Aquatic Fate and Ecotoxicology Effect of ZnS:Mn Quantum Dots on Chlorella vulgaris in Fresh Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Mn-Doped ZnS Quantum Dots Using the Polyol Method
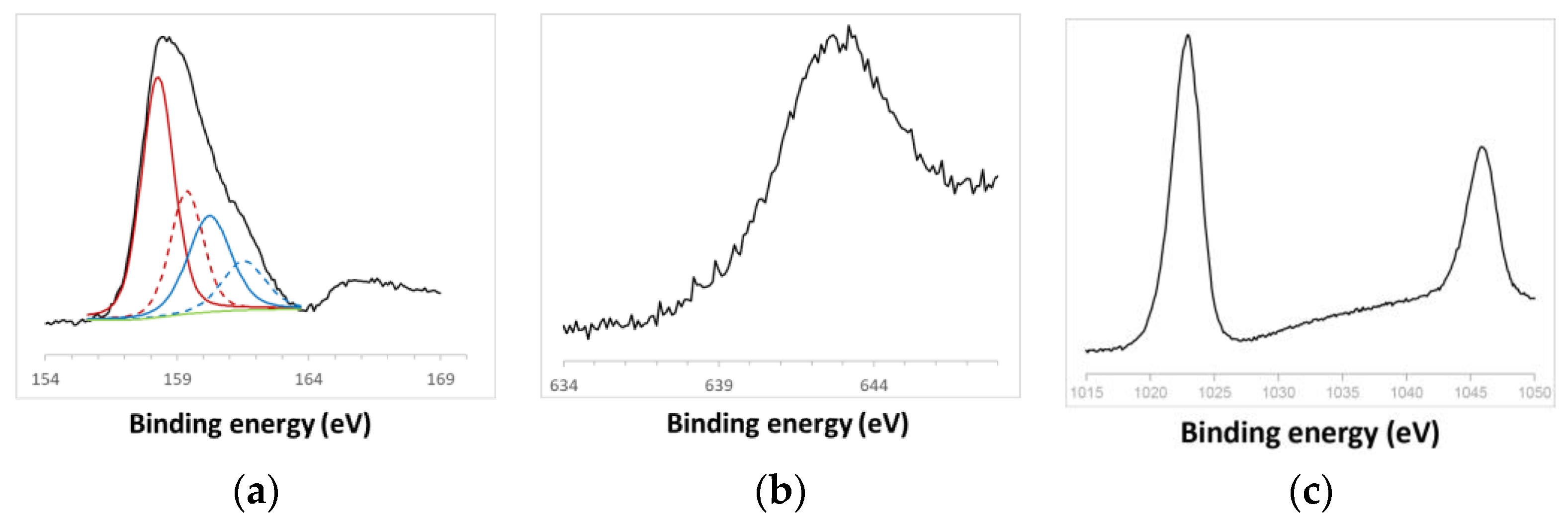
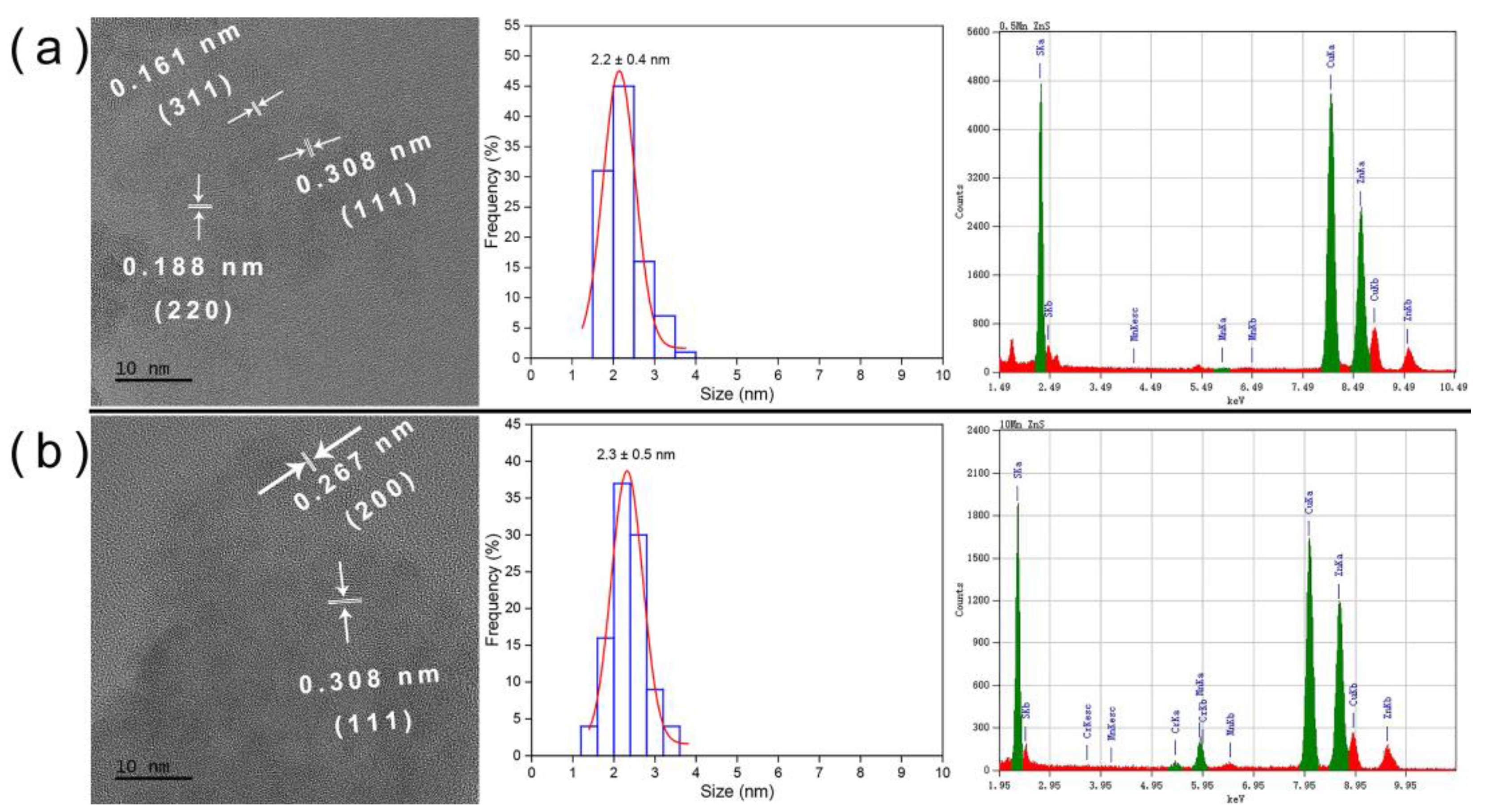
2.2. Characterization of ZnS:Mn QDs
2.3. Chlorella vulgaris Culture
2.4. Ecotoxicity Assessments
2.5. Statistical Analysis
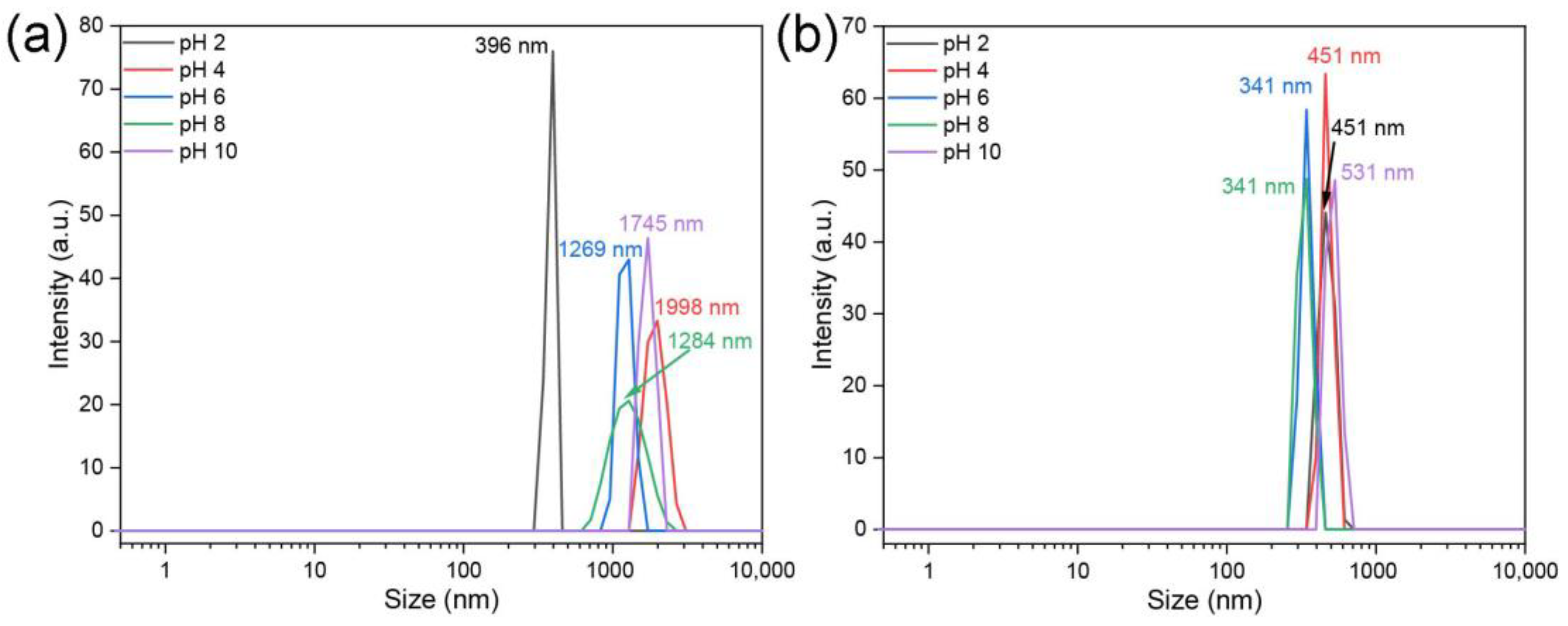
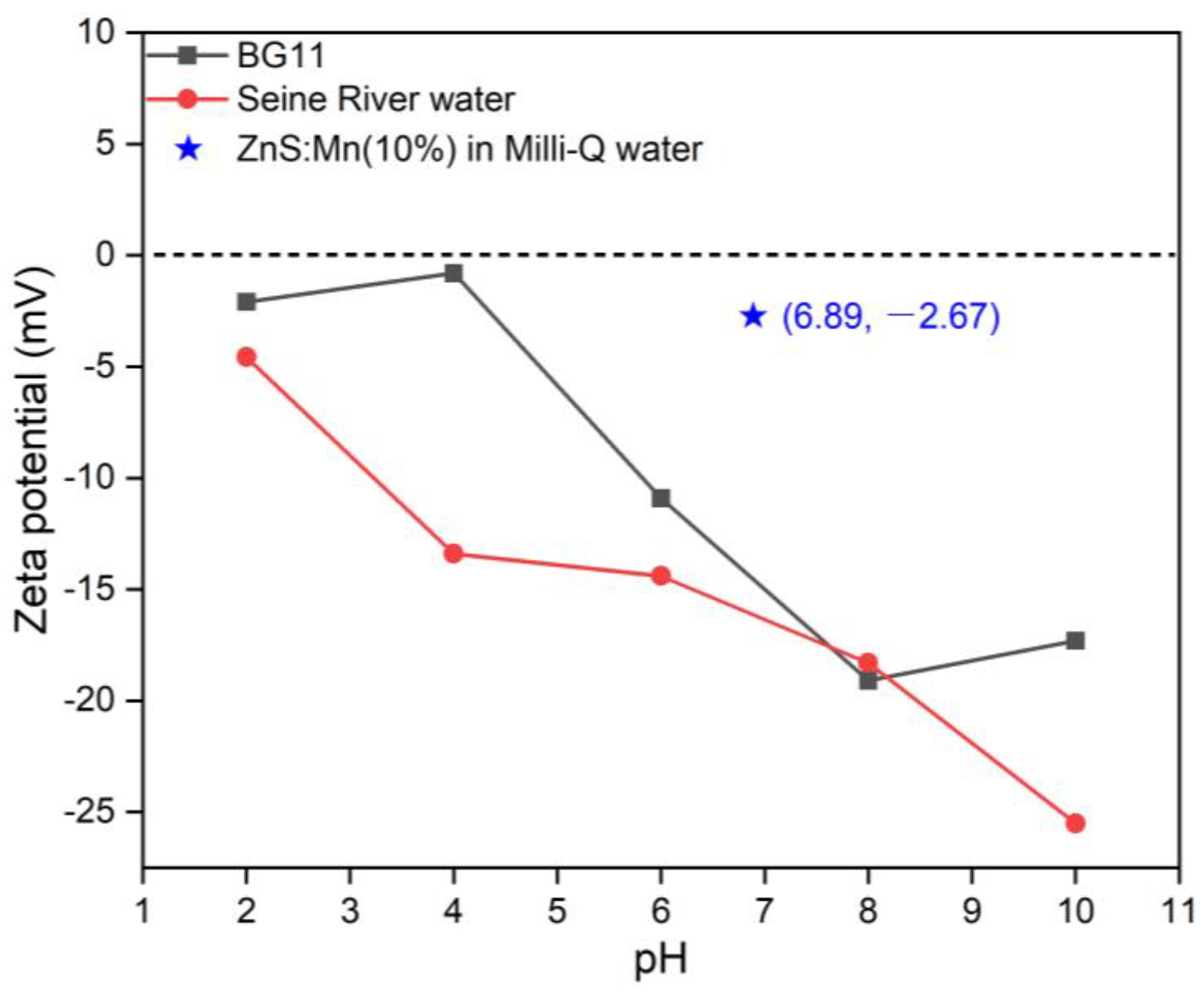
3. Results and Discussion
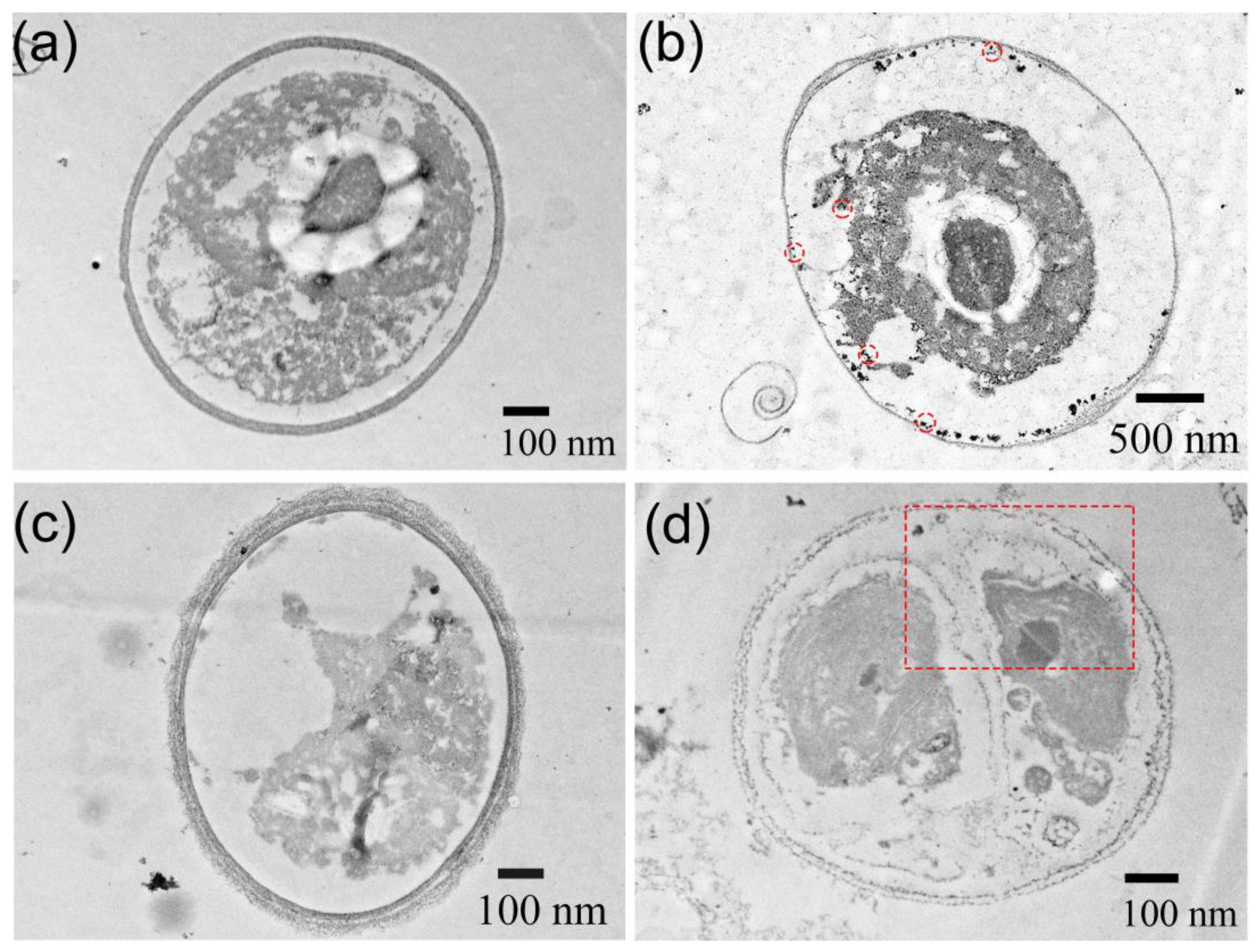
3.1. Structural Characterization of ZnS:Mn NPs
3.2. Assessment of the Toxicity of ZnS:Mn Nanoparticles
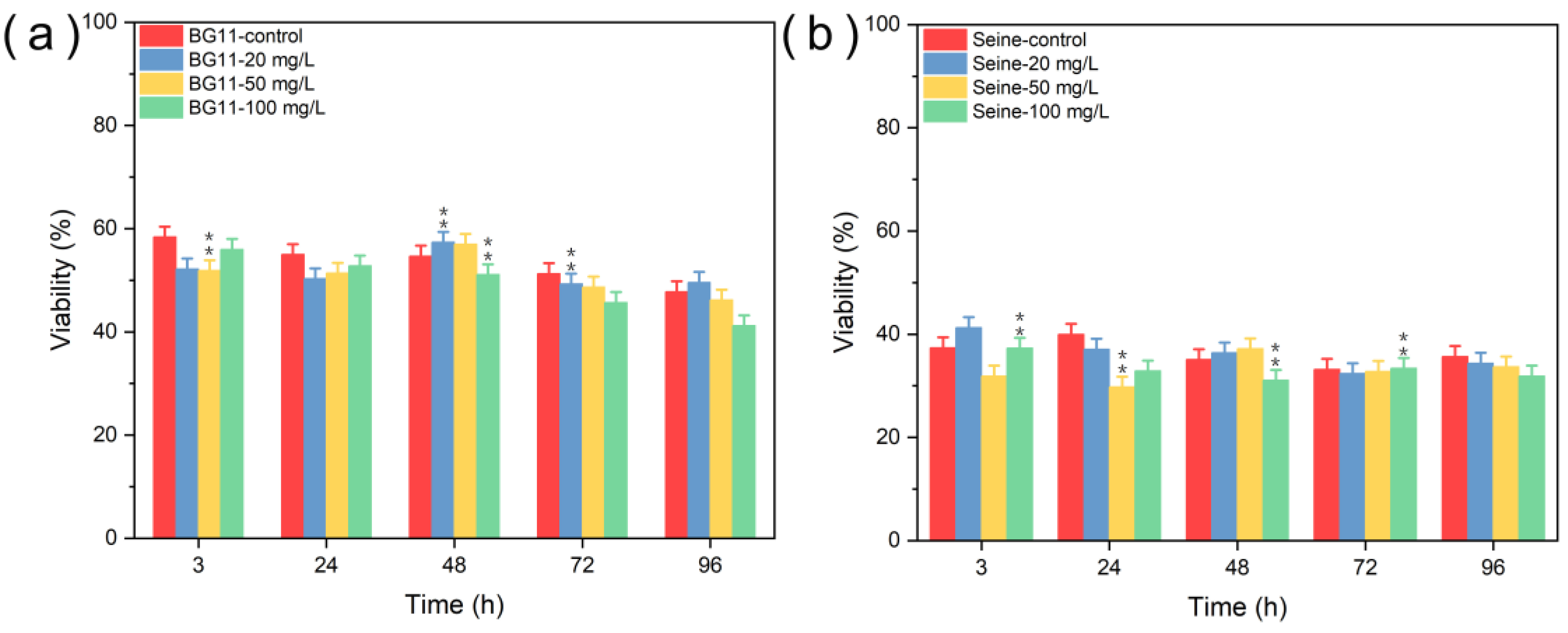
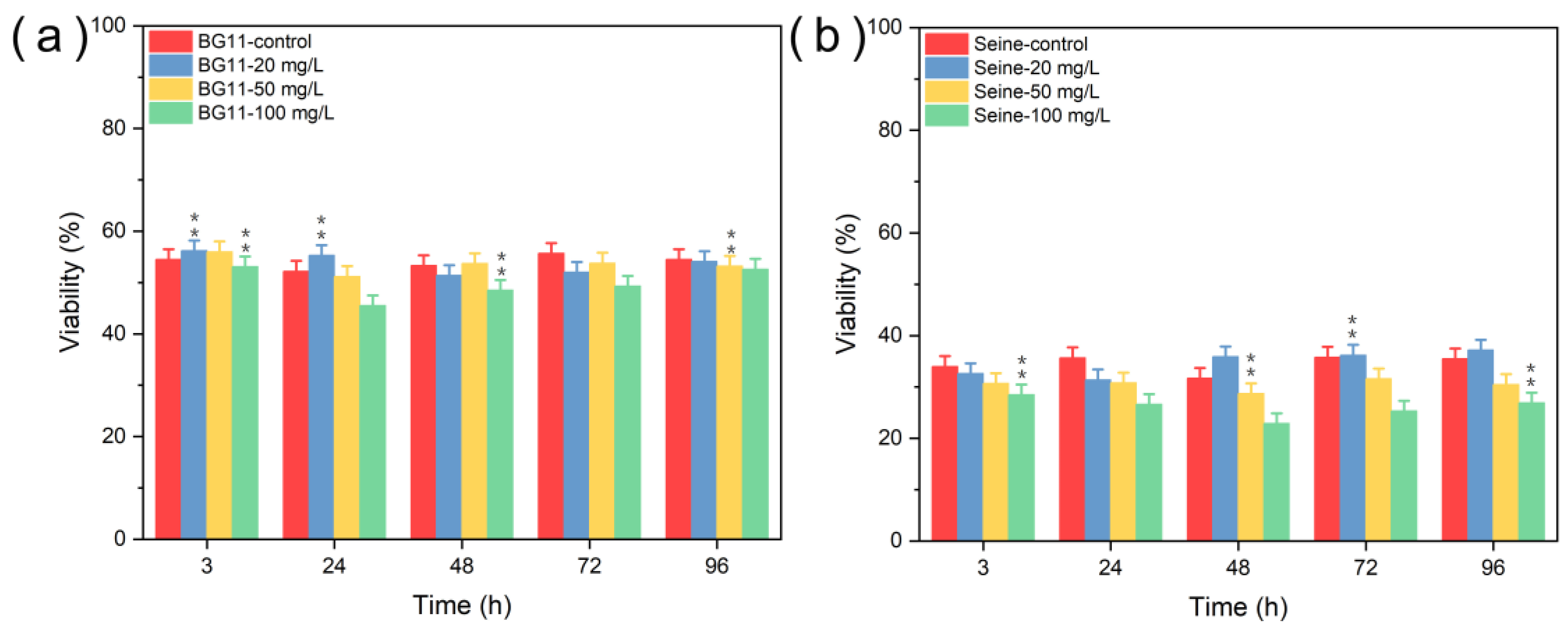
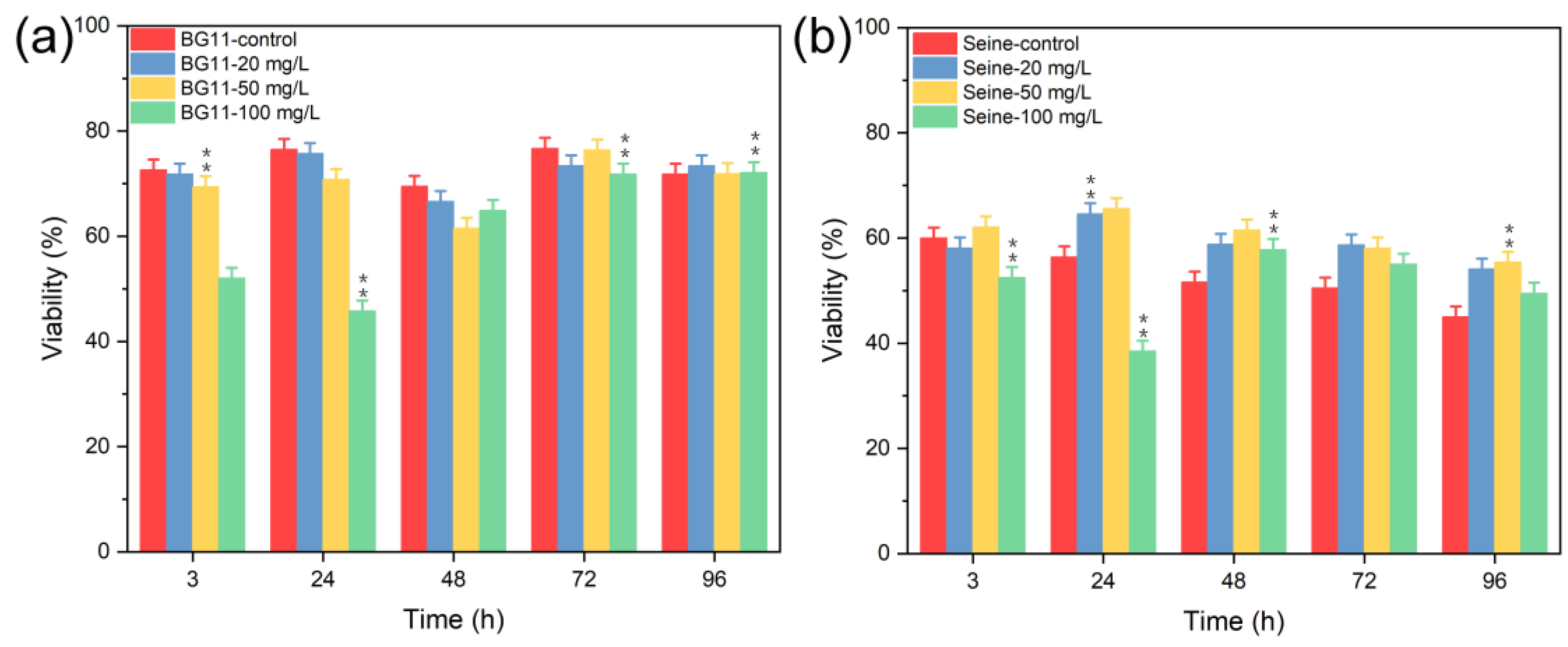
3.2.1. Toxicity of ZnS NPs
3.2.2. Toxicity of Mn2+
3.2.3. Toxicity of ZnS:Mn (10%) NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nel, A.; Xia, T. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B. The Behavior and Effects of Nanoparticles in the Environment. Environ. Pollut. 2009, 157, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Herron, N. Nanometer-Sized Semiconductor Clusters: Materials Synthesis, Quantum Size Effects, and Photophysical Properties. J. Phys. Chem. 1991, 95, 525–532. [Google Scholar] [CrossRef]
- Longo, A.V.; Notebaert, B.; Gaceur, M.; Patriarche, G.; Sciortino, A.; Cannas, M.; Messina, F.; von Bardeleben, H.J.; Battaglini, N.; Ammar, S. Photo-Activated Phosphorescence of Ultrafine ZnS:Mn Quantum Dots: On the Lattice Strain Contribution. J. Phys. Chem. C 2022, 126, 1531–1541. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Lin, Z.; Lin, L. Absorption and Luminescence of the Surface States in ZnS Nanoparticles. J. Appl. Phys. 1997, 82, 3111–3115. [Google Scholar] [CrossRef]
- Biswas, S.; Kar, S.; Chaudhuri, S. Optical and Magnetic Properties of Manganese-Incorporated Zinc Sulfide Nanorods Synthesized by a Solvothermal Process. J. Phys. Chem. B 2005, 109, 17526–17530. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Biswas, S.; Kar, S.; Chaudhuri, S. Multicolor Luminescence from Transition Metal Ion (Mn2+ and Cu2+) Doped ZnS Nanoparticles. J. Nanosci. Nanotech. 2007, 7, 3670–3676. [Google Scholar] [CrossRef]
- Mishra, P.; Ojha, K.S.; Khare, A. Structural and Optical Study of Copper-Doped Zinc Sulfide Nanoparticles. J. Appl. Spectrosc. 2018, 85, 743–748. [Google Scholar] [CrossRef]
- Manzoor, K.; Vadera, S.R.; Kumar, N.; Kutty, T.R.N. Multicolor Electroluminescent Devices Using Doped ZnS Nanocrystals. Appl. Phys. Lett. 2004, 84, 284–286. [Google Scholar] [CrossRef]
- Mei, S.; Zhang, G.; Yang, W.; Wei, X.; Zhang, W.; Zhu, J.; Guo, R. A Facile Route for Highly Efficient Color-Tunable Cu-Ga-Se/ZnSe Quantum Dots. Appl. Surf. Sci. 2018, 456, 876–881. [Google Scholar] [CrossRef]
- Wei, X.; Mei, S.; Zhang, G.; Su, D.; Xie, F.; Zhang, W.; Guo, R. Enhanced Tunable Dual Emission of Cu:InP/ZnS Quantum Dots Enabled by Introducing Ag Ions. Appl. Surf. Sci. 2019, 493, 605–612. [Google Scholar] [CrossRef]
- Chung, J.H.; Ah, C.S.; Jang, D.-J. Formation and Distinctive Decay Times of Surface- and Lattice-Bound Mn2+ Impurity Luminescence in ZnS Nanoparticles. J. Phys. Chem. B 2001, 105, 4128–4132. [Google Scholar] [CrossRef]
- Xu, S.J.; Chua, S.J.; Liu, B.; Gan, L.M.; Chew, C.H.; Xu, G.Q. Luminescence Characteristics of Impurities-Activated ZnS Nanocrystals Prepared in Microemulsion with Hydrothermal Treatment. Appl. Phys. Lett. 1998, 73, 478–480. [Google Scholar] [CrossRef]
- Rocha, A.D.; Sivry, Y.; Gelabert, A.; Beji, Z.; Benedetti, M.F.; Menguy, N.; Brayner, R. The Fate of Polyol-Made ZnO and CdS Nanoparticles in Seine River Water (Paris, France). J. Nanosci. Nanotechnol. 2015, 15, 3900–3908. [Google Scholar] [CrossRef] [PubMed]
- Planchon, M.; Jittawuttipoka, T.; Cassier-Chauvat, C.; Guyot, F.; Gelabert, A.; Benedetti, M.F.; Chauvat, F.; Spalla, O. Exopolysaccharides Protect Synechocystis against the Deleterious Effects of Titanium Dioxide Nanoparticles in Natural and Artificial Waters. J. Colloid Interface Sci. 2013, 405, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Planchon, M.; Léger, T.; Spalla, O.; Huber, G.; Ferrari, R. Metabolomic and Proteomic Investigations of Impacts of Titanium Dioxide Nanoparticles on Escherichia Coli. PLoS ONE 2017, 12, e0178437. [Google Scholar] [CrossRef] [PubMed]
- Kube, M.; Mohseni, A.; Fan, L.; Roddick, F. Impact of Alginate Selection for Wastewater Treatment by Immobilised Chlorella vulgaris. Chem. Eng. J. 2019, 358, 1601–1609. [Google Scholar] [CrossRef]
- Gao, F.; Cui, W.; Xu, J.-P.; Li, C.; Jin, W.-H.; Yang, H.-L. Lipid Accumulation Properties of Chlorella vulgaris and Scenedesmus Obliquus in Membrane Photobioreactor (MPBR) Fed with Secondary Effluent from Municipal Wastewater Treatment Plant. Renew. Energy 2019, 136, 671–676. [Google Scholar] [CrossRef]
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.; Ma, H.; Ma, A.; Terry, N. Effects of Nanoparticles on Algae: Adsorption, Distribution, Ecotoxicity and Fate. Appl. Sci. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on Cell Growth, Lipid Production and CO2 Addition of Microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Zhuang, D.; He, N.; Khoo, K.S.; Ng, E.-P.; Chew, K.W.; Ling, T.C. Application Progress of Bioactive Compounds in Microalgae on Pharmaceutical and Cosmetics. Chemosphere 2022, 291, 132932. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. A Global Approach of the Mechanism Involved in the Biosynthesis of Gold Colloids Using Micro-Algae. J. Nanopart. Res. 2014, 16, 2607. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K. ZnO Nanoparticles: Synthesis and Adsorption Study. Nat. Sci. 2009, 1, 129–135. [Google Scholar] [CrossRef]
- Pimprikar, P.S.; Joshi, S.S.; Kumar, A.R.; Zinjarde, S.S.; Kulkarni, S.K. Influence of Biomass and Gold Salt Concentration on Nanoparticle Synthesis by the Tropical Marine Yeast Yarrowia Lipolytica NCIM 3589. Colloids Surf. B Biointerfaces 2009, 74, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae Metabolites: A Rich Source for Food and Medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Mishra, B.; Tiwari, A.; Mahmoud, A.E.D. Microalgal Potential for Sustainable Aquaculture Applications: Bioremediation, Biocontrol, Aquafeed. Clean Technol. Environ. Policy 2023, 25, 675–687. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Mar. Drugs 2023, 21, 340. [Google Scholar] [CrossRef] [PubMed]
- Dziwulska, U.; Bajguz, A.; Godlewska-Żyłkiewicz, B. The Use of Algae Chlorella vulgaris Immobilized on Cellex-T Support for Separation/Preconcentration of Trace Amounts of Platinum and Palladium before GFAAS Determination. Anal. Lett. 2004, 37, 2189–2203. [Google Scholar] [CrossRef]
- Kaplan, D.; Heimer, Y.M.; Abeliovich, A.; Goldsbrough, P.B. Cadmium Toxicity and Resistance in Chlorella sp. Plant Sci. 1995, 109, 129–137. [Google Scholar] [CrossRef]
- Kim, J.; Lingaraju, B.P.; Rheaume, R.; Lee, J.-Y.; Siddiqui, K.F. Removal of Ammonia from Wastewater Effluent by Chlorella vulgaris. Tinshhua Sci. Technol. 2010, 15, 391–396. [Google Scholar] [CrossRef]
- Zhou, C.; Vitiello, V.; Pellegrini, D.; Wu, C.; Morelli, E.; Buttino, I. Toxicological Effects of CdSe/ZnS Quantum Dots on Marine Planktonic Organisms. Ecotoxicol. Environ. Saf. 2016, 123, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Ethiraj, K.R.; Chandrasekaran, N.; Mukherjee, A. Mitigating the Toxic Effects of CdSe Quantum Dots towards Freshwater Alga Scenedesmus Obliquus: Role of Eco-Corona. Environ. Pollut. 2021, 270, 116049. [Google Scholar] [CrossRef]
- Pikula, K.; Mintcheva, N.; Kulinich, S.A.; Zakharenko, A.; Markina, Z.; Chaika, V.; Orlova, T.; Mezhuev, Y.; Kokkinakis, E.; Tsatsakis, A.; et al. Aquatic Toxicity and Mode of Action of CdS and ZnS Nanoparticles in Four Microalgae Species. Environ. Res. 2020, 186, 109513. [Google Scholar] [CrossRef]
- Yu, Z.; Hao, R.; Zhang, L.; Zhu, Y. Effects of TiO2, SiO2, Ag and CdTe/CdS Quantum Dots Nanoparticles on Toxicity of Cadmium towards Chlamydomonas Reinhardtii. Ecotoxicol. Environ. Saf. 2018, 156, 75–86. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika Rajasree, S.R.; Kirubagaran, R. Evaluation of Zinc Oxide Nanoparticles Toxicity on Marine Algae Chlorella Vulgaris through Flow Cytometric, Cytotoxicity and Oxidative Stress Analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- Planchon, M.; Ferrari, R.; Guyot, F.; Gélabert, A.; Menguy, N.; Chanéac, C.; Thill, A.; Benedetti, M.F.; Spalla, O. Interaction between Escherichia Coli and TiO2 Nanoparticles in Natural and Artificial Waters. Colloids Surf. B Biointerfaces 2013, 102, 158–164. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Wang, Z.; Ye, N.; Fang, H.; Wang, D. TiO2, SiO2 and ZrO2 Nanoparticles Synergistically Provoke Cellular Oxidative Damage in Freshwater Microalgae. Nanomaterials 2018, 8, 95. [Google Scholar] [CrossRef]
- Lutterotti, L. MAUD Tutorial—Instrumental Broadening Determination. Available online: https://www.researchgate.net/publication/228868859_MAUD_tutorial-Instrumental_Broadening_Determination (accessed on 26 August 2023).
- Murugadoss, G. Synthesis and Photoluminescence Properties of Zinc Sulfide Nanoparticles Doped with Copper Using Effective Surfactants. Particuology 2013, 11, 566–573. [Google Scholar] [CrossRef]
- Sakthivel, P.; Prasanna Venkatesan, G.K.D.; Subramaniam, K.; Muthukrishnan, P. Structural, Optical, Photoluminescence and Electrochemical Behaviours of Mg, Mn Dual-Doped ZnS Quantum Dots. J. Mater. Sci. Mater. Electron. 2019, 30, 11984–11993. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Li, J.; Ma, T.-Y.; Liu, Y.-P.; Du, G.; Yuan, Z.-Y. Sonochemistry-Assisted Synthesis and Optical Properties of Mesoporous ZnS Nanomaterials. J. Mater. Chem. A 2014, 2, 1093–1101. [Google Scholar] [CrossRef]
- Do, Y.R.; Park, D.H.; Yang, H.G.; Park, W.; Wagner, B.K.; Yasuda, K.; Summers, C.J. Uniform Nanoscale SiO2 Encapsulation of ZnS Phosphors for Improved Aging Properties under Low Voltage Electron Beam Excitation. J. Electrochem. Soc. 2001, 148, G548–G551. [Google Scholar] [CrossRef]
- Labiadh, H.; Sellami, B.; Khazri, A.; Saidani, W.; Khemais, S. Optical Properties and Toxicity of Undoped and Mn-Doped ZnS Semiconductor Nanoparticles Synthesized through the Aqueous Route. Opt. Mater. 2017, 64, 179–186. [Google Scholar] [CrossRef]
- Zhou, D.-J.; Xie, X.-Y.; Zhang, Y.; Guo, D.-Y.; Zhou, Y.-J.; Xie, J.-F. Facile Synthesis of ZnS Nanorods in PEG and Their Spectral Performance. Mater. Res. Express 2016, 3, 105023. [Google Scholar] [CrossRef]
- Liu, W.; Chen, S. An Investigation of the Tribological Behaviour of Surface-Modified ZnS Nanoparticles in Liquid Paraffin. Wear 2000, 238, 120–124. [Google Scholar] [CrossRef]
- Geszke, M.; Murias, M.; Balan, L.; Medjahdi, G.; Korczynski, J.; Moritz, M.; Lulek, J.; Schneider, R. Folic Acid-Conjugated Core/Shell ZnS:Mn/ZnS Quantum Dots as Targeted Probes for Two Photon Fluorescence Imaging of Cancer Cells. Acta Biomater. 2011, 7, 1327–1338. [Google Scholar] [CrossRef]
- Kolmykov, O.; Coulon, J.; Lalevée, J.; Alem, H.; Medjahdi, G.; Schneider, R. Aqueous Synthesis of Highly Luminescent Glutathione-Capped Mn2+-Doped ZnS Quantum Dots. Mater. Sci. Eng. C 2014, 44, 17–23. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Hussein, M.Z.; Alitheen, N.B.; Abu, N. Folic Acid Targeted Mn:ZnS Quantum Dots for Theranostic Applications of Cancer Cell Imaging and Therapy. Int. J. Nanomed. 2016, 11, 413–428. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Goh, F.W.T.; Li, B.; Geng, D.; Hor, T.S.A.; Zong, Y.; Liu, Z. Manganese Oxide Catalyst Grown on Carbon Paper as an Air Cathode for High-Performance Rechargeable Zinc–Air Batteries. ChemPlusChem 2015, 80, 1341–1346. [Google Scholar] [CrossRef]
- Munk, M.; Brandão, H.M.; Nowak, S.; Mouton, L.; Gern, J.C.; Guimaraes, A.S.; Yéprémian, C.; Couté, A.; Raposo, N.R.B.; Marconcini, J.M.; et al. Direct and Indirect Toxic Effects of Cotton-Derived Cellulose Nanofibres on Filamentous Green Algae. Ecotoxicol. Environ. Saf. 2015, 122, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Ma, Y. Controlling Cellular Uptake of Nanoparticles with pH-Sensitive Polymers. Sci. Rep. 2013, 3, 2804. [Google Scholar] [CrossRef] [PubMed]
- Haniu, H.; Saito, N.; Matsuda, Y.; Tsukahara, T.; Maruyama, K.; Usui, Y.; Aoki, K.; Takanashi, S.; Kobayashi, S.; Nomura, H.; et al. Culture Medium Type Affects Endocytosis of Multi-Walled Carbon Nanotubes in BEAS-2B Cells and Subsequent Biological Response. Toxicol. In Vitro 2013, 27, 1679–1685. [Google Scholar] [CrossRef]
- Gaceur, M.; Giraud, M.; Hemadi, M.; Nowak, S.; Menguy, N.; Quisefit, J.P.; David, K.; Jahanbin, T.; Benderbous, S.; Boissière, M.; et al. Polyol-Synthesized Zn0.9Mn0.1S Nanoparticles as Potential Luminescent and Magnetic Bimodal Imaging Probes: Synthesis, Characterization, and Toxicity Study. J. Nanoparticle Res. 2012, 14, 1–5. [Google Scholar] [CrossRef]
- Jahanbin, T.; Gaceur, M.; Gros-Dagnac, H.; Benderbous, S.; Merah, S.A. High Potential of Mn-Doped ZnS Nanoparticles with Different Dopant Concentrations as Novel MRI Contrast Agents: Synthesis and in Vitro Relaxivity Studies. J. Nanoparticle Res. 2015, 17, 258. [Google Scholar] [CrossRef]
- da Rocha, A.; Menguy, N.; Yéprémian, C.; Couté, A.; Brayner, R. Ecotoxicological Studies of ZnO and CdS Nanoparticles on Chlorella vulgaris Photosynthetic Microorganism in Seine River Water. Nanomaterials 2020, 10, 227. [Google Scholar] [CrossRef]
- Furger, C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants 2021, 10, 944. [Google Scholar] [CrossRef]
- Mahana, A.; Guliy, O.I.; Mehta, S.K. Accumulation and Cellular Toxicity of Engineered Metallic Nanoparticle in Freshwater Microalgae: Current Status and Future Challenges. Ecotoxicol. Environ. Saf. 2021, 208, 111662. [Google Scholar] [CrossRef]
- Wang, X.; Liang, D.; Wang, Y.; Peijnenburg, W.J.G.M.; Monikh, F.A.; Zhao, X.; Dong, Z.; Fan, W. A Critical Review on the Biological Impact of Natural Organic Matter on Nanomaterials in the Aquatic Environment. Carbon Res. 2022, 1, 13. [Google Scholar] [CrossRef]
- Arvidsson, R.; Hansen, S.F.; Baun, A. Influence of Natural Organic Matter on the Aquatic Ecotoxicity of Engineered Nanoparticles: Recommendations for Environmental Risk Assessment. NanoImpact 2020, 20, 100263. [Google Scholar] [CrossRef]




| ×10−3 mol/L | Na+ | Mg2+ | Ca2+ | K+ | Cl− | Alkalinity | SiO2 | pH | DOC (mg/L) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seine River water | 0.429 | 0.189 | 2.366 | 0.077 | 0.617 | 3.807 | 0.334 | 0.382 | 0.06 | 8.05 | 2.445 |
| NPs | Zn | S | Mn | Mn/(Mn + Zn)exp | Mn/(Mn + Zn)XRF | |
|---|---|---|---|---|---|---|
| ZnS:Mn (0.5%) | mass (μg) | 32.315 | 11.034 | 0.048 | ||
| %mol | 58.9 | 41.0 | 0.10 | 0.005 | 0.002 | |
| ZnS:Mn (2.0%) | mass (μg) | 46.842 | 14.838 | 0.668 | ||
| %mol | 60.1 | 38.8 | 1.1 | 0.020 | 0.018 | |
| ZnS:Mn (4.0%) | mass (μg) | 22.464 | 8.427 | 0.427 | ||
| %mol | 55.9 | 42.8 | 1.3 | 0.040 | 0.023 | |
| ZnS:Mn (10%) | mass (μg) | 8.336 | 3.5 | 1.420 | ||
| %mol | 48.6 | 41.6 | 9.8 | 0.100 | 0.168 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, B.; Maaloul, R.; Nowak, S.; Sivry, Y.; Yéprémian, C.; Ammar, S.; Mammeri, F.; Brayner, R. Aquatic Fate and Ecotoxicology Effect of ZnS:Mn Quantum Dots on Chlorella vulgaris in Fresh Water. J. Xenobiot. 2024, 14, 467-483. https://doi.org/10.3390/jox14020028
Deng B, Maaloul R, Nowak S, Sivry Y, Yéprémian C, Ammar S, Mammeri F, Brayner R. Aquatic Fate and Ecotoxicology Effect of ZnS:Mn Quantum Dots on Chlorella vulgaris in Fresh Water. Journal of Xenobiotics. 2024; 14(2):467-483. https://doi.org/10.3390/jox14020028
Chicago/Turabian StyleDeng, Bingbing, Rania Maaloul, Sophie Nowak, Yann Sivry, Claude Yéprémian, Souad Ammar, Fayna Mammeri, and Roberta Brayner. 2024. "Aquatic Fate and Ecotoxicology Effect of ZnS:Mn Quantum Dots on Chlorella vulgaris in Fresh Water" Journal of Xenobiotics 14, no. 2: 467-483. https://doi.org/10.3390/jox14020028
APA StyleDeng, B., Maaloul, R., Nowak, S., Sivry, Y., Yéprémian, C., Ammar, S., Mammeri, F., & Brayner, R. (2024). Aquatic Fate and Ecotoxicology Effect of ZnS:Mn Quantum Dots on Chlorella vulgaris in Fresh Water. Journal of Xenobiotics, 14(2), 467-483. https://doi.org/10.3390/jox14020028









