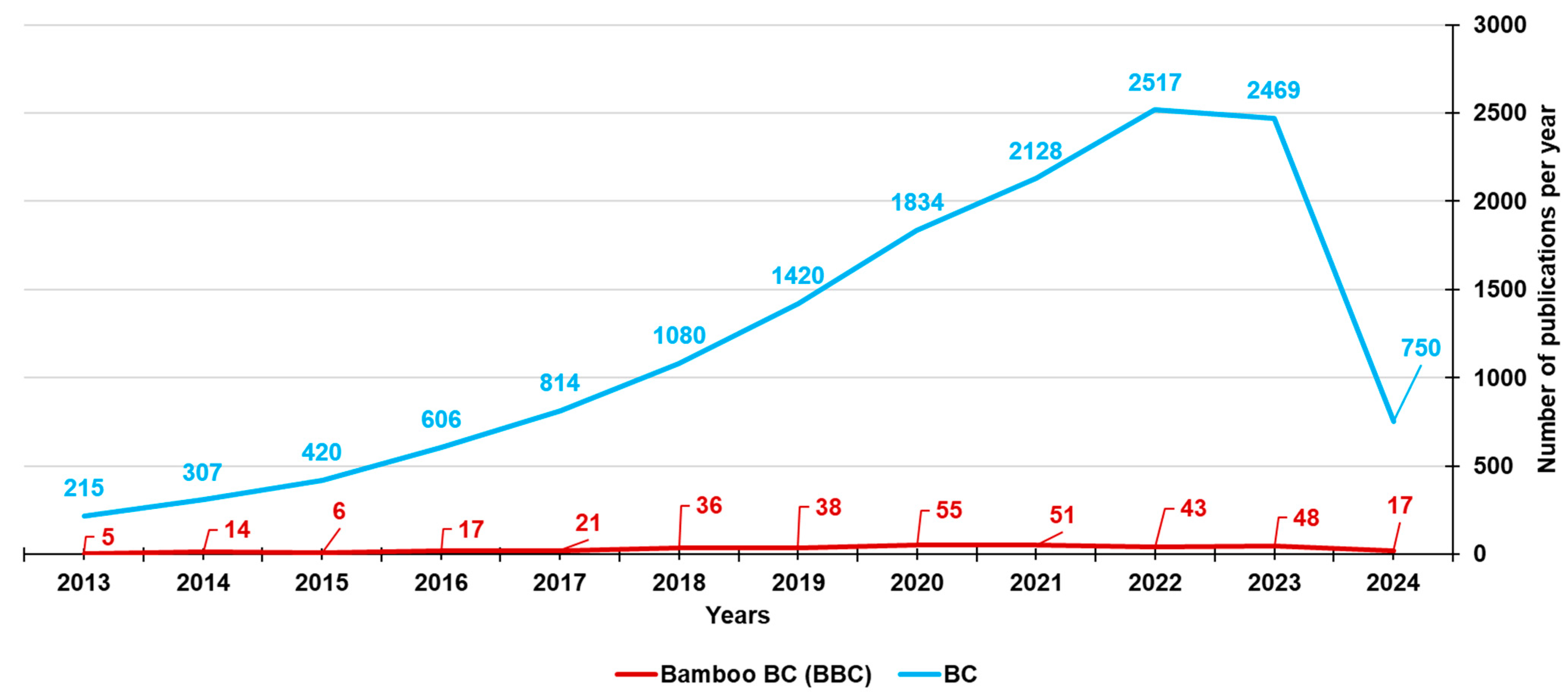
To date, the types of biomasses used to prepare BC and involved in the investigation of BC-bound PFRs mainly include lignocellulosic biomasses, such as pine needles, wheat straw, lignin, waste wood, cow manure, rice husk, and maize straw [23,35,36,37,38,39,40,41,42,43,44,45,46,47]. Lignocellulosic biomasses are made of hemicellulose, cellulose, and lignin. Despite the fact that bamboo may be a starting material almost perfect for synthesizing BC (BBC) and activated BBC (ABBC) due to its low cost, high biomass yield, and significantly accelerated growth rate, few researchers and scientists have used bamboo as a source for developing BC, as established by the number of publications on bamboo-derived BCs from the year 2013 until now (351) vs. those on BCs derived from different sources (14560) (Figure 4).
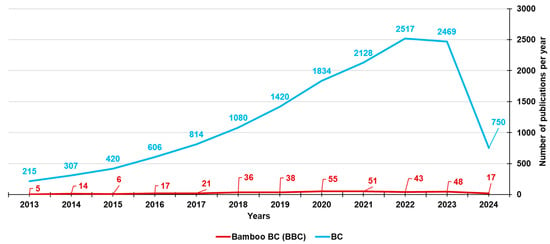
Figure 4.
Number of papers on BCs and BBCs published from the year 2013 until now, according to the PubMed dataset. The survey was carried out using the keywords biochar (light blue line) and bamboo biochar (red line).
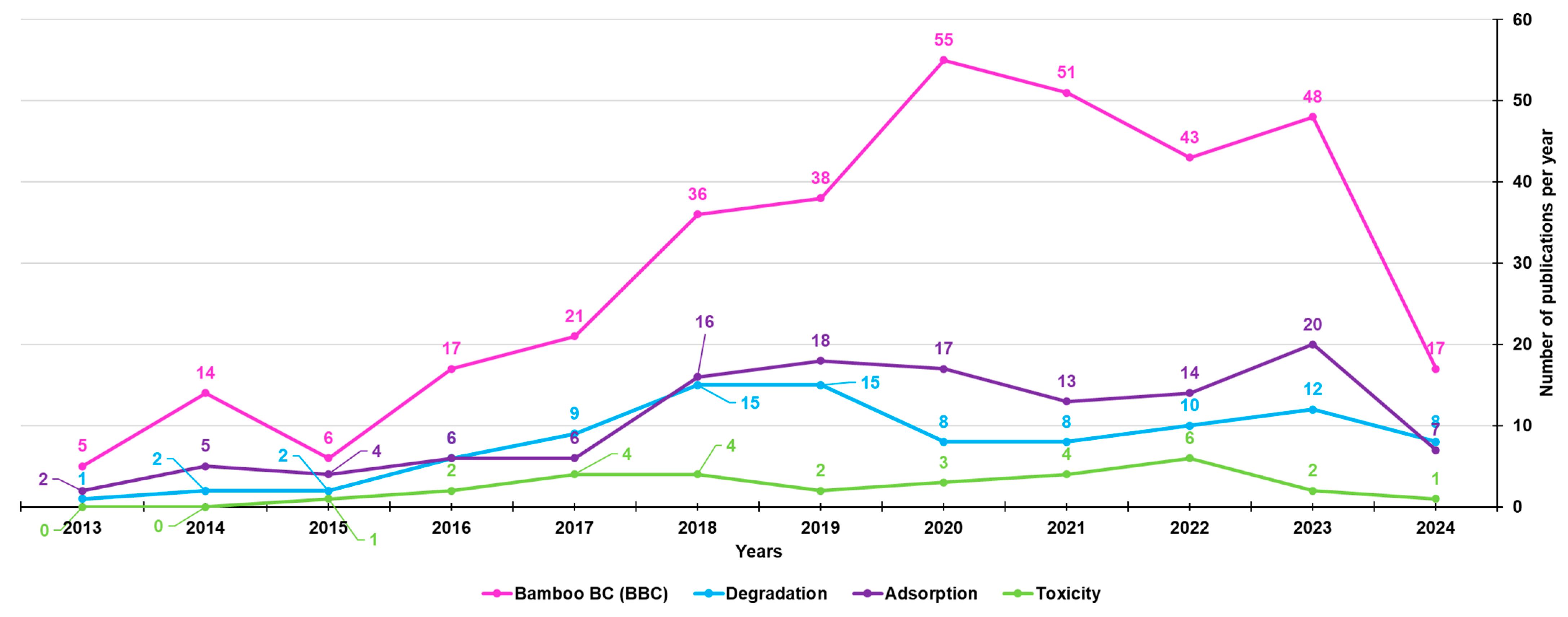
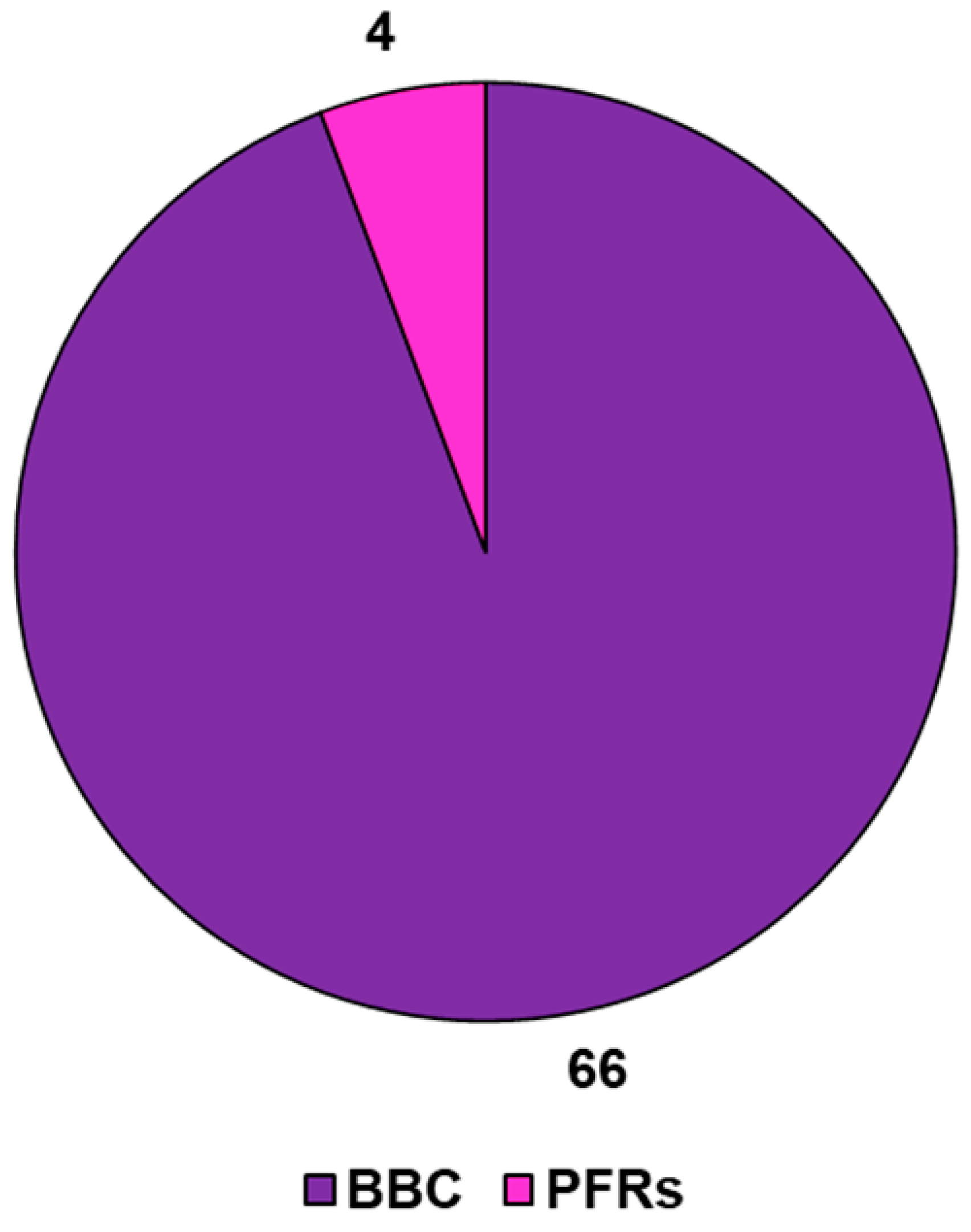
Among the 351 papers on BBC, the majority concerned its adsorption activity (128), followed by its degradation capacity (96), while fewer were studies on its possible toxic action (29) (Figure 5).
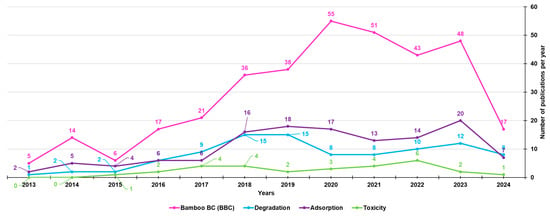
Figure 5.
Number of papers on BBCs, as well as on BBC absorption, degradation and toxic properties, published from the year 2013 until now, according to the PubMed dataset. The survey was carried out using the keywords bamboo biochar (pink line); bamboo biochar AND adsorption (purple line); bamboo biochar AND degradation (light blue line); bamboo biochar AND toxicity (light green line).
To support and sustain further research on BBCs, the possible associated PFRs, and its possible applications, the following sections of this paper first review the general characteristics of bamboo biomass and the main reported characteristics of BBCs. Secondly, the current applications of BBCs and the PFR-mediated applications of BBCs are reported and discussed.
3.1. The Use of Bamboo Biomass to Prepare BC: A Plethora of Advantages over Wood
Bamboo is a woody grass belonging to the Poaceae family, extensively occurring both in forests and in rural areas, as well as in farmlands and riverbanks [96]. Bamboo, due to its growth form, acts as a natural environmental shield and has been used as food and to construct tools and musical instruments. Moreover, bamboo has been exploited as a construction material, as an alternative to wood fuel, as a substitute for wood, and even as a substitute for non-wood forest products [96]. Due to its strength and durability, the latter applications of bamboo are typical in those world areas where the supply of wood no longer manages to meet the demand [97]. Anyway, only in recent years the interest in agricultural bamboo has exploded in Africa, North America, and even Europe. Collectively, as a regenerative agriculture and as a renewable source of energy, bamboo represents a wonder crop for producing BC. It has minimal nutrient needs, it is relatively quick to get established, and once established, it grows with unmatched vigor, using its tenacious roots to raise the water table and curb erosion [96]. Due to these characteristics and mainly thanks to its fast growth with respect to woody plants, bamboo is an optimal source of BC, more efficient and eco-friendlier than wood [97]. Bamboo is capable of proliferating without the necessity of following stringent rules for growing, harvesting, propagating and cutting [98]. Bamboo can tolerate a wide range of temperatures (−28–38 °C), is able to efficiently exploit water and soil at its disposition, and does not need fertilizers or pesticides. It is known that the possible amount of carbon content in BC deriving from a certain plant depends directly on its metabolic activity (photosynthesis), through which it ingests CO2 and releases oxygen (>30%) back into the atmosphere, thus reducing global warming. In this regard, due to its unequaled growth rate, bamboo yields more biomass and captures more atmospheric carbon per hectare than anything, thus removing more carbon from the sky and adding it to the soil. Although the usefulness of bamboo as an absorbent is not very well known, that which is cultivated in tropical and subtropical regions worldwide can be used as a renewable feedstock, as already occurs in many countries, especially in Asia (China, India, and Thailand), Central America (Costa Rica, Mexico, and Honduras), and South America (Peru, Ecuador, and Colombia) [99,100,101,102,103,104,105]. It has been reported that bamboo plantations can store four times more carbon than timber forests and that they are therefore considered as a potential alternative as a durable carbon stock [106]. Bamboo, like wood, is a promising natural template for biobased devices that take advantage of its hierarchical architecture, microarray channels, and anisotropic mechanical and electrical properties [107]. Bamboo charcoal has been recognized as a cost-effective and environmentally friendly regenerative physio-sorbing material for carbon capture [108]. Additionally, BBC, being a growing porous carbonous material with high surface area, has been used for biomass fuel, carbon capture, and containment adsorption [109]. Through pyrolysis, up to 50% of the carbon can be transferred from plant tissue to the BC, with the remaining 50% used to produce energy and fuels [110]. However, bamboo-derived biochar has not been comprehensively reported despite its good CO2 uptake [111]. Compared to wood composition, the lamellation of bamboo fiber has different fibrillar orientations surrounded by alternating narrow and broad layers [112]. The secondary cell wall is mainly made up of cellulose and lignin, with covalent linkage binding lignin phenolic acids to polysaccharide materials [112]. Bamboo culms grow and mature in a very short period of time, which can enable a continuous supply of fiber, giving it an advantage over trees. Bamboo’s mechanical and thermal properties increase its competitiveness against other forms of woody biomasses [113]. For various reasons, bamboo is a superior option to other wood planks to prepare BC, including strength, environmental friendliness, water resistance, cost, soil protection, and contribution to air quality. These benefits were the driving force behind several uses for this material, including blood purification, electromagnetic wave absorbers, and water purifiers. These benefits are influenced by the activation and carbonization procedures used to make bamboo charcoal. Green bamboo wood is roasted to a constant temperature to create activated charcoal when charcoal is exposed to oxygen by using the pyrolysis method.
3.2. Composition of Bamboo
The anatomical and chemical components of a plant, especially the plant cell wall thickness and the lignin content, are correlated with its physical and mechanical properties, which can be improved by saturated steam heat treatments [114,115], in turn influencing the characteristics of the derived BC [116,117]. In sight of having to use bamboo to produce BBC with several possible applications, it is important to have more information about bamboo chemical components.
3.2.1. Chemical Composition of Bamboo
Table 4 summarizes the main constituents of bamboo.

Table 4.
Main constituents of bamboo.
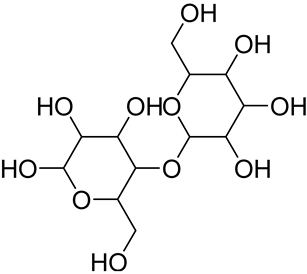
The cellulose content in bamboo stem is in the range of 40–60%, while bamboo hemicellulose (25%) is composed of more than 90% xylan (4-O-acetyl-4-O-methyl-D-glucuronoxylan, shown in Table 4), a linear short-chain polymer with a polymerization degree of 200 [116]. Xylans constitute 25% of the bamboo cell wall, which is classified between hardwood and softwood, thus being endowed with a very particular structure [120]. Lignin (20%) occurs in the compound middle lamella as well as in the primary wall structure [123]. Lignin is considered a set of correlated materials responsible for transferring the stress between fibers, which promotes traction resistance, flexibility, and rigidity to the stem longitudinal section, being a supporting element. Lignin exhibits a high molecular weight and is the second most abundant component in bamboo, emerging from several stages of lignification that are related to the plant age. However, bamboo reaches a final height between 3 and 6 months during its growth process. While the process of lignification occurs in the longitudinal direction (in the top-down direction on bamboo length) in the internodes, it occurs in the transversal direction in the inner layer of the region closest to the bark, thus completing the whole process of the growth stage. The lignin and carbohydrate proportion varies during the maturation process and tends to stabilize when the plant is about 1 year old [120]. Secondary elements in bamboo chemical composition are represented by extractives, ash, waxes, resins, and silica. The compounds known as extractives consist of aromatic organic compounds, including fatty acids, terpenes (essential oils), flavonoids, and steroids, that are distributed in the leaf, shell, stem, and other parts of the plant but do not constitute the cell wall. The variation of these secondary components is related to the plant species, age, season, and weather conditions, as well as to the soil nutrient availability and diversity and the presence of water [120,122,124]. Collectively, the content of cellulose, hemicellulose, and lignin in bamboo is similar to that of wood, while the extractives, ashes, and silica contents are higher. The soluble substances (resins, fatty acids, essential oil, tannins, etc.), ashes (inorganic substances such as potassium, calcium, silicon, and magnesium), and lignin (a polymer responsible for the plant elasticity and resistance properties) contents in nodes are lower than in the internodes. For the cellulose content, the opposite occurs [120,121].
3.2.2. Elemental Composition of Bamboo
The elemental composition of a material relates to the average contents of carbon (C), hydrogen (H), nitrogen (N), and oxygen (O), and the H/C and O/C ratios of different bamboo and other lignocellulosic species are given in Table 5.

Table 5.
Elemental composition of bamboo, wood, and other materials.
The biomasses reported in Table 5 show very similar H/C ratios (from 0.12 to 0.14), indicating that they are highly carbonized. Otherwise, the average O/C ratio of bamboo species (0.96) is lower than the O/C ratio of wood biomass (1.04), indicating a higher hydrophobicity on their surfaces, which makes bamboo biomass more stable against degradation than wood-based biomass. The average oxygen content of the bamboo species (44.04%) is lower than that of wood (47.42%), thus indicating a lower reactivity. Nevertheless, the presence of a high content of oxygen could decrease the energy density as well as the miscibility in hydrocarbon fuels. The biomass nitrogen content ranged from 0.1 to 12%, and the bamboo species had very low nitrogen content that ranged from 0.06 to 0.46%. The lowest content of nitrogen is important for environmental protection since NOx production, when burning the material, promotes environmental damage.
3.3. Bamboo-Derived Biochar (BBC)
In the last few years, researchers and scientists have used bamboo biomass from different species as a source for developing BC. Bamboo species like Dendrocalamus giganteus, Dendrocalamus latiforus Munro, Dendrocalamus asper, Phyllostachys pubescens Mazel, Phyllostachys edulis, Phyllostachys virdiglaucesons, Moso bamboo, and others have been carbonized in different conditions and in several cases chemically activated to obtain activated bamboo biochar (ABBC), which demonstrated different characteristics and properties and was studied for different applications. Table 6 summarizes useful information on the preparation of these materials and their possible uses.

Table 6.
BBCs and activated BBCs (ABBCs) developed in recent years and their applications.
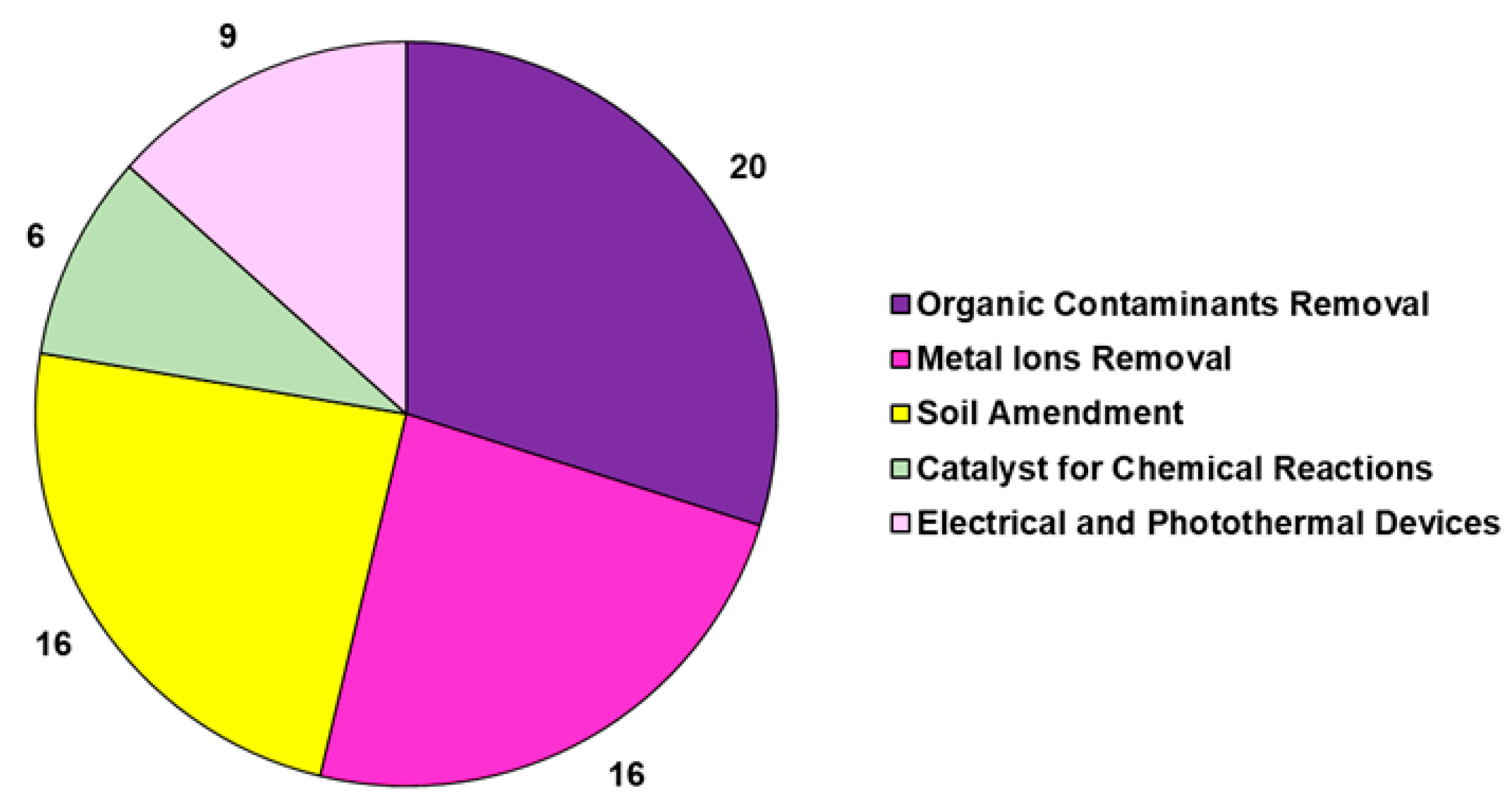
The studies reported in Table 6 are related to the transformation of the lignocellulose bamboo biomass into pyrolyzed bamboo BCs (BBCs) in a nitrogen atmosphere. In several cases, BBCs were chemically treated with alkali, acid, and oxidant solutions to increase their porosity and add new chemical functional groups, thus achieving activated BBC, namely ABBC. In the studies, BBC and ABBC were used for metal removal in an aqueous solution or gas phase for ameliorating soil quality by reducing acidity and absorbing heavy metals, as catalysts for organic chemical reactions, as energy sources, and as thermoelectrical devices, as well as for the adsorption of organic compounds, such as dyes, drugs, and aromatic compounds. To limit the problem of deforestation and to meet the pressure of avoiding the use of native forest resources for the production of BC, a woody bamboo (species Dendrocalamus giganteus Munro) was pyrolyzed by Hernandez-Mena et al. as a renewable material, and the properties of bamboo-derived BC (BBC) were studied [127]. To this end, the fast-growth bamboo was slow-pyrolyzed in a fixed-bed reactor at temperatures ranging from 300 to 600 °C and at a 10 °C/min heating rate [127]. The obtained BC was analyzed by different analytical techniques, and according to the reported results, the pyrolysis of bamboo yielded both biochar and bio-oil, thus representing a low-cost and sustainable strategy for producing energy and for agricultural applications. Using different bamboo species, including Dendrocalamus giganteus, Phyllostachys edulis, D. asper, P. pubescens Mazel, and P. edulis, and different parts of the plant as bamboo chopsticks and defoliated bamboo and even bamboo waste, BBCs were produced with electrical, electrochemical, and thermal properties [130,131,132,133,135,136,137,138]. Particularly, depending on the temperature of the pyrolysis process (700 °C), BBCs demonstrated high resistivity, thermal conductivity, and thermal heating rate, being suitable as a 3D microfluidic heater. Otherwise, BBCs obtained at 1000 °C proved to have high electric conductivity, thus being suitable as working electrodes [130]. Also, other bamboo-derived BCs were proposed as Ag-carbon electrodes for energy device applications [131]. BBCs obtained in specific conditions demonstrated excellent electrochemical performance [132] and were usable as supercapacitor electrode materials [132], as sustainable anodes for Li-ion batteries [133], as anodes for Na-ion batteries [134], as BCT-derived air cathodes for microbial fuel cells [136], as 3D solar vapor-generation devices for water desalination [137], and as efficient photothermal conversion devices [138]. The shoot shell of D. latiflorus Munro was used to produce BBCs at temperatures ranging from 300 °C to 500 °C. BBC derived at 400 °C demonstrated the best properties in soil improvement in terms of water-holding capacity (WHC), bulk density, iodine sorption, and metal adsorption. Particularly, soil adsorption capacities of Pb (II), Cr (III), and Cd (II) were improved by approximately 27%, 21%, and 29% with the addition of 2.5% wt. BBC [139]. Aiming at preparing BBCs to be used as soil improvers again, Sahoo and co-workers characterized and compared BCs obtained by pyrolysis at different temperatures (400, 500, and 600 °C) of bamboo biomass (BBCs) with those achieved by pyrolyzing pigeon pea stalk under the same conditions [113]. Briefly, the BC yield, which decreased with increasing temperature, was higher in bamboo than in pigeon pea stalk biomass due to more lignin content and low volatile matter in the former. Both BCs were shown to be highly carbonized and hydrophobic and to have low volatile matter, high porosity, and high fixed carbon, as well as proved to be beneficial for agricultural application, as they had high ash recovery [113]. The pyrolysis temperature of 600 °C was the most effective one in terms of surface area, total pore volume, and high mass fraction of carbon and fixed carbon for both the biomass materials [113]. Other authors, by pyrolyzing bamboo waste, bamboo tick, residual bamboo biomass, and bamboo agricultural by-products at temperatures ranging from 300 °C to 700 °C, prepared BBCs that were successful in enhancing soil fertility, crop growth [140], and soil acidification. Such BBCs have been demonstrated to be efficient in improving soil carbon content, promoting nutrient retention, improving microbial community abundance, and reducing CO2 emissions [141], thus being suitable for soil amendment [144]. Yanan et al. prepared three types of biochar (corn stalk biochar (CSB), rape straw biochar (RSB), and bamboo charcoal (BC)) to investigate the relationship between N2O emissions from composting and denitrifying bacterial communities on compost and BC particles [142]. The results showed that N2O emissions rates were higher in the thermophilic phase of composting and that the average emissions rate was lower upon BC treatment than after treatment with CSB and RSB [142]. The nosZ-carrying denitrifying bacterial community played a key role in reducing N2O emissions, and the study indicated that BC enhanced the efficiency of N2O emission reduction by enhancing the abundance of these key genera [142]. In another recent study by the same authors, the effects of bamboo charcoal (BC) and wheat straw biochar (WSB) on the humic acid (HA) and fulvic acid (FA) contents during pig manure composting were investigated [143]. The results evidenced that BC enhanced humification more than WSB and significantly increased the HA content and HA/FA ratio [143]. As in the study previously reported, the bacterial community structure under BC treatment differed from those under the other treatments. Particularly, BC increased the abundance of bacteria associated with the transformation of organic matter, thus influencing HA and FA concentration and improving the humification process during composting [143]. By the application of BBCs to soil obtained by the combustion of bamboo feedstocks, the quality and performance of different plants were enhanced. Particularly, the growth of mustard plants [194] and the yield of pakchoy plants [145], as well as the growth of tomato plants and the quality of their fruits, were improved [146,147]. Villagra-Mendoza et al. reported that the physicochemical changes that occurred in loam soils amended with BBC obtained by the pyrolysis of bamboo stems at 400 °C determined a significant improvement in tomato production yield [147]. Wang et al. demonstrated that the application of 5% bamboo biochar to soil reduced Cu uptake in the roots of Moso bamboo (Phyllostachys pubescens) and ameliorated soil physical properties and heavy metal solubility in soil [148]. Applications of biochar have significantly reduced the solubility of soil heavy metals. Authors evidenced that the application of BBC to soils ameliorated the quality of soils in terms of improved pH, nutrients, and abundance of beneficial fungi of Basidiomycota, Mucoromycota, and Chytridiomycota species [149], as well as reduced mobile Cd, Cu, Mn, Ni, Zn, reduced Pb, Mn, Cd, Zn, Cu, and Ni uptake in soybean shoots, and increased root nodulation, soybean growth, K and Mo uptake, and physiological performance of soybean plants [150]. Very recently, Tang et al. prepared BBCs at different pyrolysis temperatures (BB300, BB450, and BB600), which were successful in reducing As availability in paddy soil by adsorption and promoted the complexation of HCl-extractable Fe (III)/(II) and the formation of amorphous iron oxides, which, in turn, facilitated the formation of ternary complex (As-Fe-DOM) with highly stability [151]. Moreover, the abundance of Geobacteraceae and Xanthomonadaceae, which are common electroactive bacteria, was promoted in the BB450-treated paddy soil, which assisted in forming amorphous iron oxides [151]. Also, Hua et al. evidenced that the incorporation of bamboo charcoal particles prepared at 600 °C into the sludge composting material significantly reduced nitrogen loss (64.1%) and lessened the mobility of Cu and Zn [152]. Furthermore, a BBC with high adsorption capacity for CO2 capture and for low-temperature heat utilization was developed by Ji et al. by pyrolyzing bamboo sawdust at 1000 °C [153]. BBCs were also used for nitrate remediation and for the removal/degradation of hazardous inorganic pollutants. Particularly, Liu et al. immobilized a Paracoccus sp. strain YF1 on bamboo carbon obtained at 600°C to be assessed for denitrification. The results showed that denitrification was significantly improved using immobilized bacteria compared to that of free cells, where denitrification time decreased from 24 h (free cells) to 15 h (immobilized cells) [154]. Aiming at further improving the sorption characteristics of BBC in the denitrification process, a BBC/montmorillonite composite was produced in an experimental pyrolysis reactor using bamboo (P. virdiglaucesons) as biomass feedstock by Viglašová et al. This composite was successful in removing nitrates from aqueous solutions, being 1.8-fold more efficient than BBC without montmorillonite [155]. In the past, the efficient removal of nitrogen in the form of nitrate and ammonia using BBCs obtained by pyrolyzing Moso and Giant timber bamboo at temperatures in the range of 400–1000 °C in a tube furnace and charcoal kiln, respectively, have been reported by Mizuta et al. [156] and Asada et al. [157]. Particularly, the BBC prepared in the study by Asaka et al. was more efficient than commercial activated carbon in removing ammonia from water solution, and its performance improved further upon acidic treatment [157]. Concerning the environmental removal of hazardous metals, healthy dried stems without leaves of bamboo were converted in BBC in a muffle furnace at 500 °C to modulate the toxic effects of chromium on wheat plants [158], while offcuts of bamboo and bamboo residues were pyrolyzed at 900 °C and 400 °C, respectively, obtaining BBCs that were successful in the remediation of Cd (II) in Cd (II)-contaminated water and in the restoration of acidic Cd-contaminated soil [159,160]. Very recently, Ma et al. prepared BBCs and magnetic BBCs by the pyrolysis of bamboo at 600 °C and applied them for copper (Cu) immobilization in agricultural lands [161]. Particularly, magnetic BBC increased the maximum adsorption capacity and mitigated copper accumulation in lettuce. The major adsorption mechanisms of magnetic BBC were chemical precipitation, ion exchange, and metal–π complexation. Soil organic matter (SOM) facilitated the immobilization of activated copper [161]. In the same year, Zhang et al. prepared a ferrate-modified BBC and tested it as a composite system for soil amendment [162]. Particularly, the capacity of Fe-modified BBC to absorb heavy metal Cu and to reduce soil acidity was investigated [162]. An absorption efficiency of 276.12 mg/g due to single-layer surface adsorption and chemisorption processes was evidenced. Pore diffusion, electrostatic interaction, and surface interaction were the other possible mechanisms of Fe–BBC interaction with Cu2+ ions [162]. Multilayer self-assembled multifunctional bamboo shoot shell biochar microspheres (BSSBMs) showed a wide range of adsorption capacities, demonstrating the capability of absorbing heavy metals such as Ag (I) and Pd (II), antibiotics such as TC, and dyes such as MB from wastewater [163]. The maximum adsorption amounts of BSSBMs on Pd (II), Ag (I), TC, and MB were 417.3 mg/g, 222.5 mg/g, 97.2 mg/g, and 42.9 mg/g, respectively [163]. Moreover, Tan et al. investigated the adsorptive potential of a bamboo-derived BC (BBC) and of modified BBC using H2O2 for elemental mercury removal [164]. The results evidenced that BBC materials have excellent adsorption potential for elemental mercury, especially after being modified by H2O2 [164]. Batch adsorption experiments were conducted by Wang et al. to investigate the adsorption capacity of Cd (II) ions from aqueous solutions by BBC [165]. The results showed that the adsorption of Cd (II)ions was very fast initially, and equilibrium was reached after 6 h with an adsorbed capacity of 18 mg/g at pH = 8. Similarly, the batch adsorption capacity of heavy metal ions by CO2 and water steam-activated or water-activated ABBC was investigated by Wang et al. [166] and by Sheng-Fong Lo et al., respectively [167]. The optimum pH values for the adsorption capacity of heavy metal ions were 5.81–9.82 by bamboo-activated carbons. The optimum soaking time was 2–4 h for Pb2+, 4–8 h for Cu2+ and Cd2+, 4 h for Cr3+ by Moso ABBC, and 1 h for the tested heavy metal ions by Makino ABBC. However, the removal efficiency of heavy metal ions by the tested ABBCs decreased in the order Pb2+ > Cu2+ > Cr3+ > Cd2+. Specifically, depending on their interaction with anionic functional groups on the surface of activated ABBC, the adsorption capacity varies from 0.68 to 0.19 mg/g [166,167]. Moreover, water steam-activated mesoporous ABBC from the bamboo species Bambusa vulgaris striata achieved high efficiency in the removal of Cd (II), Hg (II), and Zn (II) ions from water solutions [168]. The batch studies suggested the highest adsorption capacities for an activated carbon dose of 0.6 g/L, solution pH = 9 and an equilibrium time of 16 h in static conditions were 240, 248, and 254 mg/g of cadmium, mercury, and zinc, respectively [168]. Other studies using KOH-activated bamboo derivates obtained from Melocanna baccifera species were mentioned with lower absorption capacities of 40.5 mg/g of Zn (II) [169]. A number of studies were concerned with the use of commercial bamboo charcoal, as well as that of BBCs prepared by the combustion of bamboo pieces, bamboo sawdust, or bamboo waste at different temperatures (450–1000 °C) in the remediation of hazardous organic compounds, including antibiotics and dyes. Particularly, an NaOH-activated BBC with a high percentage of surface graphitic carbon, oxygen-containing groups, and π–π interactions was used for the adsorptive removal of chloramphenicol [170]. The 100% removal of furfural, as well as the capability of removing MB by electrostatic interactions and decomposing tetracyclines by a photocatalytic process, was demonstrated by BBCs [171,172,173]. Additionally, the removal of fluoroquinolone antibiotics and MCAB-172, the in situ remediation of PCP, and the reduction of the bioavailability of DEP in soil by BBC applications has also been reported [174,175,176,177]. Furthermore, Liao et al. reported the production of not-activated and microwave-activated ABBCs starting from Moso bamboo as biomass feedstock [109,178]. BBCs were obtained by pre-carbonization at 150–270 °C for 2–3 days, carbonization at 270–450 °C for 1–2 days, calcination between 450 and 800 °C for 0.5–1 day, and finally, maintenance at 800 °C for a short time. All the processes were carried out under a nitrogen atmosphere. The obtained materials were successful in the removal of nitrogen-heterocyclic compounds (NHCs), such as pyridine, quinoline, and indole [178], as well as MB and OA7 dyes [109] from water solutions. In the first case, the spent BBC with NHC adsorption was effectively regenerated by MW radiation [178], while microwave-activated ABBCs were remarkably more efficient in removing dyes than the raw BBC (17.32 mg/g vs. 14.99 mg/g for MB and 9.28 mg/g vs. 4.91 mg/g for OA7) [109]. Anyway, a very high maximum monolayer adsorption capacity of 454.2 mg/g for MB was reported previously by Hameed et al. using a KOH-activated ABBC prepared by pyrolyzing in a two-phase process: bamboo biomass first at 700 °C for 1 h and then at 850 °C for 2 h in the presence of KOH [180]. In a recent study, two BBCs were prepared from the torrefaction of ammonium persulfate- and potassium persulfate-pretreated bamboo and then activated by cold alkali, which were named ASBC and KSBC, respectively. The two BBCs demonstrated high adsorption properties (475/881 mg/g at 303 K) over MB, mainly by electrostatic interactions [179]. As previously reported by Fan et al., Liao and colleagues succeeded in removing chloramphenicol (CAF) from water solutions using a BBC obtained from the thermal decomposition of sections of bamboo (Moso bamboo) [181]. Particularly, 4-year-old Moso bamboo cut into different sections was pre-carbonized at 150–250 °C for 1–2 d, carbonized at 250–400 °C for 0.5–1 d, calcined at 400–700 °C for 0.5–1 d, and finally, kept at 700 °C for a short time [180]. In addition to CAF, the obtained BBC was also capable of removing tetracyclines (TCs), both in batch experiments and in fixed-bed column ones [181]. A simple in situ method was used by Men et al. to load CdSe quantum dots (QDs) onto hydrothermal biochar (HTC) obtained by pyrolyzing bamboo to form CdSe/HTC composites [182]. The authors employed the as-obtained BBC-based nanomaterials in the photocatalytic degradation of tetracycline (TC). Compared with pure CdSe quantum dots, the best photocatalytic degradation efficiency of CdSe/HTC complex containing 15% HTC was 73%, which was attributed to the high carrier transport efficiency of HTC and the inhibition reorganization of photogenerated electron-hole pairs [182]. Furthermore, DBT was removed by n-octane by Zhao et al. using a commercial BBC [183], while Li and Umereweneza prepared an ABBC using bamboo sawdust that was carbonized at 873.15 K for 1 h and then activated with alkaline treatment (KOH) at 1073 K for 0.5 h [184]. The as-prepared ABBC was successful in removing NVP from water solutions [184]. The enhanced adsorption capacity towards aromatic volatile organic compounds (VOCs) by hydrophobic porous BBC produced via microwave rapid pyrolysis of bamboo in the presence of FeCl3 was recently reported by Junhao et al. [185]. Particularly, the as-obtained engineered BBC displayed better benzene and toluene adsorption capacity and humidity resistance. A BBC was also employed by Yu et al. to prepare a composite monolithic adsorbent [186]. The prepared composite adsorbent was successfully applied in a column for the reversible absorption of two coumarins from Angelicae pubescentis Radix, as well as for their determination [186]. Activated BBC (ABBC) was produced by treating the BBC with KOH, achieving a highly porous structure that was effective in extracting organic and inorganic contaminants from the air [187]. Particularly, ABBC produced at 700 °C demonstrated an air purifier’s performance efficiency for removing CO2 and PM2.5 of 91.23 and 89.19%, respectively [187]. Bamboo-derived BCs were also used as hydrolytic catalysts or as catalysts to produce aromatic and phenolic derivatives. Particularly, the combustion by the molten alkali carbonate method of bamboo power at 450–600 °C (450 °C being the optimal temperature) in a 2 L cylindrical reactor yielded a BBC that was used to support sulfonic acid groups, achieving a BBC-supported sulfonic acid catalyst that performed well in cellulose hydrolysis [188]. Similarly, a bamboo-based biochar sulfonic acid (BCSA) catalyst bearing polyamide moieties (BCSA-PA) with acidic and alkaline sites was prepared by the Yin group; it showed high activity and good selectivity for the conversion of glucose to 5-hydroxymethylfurfural (HMF) in pure water at 90 °C under microwave irradiation [189]. Chen et al. used N-doped BC catalysts derived by the fast pyrolysis (30 min) of bamboo at 600 °C in the presence of variant NH3 concentrations for the catalytic pyrolysis of bamboo wastes to prepare phenols [190]. High-concentration aromatic and phenolic derivatives were prepared by Chen et al. through the catalytic deoxygenation co-pyrolysis of bamboo wastes and microalgae using a BBC catalyst, in turn achieved by bamboo waste pyrolysis [191]. Similarly, Yang et al. produced high-concentration phenols (67%) through the catalytic fast pyrolysis of bamboo wastes at 600 °C using a BBC catalyst activated with KOH, K2CO3, KHCO3, or CH3COOK at 800 °C, in turn achieved by bamboo waste pyrolysis at 600 °C [192]. Also, Niu et al. prepared a sulfonated heterogeneous acid catalyst from bamboo-activated carbon (BAC) through arylation. This BBC-based catalyst was used in the catalytic esterification of oleic acid with ethanol to produce biodiesel with 96% efficiency [193]. Collectively, information reported in Table 6 has evidenced that the majority of case studies on BBC and ABBC were concerned with their use in the removal of organic and inorganic xenobiotics from wastewater and in soil amendment, followed by their application in developing electrical and photochemical devices. Otherwise, their employment as catalysts for organic chemical reactions remains limited (Figure 6). In fact, unlike BC-based materials obtained from other biomasses, which have been employed as heterogeneous catalysts for organic reactions, including reduction, oxidations, esterification, C-C and C-N coupling, alkylation, epoxidation, cycloadditions, and multi-component reactions [195], the reactions catalyzed by BBCs include only those reported in Table 6, such as cellulose hydrolysis, pyrolytic production of phenols and aromatic compounds, and esterification reactions [188,189,190,191,192,193].
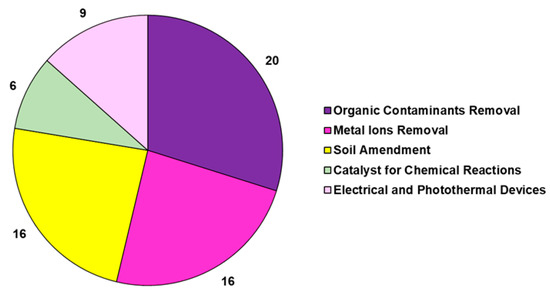
Figure 6.
Main relative applications of bamboo-derived BC. Numbers refer to the existing publications reporting that specific application.
4. BBC-Derived Persistent Free Radicals
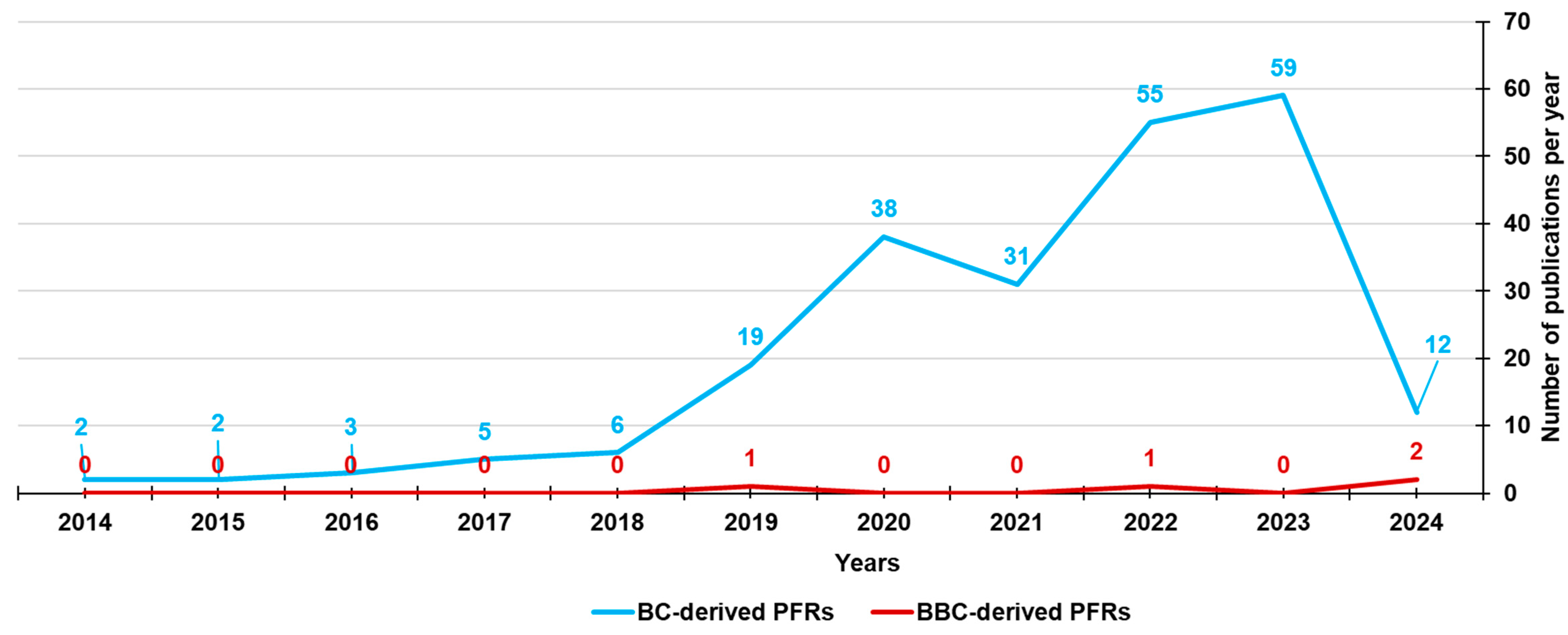
While the formation and presence of PFRs in the BC produced by several feedstock biomasses has been widely documented and studied since 2014, the literature documentation regarding those found in bamboo-derived biochar (BBC) is limited to four recent publications (Figure 7), which are reported in Table 7.
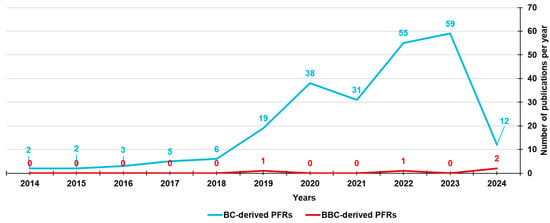
Figure 7.
Number of papers on BC-derived PFRs and BBC-derived PFRs from 2014 until now, according to Scopus and PubMed datasets (reviews included). The survey was carried out using the following keywords: persistent AND free AND radicals AND biochar (light blue line) and persistent AND free AND radicals AND biochar AND bamboo (red line).

Table 7.
Bamboo-BC (BBC)-derived EPFRs and their applications.
Briefly, Huang et al., to assess the non-negligible role of biomass types and their compositions on the formation of PFRs in the related BC, selected three different biomass feedstocks, including bamboo [196]. Biomass was pyrolyzed in a tube furnace for 2 h at a temperature of 500 °C, and BBC was stored in an anaerobic glovebox purged with N2 to avoid the effects of oxygen molecules on the PFR concentrations contained therein. The as-obtained BBC was tested in a BBC/H2O2 Fenton-like system to catalyze the oxidative degradation of tetracycline, evidencing high degradation efficiency by an electron transfer pathway via ROS production. The authors demonstrated that the catalytic property of BBC strongly depended on the amounts of PFRs [196]. The second and last experimental work found in literature concerning BBC-related PFRs was that by Zhang et al., who prepared N-doped (NBC), S-doped (SBC), and NS-doped (NSBC) BBC using Moso bamboo [197]. Bamboo biomass was pyrolyzed at temperatures in the range of 300–700 °C, giving the highest concentration of PFRs at 500 °C. The as-prepared BBCs were used to activate persulfate to produce both radical (SO4•−, •OH, and O2•−) and non-radical (1O2) species, which catalyzed the degradation of different antibiotics. Particularly, both EPFRs enhanced by the N-or S-doping, and non-radical species catalyzed the degradation of different antibiotics, including PFX, OTC, and CTC [197]. More recently, the photodegradation processes under simulated solar illuminations of 2,4,4′-trichlorobiphenyl (PCB28) adsorbed on biochar colloids (BCCs) released from bulk BBCs were studied by Wang et al. as a function of pyrolysis temperature (300, 500, or 700 °C) [198]. It was demonstrated that PCB28 adsorbed on the low-temperature BCCs degraded mainly by accepting electrons from the BBC-associated PFRs. Such electron transfer led to PCB28 dechlorination, followed by ring-opening oxidation through hydroxyl radical attack, ultimately resulting in progressive mineralization [198]. Otherwise, singlet oxygen non-radical mechanisms caused preferential ring opening of adsorbed PCB28 on the high-temperature BCCs, preceding dichlorination [198]. Lignocellulosic bamboo biomass was used as raw material to prepare Fe-N-BBC for persulfate activation and sulfamethoxazole (SMX) and TOC degradation [199]. BBCs were capable of activating peroxydisulfate (PDS) and degraded SMX and TOC, mainly via the oxidative action of four active species, such as •OH, SO4•−, •O2, and 1O2 [199]. Based on these data, if the research on BBC is still limited compared to that on BC, the research studying the possible presence of PFRs in BBC, their characteristics, their possible application, their environmental transformation, and above all, their possible toxicity to humans and the environment is even more minimal (Figure 8).
5. Conclusions and Future Perspectives
In this review, the different applications of biochar (BC) and activated BC obtained by the thermal decomposition of several bamboo species (BBC and ABBC) have been schematically highlighted and extensively discussed. Bambusa vulgaris, Gigantochloa albociliata, Moso bamboo (Phyllostachys edulis), Dendrocalamus giganteus, Bamboo Dendrocatiflorus Munro, dry bamboo stalks, Phyllostachys virdiglaucesons, etc. are the most used bamboo species for developing BBC and ABBC. The interest in bamboo as a source for producing BC is quite young and still too limited, as evidenced by the lower number of existing experimental works concerning BBC compared to those concerning the production of BC from other biomasses. Anyway, BBC has revealed to be promising as an electromagnetic wave absorber, for constructing electrodes to be used in dye-sensitized solar cells, and for water and soil purification by absorption of organic xenobiotics and inorganic pollutants. Additionally, BBC has been helpful for the release of nutrients and gas remediation, and it has been largely demonstrated that BBC positively affects soil properties and greenhouse gas emissions, thus representing a nonpareil natural gift source for BC. Anyway, in our perspective, the more extensive use of bamboo and BBC in the urban, green building, and gardening industries to increase the nation’s economic aspects and reduce ecotoxicity, the improvement of the properties of BC developable from bamboo, and more in-depth knowledge about the possible existence of BBC-associated PFRs is mandatory. In this context, the main scope of this review was to support increasing production of BBC and ABBC and to stimulate further studies about their possible applications, thus enlarging the current knowledge about these materials and allowing their more rational, safer, and optimized application, especially in the removal of xenobiotics.
Author Contributions
Authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Fundação Carlos Chagas Filho Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ project n.SEI-260003/001227/2020), the European Union—Next Generation EU-the Italian Ministry of University (MUR) (PRIN2022, project n. 2022JM3LZ3_(SUST-CARB)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data and information reported in this work have been obtained by literature using Scopus, PubMed, and Scifinder databases.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Biochar Initiative. Available online: https://biochar-international.org/about-biochar/faqs/ (accessed on 11 January 2024).
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive Review on Production and Utilization of Biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Danesh, P.; Niaparast, P.; Ghorbannezhad, P.; Ali, I. Biochar Production: Recent Developments, Applications, and Challenges. Fuel 2023, 337, 126889. [Google Scholar] [CrossRef]
- Alfei, S.; Pandoli, O.G. Biochar-Derived Persistent Free Radicals: A Plethora of Environmental Applications in a Lights and Shadows Scenario. Preprints 2024. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Arunachalam, K. Biochar: Production, Properties and Emerging Role as a Support for Enzyme Immobilization. J. Clean. Prod. 2020, 255, 120267. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Bridgwater, A.V. The Production of Biofuels and Renewable Chemicals by Fast Pyrolysis of Biomass. Int. J. Glob. Energy Issues 2007, 27, 160–203. [Google Scholar] [CrossRef]
- DeSisto, W.J.; Hill, N.; Beis, S.H.; Mukkamala, S.; Joseph, J.; Baker, C.; Ong, T.-H.; Stemmler, E.A.; Wheeler, M.C.; Frederick, B.G.; et al. Fast Pyrolysis of Pine Sawdust in a Fluidized-Bed Reactor. Energy Fuels 2010, 24, 2642–2651. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A Review of Pyrolysis Technologies and Feedstock: A Blending Approach for Plastic and Biomass towards Optimum Biochar Yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- National Energy Technology Laboratory. Syngas Composition. Available online: https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/syngas-composition (accessed on 16 January 2024).
- Machado, H.; Cristino, A.F.; Orišková, S.; Galhano dos Santos, R. Bio-Oil: The Next-Generation Source of Chemicals. Reactions 2022, 3, 118–137. [Google Scholar] [CrossRef]
- Shan Ahamed, T.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of Bio-Oil from Thermochemical Conversion of Various Biomass—Mechanism, Challenges and Opportunities. Fuel 2021, 287, 119329. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the Applications of Bio-Oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y.; Oshiki, T.; Kishimoto, T. Low-Temperature Catalytic Hydrothermal Treatment of Wood Biomass: Analysis of Liquid Products. Chem. Eng. J. 2005, 108, 127–137. [Google Scholar] [CrossRef]
- Alok, J. ‘Biochar’ Goes Industrial with Giant Microwaves to Lock Carbon in Charcoal. The Guardian. 13 March 2009. Available online: https://www.theguardian.com/environment/2009/mar/13/charcoal-carbon (accessed on 15 January 2024).
- Ng, W.C.; You, S.; Ling, R.; Gin, K.Y.-H.; Dai, Y.; Wang, C.-H. Co-Gasification of Woody Biomass and Chicken Manure: Syngas Production, Biochar Reutilization, and Cost-Benefit Analysis. Energy 2017, 139, 732–742. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Klinghoffer, N.B.; Castaldi, M.J.; Nzihou, A. Influence of Char Composition and Inorganics on Catalytic Activity of Char from Biomass Gasification. Fuel 2015, 157, 37–47. [Google Scholar] [CrossRef]
- Nunoura, T.; Wade, S.R.; Bourke, J.P.; Antal, M.J. Studies of the Flash Carbonization Process. 1. Propagation of the Flaming Pyrolysis Reaction and Performance of a Catalytic Afterburner. Ind. Eng. Chem. Res. 2006, 45, 585–599. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, J.; Liu, B.; Mei, Q.; Luo, Z. Influence of Torrefaction Pretreatment on Biomass Gasification Technology. Chin. Sci. Bull. 2011, 56, 1449–1456. [Google Scholar] [CrossRef]
- Smidth, A.N. A New Boost for Biochar as a Natural Climate Solution. 2023. Available online: https://blog.nature.org/science-brief/a-new-boost-for-biochar-as-a-natural-climate-solution/#:~:text=biochar%20is%20a%20type%20of%20charcoal%20made%20from,other%20biomass%20would%2c%20leading%20to%20more%20carbon%20sequestration (accessed on 27 December 2023).
- Luo, L.; Wang, J.; Lv, J.; Liu, Z.; Sun, T.; Yang, Y.; Zhu, Y.-G. Carbon Sequestration Strategies in Soil Using Biochar: Advances, Challenges, and Opportunities. Environ. Sci. Technol. 2023, 57, 11357–11372. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for Soil Amendment. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–146. [Google Scholar]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for Composting Improvement and Contaminants Reduction. A Review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Koh, H.J. Application of Biochar from Food and Wood Waste as Green Admixture for Cement Mortar. Sci. Total Environ. 2018, 619–620, 419–435. [Google Scholar] [CrossRef]
- Suarez-Riera, D.; Restuccia, L.; Ferro, G.A. The Use of Biochar to Reduce the Carbon Footprint of Cement-Based Materials. Procedia Struct. Integr. 2020, 26, 199–210. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of Biochar for the Adsorption of Organic Pollutants from Wastewater: Modification Strategies, Mechanisms and Challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Kalu, S.; Kulmala, L.; Zrim, J.; Peltokangas, K.; Tammeorg, P.; Rasa, K.; Kitzler, B.; Pihlatie, M.; Karhu, K. Potential of Biochar to Reduce Greenhouse Gas Emissions and Increase Nitrogen Use Efficiency in Boreal Arable Soils in the Long-Term. Front. Environ. Sci. 2022, 10, 914766. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, B.; Gao, B.; Cheng, N.; Feng, Q.; Chen, M.; Wang, S. Degradation of Organic Pollutants from Water by Biochar-Assisted Advanced Oxidation Processes: Mechanisms and Applications. J. Hazard. Mater. 2023, 442, 130075. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of Biochar and Its Composites in Catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Hagemann, N.; Draper, K.; Kammann, C. The Use of Biochar in Animal Feeding. PeerJ 2019, 7, e7373. [Google Scholar] [CrossRef]
- Yu, D.; Yu, Y.; Tang, J.; Li, X.; Ke, C.; Yao, Z. Application Fields of Kitchen Waste Biochar and Its Prospects as Catalytic Material: A Review. Sci. Total Environ. 2022, 810, 152171. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A Review on Recent Advances of Biochar from Agricultural and Forestry Wastes: Preparation, Modification and Applications in Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 111638. [Google Scholar] [CrossRef]
- Wijitkosum, S. Biochar Derived from Agricultural Wastes and Wood Residues for Sustainable Agricultural and Environmental Applications. Int. Soil Water Conserv. Res. 2022, 10, 335–341. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar Derived from Anaerobically Digested Sugar Beet Tailings: Characterization and Phosphate Removal Potential. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Puettmann, M.; Sahoo, K.; Wilson, K.; Oneil, E. Life Cycle Assessment of Biochar Produced from Forest Residues Using Portable Systems. J. Clean. Prod. 2020, 250, 119564. [Google Scholar] [CrossRef]
- Papageorgiou, A.; Azzi, E.S.; Enell, A.; Sundberg, C. Biochar Produced from Wood Waste for Soil Remediation in Sweden: Carbon Sequestration and Other Environmental Impacts. Sci. Total Environ. 2021, 776, 145953. [Google Scholar] [CrossRef] [PubMed]
- Laird, D. Biochar Amendments Make the Harvesting of Crop Residue for Bioenergy Production Sustainable. Nutr. Cycl. Agroecosyst. 2023. [Google Scholar] [CrossRef]
- Li, N.; He, M.; Lu, X.; Yan, B.; Duan, X.; Chen, G.; Wang, S.; Hou, L. Municipal Solid Waste Derived Biochars for Wastewater Treatment: Production, Properties and Applications. Resour. Conserv. Recycl. 2022, 177, 106003. [Google Scholar] [CrossRef]
- Shi, W.; Wang, H.; Yan, J.; Shan, L.; Quan, G.; Pan, X.; Cui, L. Wheat Straw Derived Biochar with Hierarchically Porous Structure for Bisphenol A Removal: Preparation, Characterization, and Adsorption Properties. Sep. Purif. Technol. 2022, 289, 120796. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Chin, B.L.F.; Lock, S.S.M.; Yee, C.Y.; Yiin, C.L.; Peng, W.; Lam, S.S. Production of Biochar from Rice Straw and Its Application for Wastewater Remediation—An Overview. Bioresour. Technol. 2022, 360, 127588. [Google Scholar] [CrossRef]
- Gunamantha, I.M.; Widana, G.A.B. Characterization the Potential of Biochar from Cow and Pig Manure for Geoecology Application. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012055. [Google Scholar] [CrossRef]
- Rathnayake, D.; Schmidt, H.; Leifeld, J.; Mayer, J.; Epper, C.A.; Bucheli, T.D.; Hagemann, N. Biochar from Animal Manure: A Critical Assessment on Technical Feasibility, Economic Viability, and Ecological Impact. GCB Bioenergy 2023, 15, 1078–1104. [Google Scholar] [CrossRef]
- Drané, M.; Zbair, M.; Hajjar-Garreau, S.; Josien, L.; Michelin, L.; Bennici, S.; Limousy, L. Unveiling the Potential of Corn Cob Biochar: Analysis of Microstructure and Composition with Emphasis on Interaction with NO2. Materials 2023, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Sudarsan, J.S.; Prasanna, K.; Shiam Babu, R.; Sai Krishna, V.M.V. Biochar: A Sustainable Solution for Organic Waste Management a Way Forward towards Circular Economy. In Recent Trends in Solid Waste Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 215–230. [Google Scholar]
- Rawat, S.; Wang, C.-T.; Lay, C.-H.; Hotha, S.; Bhaskar, T. Sustainable Biochar for Advanced Electrochemical/Energy Storage Applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Daly, J. Poo-Eating Beetles and Charcoal Used by WA Farmer to Combat Climate Change. ABC RURAL. 18 October 2019. Available online: https://www.abc.net.au/news/rural/2019-10-18/wa-farmer-uses-beetles-and-charcoal-to-combat-climate-change/11613846 (accessed on 16 January 2024).
- Making Concrete Change: Innovation in Low-Carbon Cement and Concrete. Chatham House—International Affairs Think Tank. Available online: https://www.chathamhouse.org/2018/06/making-concrete-change-innovation-low-carbon-cement-and-concrete (accessed on 22 January 2024).
- Arvaniti, E.C.; Juenger, M.C.G.; Bernal, S.A.; Duchesne, J.; Courard, L.; Leroy, S.; Provis, J.L.; Klemm, A.; De Belie, N. Physical Characterization Methods for Supplementary Cementitious Materials. Mater. Struct. 2015, 48, 3675–3686. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Pang, S.D. Effect of Biochar on Mechanical and Permeability Properties of Concrete Exposed to Elevated Temperature. Constr. Build. Mater. 2020, 234, 117338. [Google Scholar] [CrossRef]
- Varjani, S.; Kumar, G.; Rene, E.R. Developments in Biochar Application for Pesticide Remediation: Current Knowledge and Future Research Directions. J. Environ. Manag. 2019, 232, 505–513. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-Based Engineered Composites for Sorptive Decontamination of Water: A Review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A Review of Biochars’ Potential Role in the Remediation, Revegetation and Restoration of Contaminated Soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic Properties of Biochars Produced at Different Temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.; Gao, Y.; Zhang, L.; Ok, Y.S.; An, T. Persistent Free Radicals in Carbon-Based Materials on Transformation of Refractory Organic Contaminants (ROCs) in Water: A Critical Review. Water Res. 2018, 137, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Schmidt, H.-P.; Gerten, D.; Lucht, W.; Kammann, C. Biogeochemical Potential of Biomass Pyrolysis Systems for Limiting Global Warming to 1.5 °C. Environ. Res. Lett. 2018, 13, 044036. [Google Scholar] [CrossRef]
- Yousaf, B.; Liu, G.; Wang, R.; Abbas, Q.; Imtiaz, M.; Liu, R. Investigating the Biochar Effects on C-mineralization and Sequestration of Carbon in Soil Compared with Conventional Amendments Using the Stable Isotope (δ13C) Approach. GCB Bioenergy 2017, 9, 1085–1099. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable Biochar to Mitigate Global Climate Change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Cocora, L.; Brand, K.; Cocora, L.; Brand, K. United Nations University: Japan’s Charcoal Making Traditions Still Alive. Available online: https://ourworld.unu.edu/en/japans-charcoal-making-traditions-still-alive (accessed on 16 January 2024).
- Arshad, U.; Altaf, M.T.; Liaqat, W.; Ali, M.; Shah, M.N.; Jabran, M.; Ali, M.A. Biochar: Black Gold for Sustainable Agriculture and Fortification Against Plant Pathogens—A Review. Gesunde Pflanz. 2023. [Google Scholar] [CrossRef]
- Hamidzadeh, Z.; Ghorbannezhad, P.; Ketabchi, M.R.; Yeganeh, B. Biomass-Derived Biochar and Its Application in Agriculture. Fuel 2023, 341, 127701. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Pignatello, J.J. Laboratory Tests of Biochars as Absorbents for Use in Recovery or Containment of Marine Crude Oil Spills. Environ. Eng. Sci. 2013, 30, 374–380. [Google Scholar] [CrossRef]
- Jothirani, R.; Kumar, P.S.; Saravanan, A.; Narayan, A.S.; Dutta, A. Ultrasonic Modified Corn Pith for the Sequestration of Dye from Aqueous Solution. J. Ind. Eng. Chem. 2016, 39, 162–175. [Google Scholar] [CrossRef]
- Suganya, S.; Senthil Kumar, P.; Saravanan, A.; Sundar Rajan, P.; Ravikumar, C. Computation of Adsorption Parameters for the Removal of Dye from Wastewater by Microwave Assisted Sawdust: Theoretical and Experimental Analysis. Environ. Toxicol. Pharmacol. 2017, 50, 45–57. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Renita, A.A. Hybrid Synthesis of Novel Material through Acid Modification Followed Ultrasonication to Improve Adsorption Capacity for Zinc Removal. J. Clean. Prod. 2018, 172, 92–105. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel Insights into the Adsorption of Organic Contaminants by Biochar: A Review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, P.; Li, H.; Yang, Q.; Oleszczuk, P.; Pan, B. Generation Mechanism of Persistent Free Radicals in Lignocellulose-Derived Biochar: Roles of Reducible Carbonyls. Environ. Sci. Technol. 2022, 56, 10638–10645. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhu, Y.; Zeng, G.; Zhang, Y.; Wang, Y.; Zhang, M.; Long, H.; Tang, W. Efficient Removal of Perfluorooctanoic Acid by Persulfate Advanced Oxidative Degradation: Inherent Roles of Iron-Porphyrin and Persistent Free Radicals. Chem. Eng. J. 2020, 392, 123640. [Google Scholar] [CrossRef]
- Cao, W.; Zeng, C.; Guo, X.; Liu, Q.; Zhang, X.; Mameda, N. Enhanced Electrochemical Degradation of 2,4-Dichlorophenol with the Assist of Hydrochar. Chemosphere 2020, 260, 127643. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Y.; Li, J.; Hao, Q.; Li, X.; Liu, F. Heterogeneous Activation of Peroxymonosulfate by a Biochar-Supported Co3O4 Composite for Efficient Degradation of Chloramphenicols. Environ. Pollut. 2020, 257, 113610. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-Efficiency Degradation of Organic Pollutants with Fe, N Co-Doped Biochar Catalysts via Persulfate Activation. J. Hazard. Mater. 2020, 397, 122764. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hu, W.; Wang, X.; Tong, W.; Li, P.; Zhou, H.; Wang, Y.; Zhang, Y. Molten Salt Induced Nitrogen-Doped Biochar Nanosheets as Highly Efficient Peroxymonosulfate Catalyst for Organic Pollutant Degradation. Environ. Pollut. 2020, 260, 114053. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, L.; Qian, L.; Han, L.; Chen, M. Nano-Magnetite Supported by Biochar Pyrolyzed at Different Temperatures as Hydrogen Peroxide Activator: Synthesis Mechanism and the Effects on Ethylbenzene Removal. Environ. Pollut. 2020, 261, 114020. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.M.; Liu, G.; Zhou, H. Preparation of N-Doped Biochar from Sewage Sludge and Melamine for Peroxymonosulfate Activation: N-Functionality and Catalytic Mechanisms. Sci. Total Environ. 2020, 744, 140862. [Google Scholar] [CrossRef]
- da Silva Veiga, P.A.; Schultz, J.; da Silva Matos, T.T.; Fornari, M.R.; Costa, T.G.; Meurer, L.; Mangrich, A.S. Production of High-Performance Biochar Using a Simple and Low-Cost Method: Optimization of Pyrolysis Parameters and Evaluation for Water Treatment. J. Anal. Appl. Pyrolysis 2020, 148, 104823. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, K.-K.; Zhang, Y.; Zhang, Y.-R. Sunflower-Straw-Derived Biochar-Enhanced Fe(III)/S2O82− System for Degradation of Benzoic Acid. Huan Jing Ke Xue 2020, 41, 2301–2309. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, Q.; Tan, Y.; Lan, P.; Zhou, D.; Wu, M.; Liang, N.; Pan, B.; Xing, B. Dual Roles of Biochar Redox Property in Mediating 2,4-Dichlorophenol Degradation in the Presence of Fe3+ and Persulfate. Chemosphere 2021, 279, 130456. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, Q.; Gao, Y.; Jian, H.; Wang, C.; Sun, H. Effects of Biochar-Dissolved Organic Matter on the Photodegradation of Sulfamethoxazole and Chloramphenicol in Biochar Solutions as Revealed by Oxygen Reduction Performances and Free Radicals. Sci. Total Environ. 2021, 781, 146807. [Google Scholar] [CrossRef]
- Zhong, D.; Jiang, Y.; Zhao, Z.; Wang, L.; Chen, J.; Ren, S.; Liu, Z.; Zhang, Y.; Tsang, D.C.W.; Crittenden, J.C. PH Dependence of Arsenic Oxidation by Rice-Husk-Derived Biochar: Roles of Redox-Active Moieties. Environ. Sci. Technol. 2019, 53, 9034–9044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, P.; Zhang, Y. Decontamination of Cr(VI) Facilitated Formation of Persistent Free Radicals on Rice Husk Derived Biochar. Front. Environ. Sci. Eng. 2019, 13, 22. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Yang, X.; Peng, P.; Li, Z.; Jin, C. Enhanced Transformation of Cr(VI) by Heterocyclic-N within Nitrogen-Doped Biochar: Impact of Surface Modulatory Persistent Free Radicals (PFRs). Environ. Sci. Technol. 2020, 54, 8123–8132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, J.; Liu, J.; Liu, J.; Xia, F.; Wang, C.; Dahlgren, R.A.; Liu, W. Mechanism of Cr(VI) Removal by Magnetic Greigite/Biochar Composites. Sci. Total Environ. 2020, 700, 134414. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kong, Y.; Zhao, S.; Jia, H.; Vione, D.; Kang, Y.; Gao, P. Enhancement of Cr(VI) Decontamination by Irradiated Sludge Biochar in Neutral Conditions: Evidence of a Possible Role of Persistent Free Radicals. Sep. Purif. Technol. 2021, 277, 119414. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Z.; Zhang, H.; Chen, T.; Qiu, Y.; Xu, Z.; Yin, D. Efficient Removal of Several Estrogens in Water by Fe-Hydrochar Composite and Related Interactive Effect Mechanism of H2O2 and Iron with Persistent Free Radicals from Hydrochar of Pinewood. Sci. Total Environ. 2019, 658, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, C.; Bu, L.; Tang, C.; Wang, S.; Duan, P.; Yao, L.; Tang, J.; Dionysiou, D.D.; Wu, Y. Bismuth Impregnated Biochar for Efficient Estrone Degradation: The Synergistic Effect between Biochar and Bi/Bi2O3 for a High Photocatalytic Performance. J. Hazard. Mater. 2020, 384, 121258. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhang, C.; Wang, S.; Ren, N.; Ho, S.-H. Graphitic Biochar Catalysts from Anaerobic Digestion Sludge for Nonradical Degradation of Micropollutants and Disinfection. Chem. Eng. J. 2020, 384, 123244. [Google Scholar] [CrossRef]
- Xu, H.; Han, Y.; Wang, G.; Deng, P.; Feng, L. Walnut Shell Biochar Based Sorptive Remediation of Estrogens Polluted Simulated Wastewater: Characterization, Adsorption Mechanism and Degradation by Persistent Free Radicals. Environ. Technol. Innov. 2022, 28, 102870. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-Light-Driven Photocatalytic Degradation of Dye and Antibiotics by Activated Biochar Composited with K+ Doped g-C3N4: Effects, Mechanisms, Actual Wastewater Treatment and Disinfection. Sci. Total Environ. 2022, 839, 155955. [Google Scholar] [CrossRef] [PubMed]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, Formation, Environmental Fate and Risks of Environmentally Persistent Free Radicals in Biochars. Environ. Int. 2020, 134, 105172. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of Persistent Free Radicals in Biochar To Activate Persulfate for Contaminant Degradation. Environ. Sci. Technol. 2015, 49, 5645–5653. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Upadhyay, A.; Ding, Y.; Emamverdian, A.; Shahzad, A. Bamboo: Origin, Habitat, Distributions and Global Prospective. In Biotechnological Advances in Bamboo; Springer: Singapore, 2021; pp. 1–31. [Google Scholar]
- Yadav, M.; Mathur, A. Bamboo as a Sustainable Material in the Construction Industry: An Overview. Mater. Today Proc. 2021, 43, 2872–2876. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Singhwane, A.; Dhangar, M.; Mili, M.; Gorhae, N.; Naik, A.; Prashant, N.; Srivastava, A.K.; Verma, S. Bamboo for Producing Charcoal and Biochar for Versatile Applications. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Q.; Sun, W.; Zhao, Y.; Wang, X.; Liu, X.; Li, Y. Bamboo Flattening Technique: A Literature and Patent Review. Eur. J. Wood Wood Prod. 2021, 79, 1035–1048. [Google Scholar] [CrossRef]
- Yang, L.; Lou, Z.; Han, X.; Liu, J.; Wang, Z.; Zhang, Y.; Wu, X.; Yuan, C.; Li, Y. Fabrication of a Novel Magnetic Reconstituted Bamboo with Mildew Resistance Properties. Mater. Today Commun. 2020, 23, 101086. [Google Scholar] [CrossRef]
- da Silveira, E.S.; Roel, A.R.; dos Santos Brito, V.H.; Pistori, H.; Cereda, M.P. Influência de Espécies de Bambu Como Alimento No Crescimento Populacional e Na Preferência Alimentar Do Caruncho-Do-Bambu. I. In Bambus no Brasil: Da Biologia à Tecnologia; Drumond, P.M., Wiedman, G., Eds.; Instituto Ciência Hoje—ICH: Rio de Janeiro, Brazil, 2017; pp. 130–144. [Google Scholar]
- Darabant, A.; Haruthaithanasan, M.; Atkla, W.; Phudphong, T.; Thanavat, E.; Haruthaithanasan, K. Bamboo Biomass Yield and Feedstock Characteristics of Energy Plantations in Thailand. Energy Procedia 2014, 59, 134–141. [Google Scholar] [CrossRef]
- Romero Millán, L.M.; Sierra Vargas, F.E.; Nzihou, A. Characterization of Steam Gasification Biochars from Lignocellulosic Agrowaste towards Soil Applications. Waste Biomass Valoriz. 2021, 12, 4141–4155. [Google Scholar] [CrossRef]
- Masís-Meléndez, F.; Segura-Chavarría, D.; García-González, C.A.; Quesada-Kimsey, J.; Villagra-Mendoza, K. Variability of Physical and Chemical Properties of TLUD Stove Derived Biochars. Appl. Sci. 2020, 10, 507. [Google Scholar] [CrossRef]
- Patel, B.; Patel, M.; Gami, B.; Patel, A. Cultivation of Bioenergy Crops in Gujarat State: A Consultative Survey Process to Understand the Current Practices of Landowners. Environ. Dev. Sustain. 2021, 23, 8991–9013. [Google Scholar] [CrossRef]
- Odega, C.A.; Ayodele, O.O.; Ogutuga, S.O.; Anguruwa, G.T.; Adekunle, A.E.; Fakorede, C.O. Potential Application and Regeneration of Bamboo Biochar for Wastewater Treatment: A Review. Adv. Bamboo Sci. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Ginoble Pandoli, O.; Santos de Sá, D.; Nogueira Barbosa Junior, M.; Paciornik, S. Bamboo-Based Lignocellulose Biomass as Catalytic Support for Organic Synthesis and Water Treatments. In Bamboo Science and Technology; Springer: Singapore, 2023; pp. 297–327. [Google Scholar] [CrossRef]
- Kawakami, M.; Karato, T.; Takenaka, T.; Yokoyama, S. Structure Analysis of Coke, Wood Charcoal and Bamboo Charcoal by Raman Spectroscopy and Their Reaction Rate with CO2. ISIJ Int. 2005, 45, 1027–1034. [Google Scholar] [CrossRef]
- Liao, P.; Malik Ismael, Z.; Zhang, W.; Yuan, S.; Tong, M.; Wang, K.; Bao, J. Adsorption of Dyes from Aqueous Solutions by Microwave Modified Bamboo Charcoal. Chem. Eng. J. 2012, 195–196, 339–346. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-Energy in the Black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2 Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Anderson, D.P.; Chang, P.R.; Hua, Y. Bamboo Fiber and Its Reinforced Composites: Structure and Properties. Cellulose 2012, 19, 1449–1480. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and Characterization of Biochar Produced from Slow Pyrolysis of Pigeon Pea Stalk and Bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Yuan, C.; Lou, Z.; Li, Y. Effect of Saturated Steam Heat Treatment on Physical and Chemical Properties of Bamboo. Molecules 2020, 25, 1999. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Yuan, T.; Wang, Q.; Wu, X.; Hu, S.; Hao, X.; Liu, X.; Li, Y. Fabrication of Crack-Free Flattened Bamboo and Its Macro-/MicroMorphological and Mechanical Properties. J. Renew. Mater. 2021, 9, 959–977. [Google Scholar] [CrossRef]
- Liese, W. The Anatomy of Bamboo Culms; INBAR: Beijing, China, 1998; 208p. [Google Scholar]
- Lee, C.-H.; Chung, M.-J.; Lin, C.-H.; Yang, T.-H. Effects of Layered Structure on the Physical and Mechanical Properties of Laminated Moso Bamboo (Phyllosachys edulis) Flooring. Constr. Build. Mater. 2012, 28, 31–35. [Google Scholar] [CrossRef]
- Wastowski, A.D. Química Da Madeira Editora Interciência, 1st ed.; Editora Interciência: Rio de Janeiro, Brazil, 2018; 556p. [Google Scholar]
- Yan, L.; Kasal, B.; Huang, L. A Review of Recent Research on the Use of Cellulosic Fibres, Their Fibre Fabric Reinforced Cementitious, Geo-Polymer and Polymer Composites in Civil Engineering. Compos. B Eng. 2016, 92, 94–132. [Google Scholar] [CrossRef]
- Liese, W.; Tang, T.K.H. Properties of the Bamboo Culm. In Bamboo; Springer: Cham, Switzerland, 2015; pp. 227–256. [Google Scholar] [CrossRef]
- Nayak, L.; Mishra, S.P. Prospect of Bamboo as a Renewable Textile Fiber, Historical Overview, Labeling, Controversies and Regulation. Fash. Text. 2016, 3, 2. [Google Scholar] [CrossRef]
- Wan, J.; Wang, Y.; Xiao, Q. Effects of Hemicellulose Removal on Cellulose Fiber Structure and Recycling Characteristics of Eucalyptus Pulp. Bioresour. Technol. 2010, 101, 4577–4583. [Google Scholar] [CrossRef]
- Mohamad Ibrahim, M.N.; Zakaria, N.; Sipaut, C.S.; Sulaiman, O.; Hashim, R. Chemical and Thermal Properties of Lignins from Oil Palm Biomass as a Substitute for Phenol in a Phenol Formaldehyde Resin Production. Carbohydr. Polym. 2011, 86, 112–119. [Google Scholar] [CrossRef]
- Nogueira, C.d.L. Painel de Bambu Laminado Colado Estrutural. Master’s Thesis, Universidade de São Paulo, Piracicaba, Brazil, 2008. [Google Scholar]
- Macedo, L.A.; Rousset, P.L.A.; Vale, A.T. Influência Da Composição Da Biomassa No Rendimento Em Condensáveis Da Torrefação de Resíduos Vegetais. Pesqui. Florest. Bras. 2014, 34, 417–424. [Google Scholar] [CrossRef]
- Rousset, P.; Aguiar, C.; Labbé, N.; Commandré, J.-M. Enhancing the Combustible Properties of Bamboo by Torrefaction. Bioresour. Technol. 2011, 102, 8225–8231. [Google Scholar] [CrossRef]
- Hernandez-Mena, L.E.; Pécoraa, A.A.B.; Beraldo, A.L. Slow Pyrolysis of Bamboo Biomass: Analysis of Biochar Properties. Chem. Eng. Trans. 2014, 37, 115–120. [Google Scholar] [CrossRef]
- Lin, L.-D.; Chang, F.-C.; Ko, C.-H.; Wang, C.-T. Bamboo-Derived Fuel from Dendrocalamus latiflorus, Phyllostachys makinoi, and Phyllostachys pubescens Waste. Bioresources 2016, 11, 8425–8434. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and Prediction of Biomass Pyrolysis Products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Gontijo, L.O.L.; Junior, M.N.B.; de Sá, D.S.; Letichevsky, S.; Pedrozo-Peñafiel, M.J.; Aucélio, R.Q.; Bott, I.S.; Diniz Lopes Alves, H.; Fragneaud, B.; Oliveira Maciel, I.; et al. 3D Conductive Monolithic Carbons from Pyrolyzed Bamboo for Microfluidic Self-Heating System. Carbon 2023, 213, 118214. [Google Scholar] [CrossRef]
- Lin, Q.; Gao, R.; Li, D.; Lu, Y.; Liu, S.; Yu, Y.; Huang, Y.; Yu, W. Bamboo-Inspired Cell-Scale Assembly for Energy Device Applications. NPJ Flex. Electron. 2022, 6, 13. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Xu, F.; Li, M.; Han, C.; Gong, Y.; Wang, H.; Wang, Y. Inspired by Bread Leavening: One-Pot Synthesis of Hierarchically Porous Carbon for Supercapacitors. Green Chem. 2015, 17, 4053–4060. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, J.; Ai, W.; Fan, Z.; Shen, X.; Zou, C.; Liu, J.; Zhang, H.; Yu, T. Evolution of Disposable Bamboo Chopsticks into Uniform Carbon Fibers: A Smart Strategy to Fabricate Sustainable Anodes for Li-Ion Batteries. Energy Environ. Sci. 2014, 7, 2670–2679. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, Z.; Xu, L.; Li, C.; Han, D. Preparation of Green High-performance Biomass-Derived Hard Carbon Materials from Bamboo Powder Waste. ChemistryOpen 2024. [Google Scholar] [CrossRef]
- Subyakto, S.; Budiman, I.; Pari, G. Effects of Temperature and Time of Carbonization on the Properties of Bamboo (Dendrocalamus asper) Carbon. Wood Res. J. 2017, 4, 68–73. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Zhang, L.; Zhu, X.; Liao, Q. A Monolithic Air Cathode Derived from Bamboo for Microbial Fuel Cells. RSC Adv. 2017, 7, 28469–28475. [Google Scholar] [CrossRef]
- Bian, Y.; Du, Q.; Tang, K.; Shen, Y.; Hao, L.; Zhou, D.; Wang, X.; Xu, Z.; Zhang, H.; Zhao, L.; et al. Carbonized Bamboos as Excellent 3D Solar Vapor-Generation Devices. Adv. Mater. Technol. 2019, 4, 1800593. [Google Scholar] [CrossRef]
- Liu, J.; Yao, J.; Yuan, Y.; Liu, Q.; Zhang, W.; Zhang, X.; Gu, J. Surface-Carbonized Bamboos with Multilevel Functional Biostructures Deliver High Photothermal Water Evaporation Performance. Adv. Sustain. Syst. 2020, 4, 2000126. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Zhao, J.; Luo, Z.; Tu, S.; Yin, Y. Properties of Biochar Obtained from Pyrolysis of Bamboo Shoot Shell. J. Anal. Appl. Pyrolysis 2015, 114, 172–178. [Google Scholar] [CrossRef]
- Aggangan, N.S.; Cortes, A.D.; Reaño, C.E. Growth Response of Cacao (Theobroma cacao L.) Plant as Affected by Bamboo Biochar and Arbuscular Mycorrhizal Fungi in Sterilized and Unsterilized Soil. Biocatal. Agric. Biotechnol. 2019, 22, 101347. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response of Microbial Community Structure and Function to Short-Term Biochar Amendment in an Intensively Managed Bamboo (Phyllostachys praecox) Plantation Soil: Effect of Particle Size and Addition Rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef]
- Yin, Y.; Gu, M.; Zhang, W.; Yang, C.; Li, H.; Wang, X.; Chen, R. Relationships between Different Types of Biochar and N2O Emissions during Composting Based on Roles of NosZ-Carrying Denitrifying Bacterial Communities Enriched on Compost and Biochar Particles. Bioresour. Technol. 2024, 394, 130214. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tao, X.; Du, Y.; Li, M.; Yang, S.; Zhang, W.; Yang, C.; Li, H.; Wang, X.; Chen, R. Biochar Improves the Humification Process during Pig Manure Composting: Insights into Roles of the Bacterial Community and Metabolic Functions. J. Environ. Manag. 2024, 355, 120463. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Méndez, A.S.; Ortega-Ramírez, E.; Lucho-Constantino, C.A.; Arce-Cervantes, O.; Vázquez-Rodríguez, G.A.; Coronel-Olivares, C.; Beltrán-Hernández, R.I. Bamboo Biochar and a Nopal-Based Biofertilizer as Improvers of Alkaline Soils with Low Buffer Capacity. Appl. Sci. 2021, 11, 6502. [Google Scholar] [CrossRef]
- Situmeang, Y.P.; Sudewa, K.A.; Holo, P.P. Utilization Biochar of Bamboo and Compost in Improving Yield of Pakchoy Plant. J. Biol. Chem. Res. 2017, 34, 713–722. [Google Scholar]
- Suthar, R.; Wang, C.; Nunes, M.; Chen, J.; Sargent, S.; Bucklin, R.; Gao, B. Bamboo Biochar Pyrolyzed at Low Temperature Improves Tomato Plant Growth and Fruit Quality. Agriculture 2018, 8, 153. [Google Scholar] [CrossRef]
- Villagra-Mendoza, K.; Masís-Meléndez, F.; Quesada-Kimsey, J.; García-González, C.A.; Horn, R. Physicochemical Changes in Loam Soils Amended with Bamboo Biochar and Their Influence in Tomato Production Yield. Agronomy 2021, 11, 2052. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, B.; Shafi, M.; Ma, J.; Guo, J.; Wu, J.; Ye, Z.; Liu, D.; Jin, H. Effects of Biochar on Growth, and Heavy Metals Accumulation of Moso Bamboo (Phyllostachy pubescens), Soil Physical Properties, and Heavy Metals Solubility in Soil. Chemosphere 2019, 219, 510–516. [Google Scholar] [CrossRef]
- Tarin, M.W.K.; Fan, L.; Xie, D.; Tayyab, M.; Rong, J.; Chen, L.; Muneer, M.A.; Zheng, Y. Response of Soil Fungal Diversity and Community Composition to Varying Levels of Bamboo Biochar in Red Soils. Microorganisms 2021, 9, 1385. [Google Scholar] [CrossRef]
- Wang, C.; Alidoust, D.; Yang, X.; Isoda, A. Effects of Bamboo Biochar on Soybean Root Nodulation in Multi-Elements Contaminated Soils. Ecotoxicol. Environ. Saf. 2018, 150, 62–69. [Google Scholar] [CrossRef]
- Tang, L.; Xiong, L.; Zhang, H.; Joseph, A.; Wang, Y.; Li, J.; Yuan, X.; Rene, E.R.; Zhu, N. Reduced Arsenic Availability in Paddy Soil through Fe-Organic Ligand Complexation Mediated by Bamboo Biochar. Chemosphere 2024, 349, 140790. [Google Scholar] [CrossRef]
- Hua, L.; Wu, W.; Liu, Y.; McBride, M.B.; Chen, Y. Reduction of Nitrogen Loss and Cu and Zn Mobility during Sludge Composting with Bamboo Charcoal Amendment. Environ. Sci. Pollut. Res. 2009, 16, 1–9. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, C.; Zhang, X.J.; Xie, P.F.; Wu, C.; Jiang, L. A High Adsorption Capacity Bamboo Biochar for CO2 Capture for Low Temperature Heat Utilization. Sep. Purif. Technol. 2022, 293, 121131. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, L.; Chen, Z.; Megharaj, M.; Naidu, R. Removal of Nitrate Using Paracoccus sp. YF1 Immobilized on Bamboo Carbon. J. Hazard. Mater. 2012, 229–230, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Viglašová, E.; Galamboš, M.; Danková, Z.; Krivosudský, L.; Lengauer, C.L.; Hood-Nowotny, R.; Soja, G.; Rompel, A.; Matík, M.; Briančin, J. Production, Characterization and Adsorption Studies of Bamboo-Based Biochar/Montmorillonite Composite for Nitrate Removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, K.; Matsumoto, T.; Hatate, Y.; Nishihara, K.; Nakanishi, T. Removal of Nitrate-Nitrogen from Drinking Water Using Bamboo Powder Charcoal. Bioresour. Technol. 2004, 95, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Asada, T.; Ohkubo, T.; Kawata, K.; Oikawa, K. Ammonia Adsorption on Bamboo Charcoal with Acid Treatment. J. Health Sci. 2006, 52, 585–589. [Google Scholar] [CrossRef]
- Deng, P.; Wan, W.; Azeem, M.; Riaz, L.; Zhang, W.; Yang, Y.; Li, C.; Yuan, W. Characterization of Biochar Derived from Bamboo and Its Application to Modulate the Toxic Effects of Chromium on Wheat Plant. Biomass Convers. Biorefin. 2024, 14, 7643–7658. [Google Scholar] [CrossRef]
- Huang, A.; Bai, W.; Yang, S.; Wang, Z.; Wu, N.; Zhang, Y.; Ji, N.; Li, D. Adsorption Characteristics of Chitosan-Modified Bamboo Biochar in Cd(II) Contaminated Water. J. Chem. 2022, 2022, 6303252. [Google Scholar] [CrossRef]
- Mohamed, I.; Zhang, G.; Li, Z.; Liu, Y.; Chen, F.; Dai, K. Ecological Restoration of an Acidic Cd Contaminated Soil Using Bamboo Biochar Application. Ecol. Eng. 2015, 84, 67–76. [Google Scholar] [CrossRef]
- Ma, W.; Han, R.; Zhang, W.; Zhang, H.; Chen, L.; Zhu, L. Magnetic Biochar Enhanced Copper Immobilization in Agricultural Lands: Insights from Adsorption Precipitation and Redox. J. Environ. Manag. 2024, 352, 120058. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, J.; Han, H.; Wang, Y. Study on Improvement of Copper Sulfide Acid Soil Properties and Mechanism of Metal Ion Fixation Based on Fe-Biochar Composite. Sci. Rep. 2024, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Long, Q.; Zhu, Y.; Lin, C.; Xu, X.; Pan, B.; Shi, W.; Guo, Y.; Deng, J.; Yao, Q.; et al. Multifunctional Self-Assembled Adsorption Microspheres Based on Waste Bamboo Shoot Shells for Multi-Pollutant Water Purification. Environ. Res. 2024, 249, 118452. [Google Scholar] [CrossRef]
- Tan, Z.; Qiu, J.; Zeng, H.; Liu, H.; Xiang, J. Removal of Elemental Mercury by Bamboo Charcoal Impregnated with H2O2. Fuel 2011, 90, 1471–1475. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of Cadmium (II) Ions from Aqueous Solution by a New Low-Cost Adsorbent—Bamboo Charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef]
- Wang, S.Y.; Tsai, M.H.; Lo, S.F.; Tsai, M.J. Effects of Manufacturing Conditions on the Adsorption Capacity of Heavy Metal Ions by Makino Bamboo Charcoal. Bioresour. Technol. 2008, 99, 7027–7033. [Google Scholar] [CrossRef]
- Lo, S.-F.; Wang, S.-Y.; Tsai, M.-J.; Lin, L.-D. Adsorption Capacity and Removal Efficiency of Heavy Metal Ions by Moso and Ma Bamboo Activated Carbons. Chem. Eng. Res. Des. 2012, 90, 1397–1406. [Google Scholar] [CrossRef]
- González, P.G.; Pliego-Cuervo, Y.B. Adsorption of Cd(II), Hg(II) and Zn(II) from Aqueous Solution Using Mesoporous Activated Carbon Produced from Bambusa vulgaris Striata. Chem. Eng. Res. Des. 2014, 92, 2715–2724. [Google Scholar] [CrossRef]
- Lalhruaitluanga, H.; Prasad, M.N.V.; Radha, K. Potential of Chemically Activated and Raw Charcoals of Melocanna baccifera for Removal of Ni(II) and Zn(II) from Aqueous Solutions. Desalination 2011, 271, 301–308. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, B.; Yuan, S.; Wu, X.; Chen, J.; Wang, L. Adsorptive Removal of Chloramphenicol from Wastewater by NaOH Modified Bamboo Charcoal. Bioresour. Technol. 2010, 101, 7661–7664. [Google Scholar] [CrossRef]
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of Modified Biochars Derived from Bamboo Pyrolysis and Their Utilization for Target Component (Furfural) Adsorption. Energy Fuels 2014, 28, 5119–5127. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Fang, J.; Zhang, M.; Chen, H.; Zhou, Y.; Creamer, A.E.; Sun, Y.; Yang, L. Characterization and Environmental Applications of Clay–Biochar Composites. Chem. Eng. J. 2014, 242, 136–143. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Ma, C.; Liu, Y.; Dong, H.; Ma, W.; Liu, Z.; Wei, M.; Li, C.; Yan, Y. A Two Step Hydrothermal Process to Prepare Carbon Spheres from Bamboo for Construction of Core–Shell Non-Metallic Photocatalysts. New J. Chem. 2018, 42, 6515–6524. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Wu, J.; Liu, Q.; Zhang, H.; Jin, S. Adsorptive Removal of Fluoroquinolone Antibiotics Using Bamboo Biochar. Sustainability 2015, 7, 12947–12957. [Google Scholar] [CrossRef]
- Xu, T.; Lou, L.; Luo, L.; Cao, R.; Duan, D.; Chen, Y. Effect of Bamboo Biochar on Pentachlorophenol Leachability and Bioavailability in Agricultural Soil. Sci. Total Environ. 2012, 414, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, X.; Wei, B.; Zhao, Y.; Wang, J. Evaluation of Adsorption Potential of Bamboo Biochar for Metal-Complex Dye: Equilibrium, Kinetics and Artificial Neural Network Modeling. Int. J. Environ. Sci. Technol. 2014, 11, 1093–1100. [Google Scholar] [CrossRef]
- Zhang, X.; Sarmah, A.K.; Bolan, N.S.; He, L.; Lin, X.; Che, L.; Tang, C.; Wang, H. Effect of Aging Process on Adsorption of Diethyl Phthalate in Soils Amended with Bamboo Biochar. Chemosphere 2016, 142, 28–34. [Google Scholar] [CrossRef]
- Liao, P.; Yuan, S.; Xie, W.; Zhang, W.; Tong, M.; Wang, K. Adsorption of Nitrogen-Heterocyclic Compounds on Bamboo Charcoal: Kinetics, Thermodynamics, and Microwave Regeneration. J. Colloid Interface Sci. 2013, 390, 189–195. [Google Scholar] [CrossRef]
- Liu, X.-M.; Huan, W.-W.; Kang, Y.; Guo, J.-Z.; Wang, Y.-X.; Li, F.-H.; Li, B. Effects of Cation Types in Persulfate on Physicochemical and Adsorptive Properties of Biochar Prepared from Persulfate-Pretreated Bamboo. Bioresour. Technol. 2024, 393, 130140. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of Methylene Blue onto Bamboo-Based Activated Carbon: Kinetics and Equilibrium Studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Zhan, Z.; Dai, J.; Wu, X.; Zhang, W.; Wang, K.; Yuan, S. Adsorption of Tetracycline and Chloramphenicol in Aqueous Solutions by Bamboo Charcoal: A Batch and Fixed-Bed Column Study. Chem. Eng. J. 2013, 228, 496–505. [Google Scholar] [CrossRef]
- Men, Q.; Wang, T.; Ma, C.; Yang, L.; Liu, Y.; Huo, P.; Yan, Y. In-Suit Preparation of CdSe Quantum Dots/Porous Channel Biochar for Improving Photocatalytic Activity for Degradation of Tetracycline. J. Taiwan Inst. Chem. Eng. 2019, 99, 180–192. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, J.; Duan, E.; Wang, J. Adsorption Equilibrium and Kinetics of Dibenzothiophene from N-Octane on Bamboo Charcoal. Appl. Surf. Sci. 2008, 254, 3242–3247. [Google Scholar] [CrossRef]
- Li, S.; Umereweneza, D. Adsorption of N-Vinylpyrrolidone from Polyvinylpyrrolidone Solution onto Bamboo-Based Activated Carbon. Sep. Sci. Technol. 2012, 47, 104–111. [Google Scholar] [CrossRef]
- Lin, J.; Xu, Z.; Zhang, Q.; Cao, Y.; Mašek, O.; Lei, H.; Tsang, D.C.W. Enhanced Adsorption of Aromatic VOCs on Hydrophobic Porous Biochar Produced via Microwave Rapid Pyrolysis. Bioresour. Technol. 2024, 393, 130085. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xie, T.; Liu, S.; Bai, L. Fabrication of a Biochar-Doped Monolithic Adsorbent and Its Application for the Extraction and Determination of Coumarins from Angelicae pubescentis Radix. J. Chromatogr. A 2024, 1714, 464564. [Google Scholar] [CrossRef] [PubMed]
- Jagnade, P.; Panwar, N.L. Experiment Investigation on the Performance of Activated Bamboo Biochar for the Adsorption of CO2 and PM2.5. Emergent Mater. 2024. [Google Scholar] [CrossRef]
- Wei, Y.; Shen, C.; Xie, J.; Bu, Q. Study on Reaction Mechanism of Superior Bamboo Biochar Catalyst Production by Molten Alkali Carbonates Pyrolysis and Its Application for Cellulose Hydrolysis. Sci. Total Environ. 2020, 712, 136435. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Q.; Xiao, Y.; Zhang, C.; Fu, Z.; Liu, Y.; Yi, X.; Zheng, A.; Li, C.; Yin, D. Acid–Base Synergistic Catalysis of Biochar Sulfonic Acid Bearing Polyamide for Microwave-Assisted Hydrolysis of Cellulose in Water. Cellulose 2019, 26, 751–762. [Google Scholar] [CrossRef]
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo Wastes Catalytic Pyrolysis with N-Doped Biochar Catalyst for Phenols Products. Appl. Energy 2020, 260, 114242. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.; Xia, M.; Yang, H.; Chen, Y.; Chen, X.; Che, Q.; Chen, H. Catalytic Deoxygenation Co-Pyrolysis of Bamboo Wastes and Microalgae with Biochar Catalyst. Energy 2018, 157, 472–482. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Chen, W.; Chen, Y.; Wang, X.; Chen, H. Role of Porous Structure and Active O-Containing Groups of Activated Biochar Catalyst during Biomass Catalytic Pyrolysis. Energy 2020, 210, 118646. [Google Scholar] [CrossRef]
- Niu, S.; Ning, Y.; Lu, C.; Han, K.; Yu, H.; Zhou, Y. Esterification of Oleic Acid to Produce Biodiesel Catalyzed by Sulfonated Activated Carbon from Bamboo. Energy Convers. Manag. 2018, 163, 59–65. [Google Scholar] [CrossRef]
- Situmeang, Y.P. The Use of Bamboo Biochar as a Soil Improver on the Growth and Yield of Mustard Plants. Agriwar J. 2022, 2, 14–18. [Google Scholar] [CrossRef]
- Zhang, S.-Z.; Cui, Z.-S.; Zhang, M.; Zhang, Z.-H. Biochar-Based Functional Materials as Heterogeneous Catalysts for Organic Reactions. Curr. Opin. Green Sustain. Chem. 2022, 38, 100713. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible Role of Biomass Types and Its Compositions on the Formation of Persistent Free Radicals in Biochar: Insight into the Influences on Fenton-like Process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; He, R.; Zhao, J.; Kang, W.; Lv, J. Effect of Pyrolysis Temperature on the Activated Permonosulfate Degradation of Antibiotics in Nitrogen and Sulfur-Doping Biochar: Key Role of Environmentally Persistent Free Radicals. Chemosphere 2022, 294, 133737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, X.; Yang, K.; Lin, D. Differential Photodegradation Processes of Adsorbed Polychlorinated Biphenyls on Biochar Colloids with Various Pyrolysis Temperatures. Water Res. 2024, 251, 121174. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ge, Q.; Wan, J.; Wang, Y.; Zhu, C. Insights into the Influence and Mechanism of Biomass Substrate and Thermal Conversion Conditions on Fe N Doped Biochar as a Persulfate Activator for Sulfamethoxazole Removal. Sci. Total Environ. 2024, 907, 168101. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).