Prenatal Exposure to Bisphenol A and/or Diethylhexyl Phthalate Impacts Brain Monoamine Levels in Rat Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
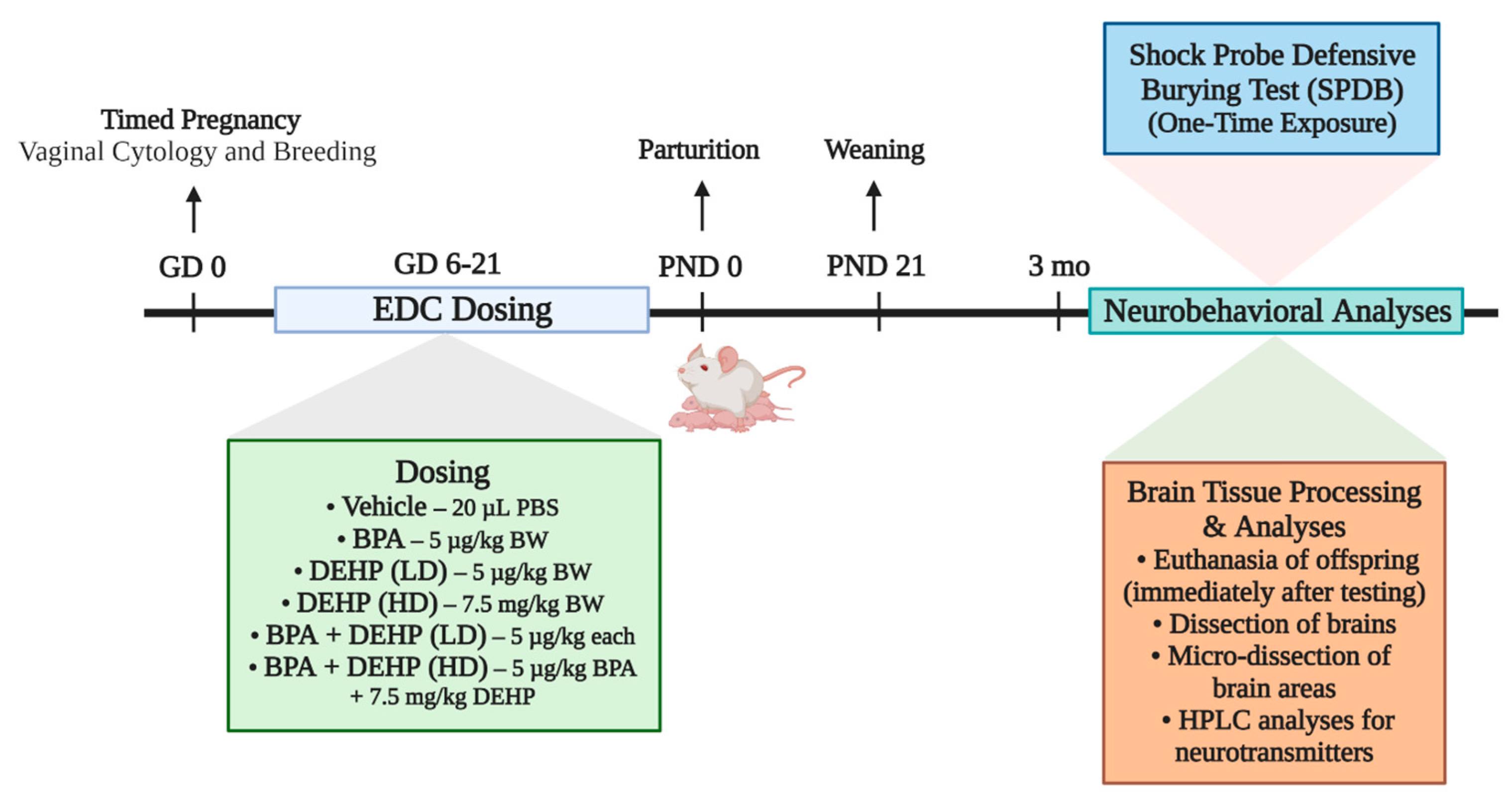
2.3. Experimental Design
2.4. Shock Probe Defensive Burying Test (SPDB)
2.5. Tissue Collection and Preparation
2.6. Brain Sectioning, Microdissection, and Neurotransmitter Analysis
2.7. Neurotransmitter Analysis by HPLC-EC
2.8. Statistical Analysis
3. Results
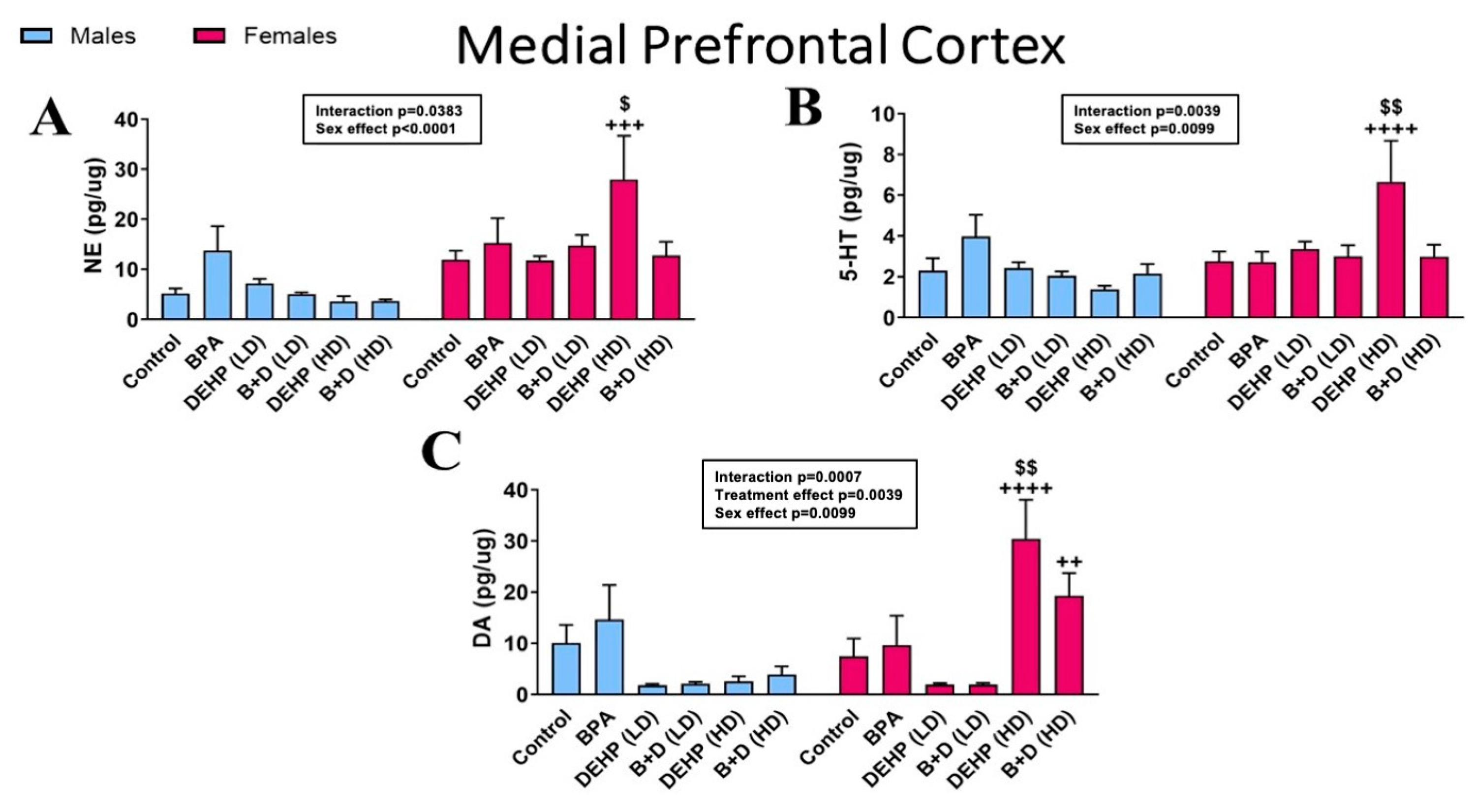
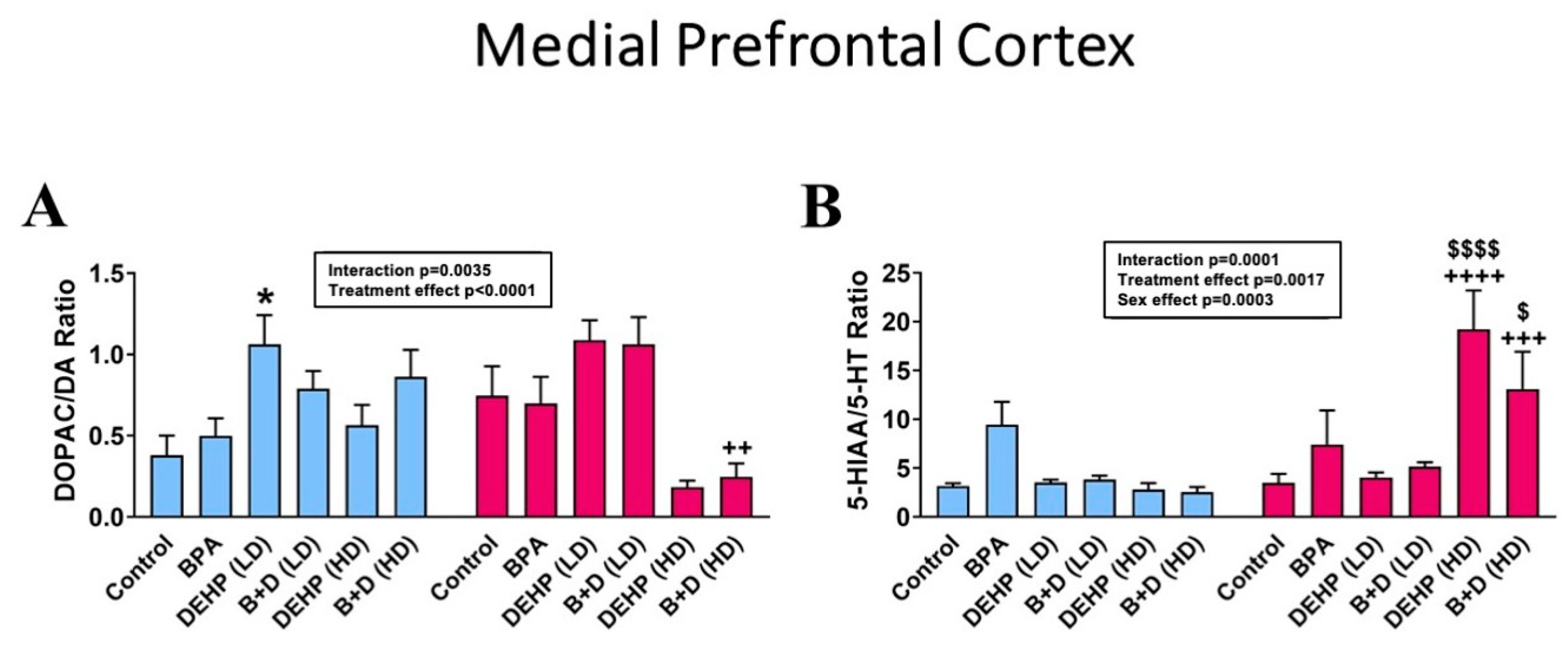
3.1. Medial Prefrontal Cortex
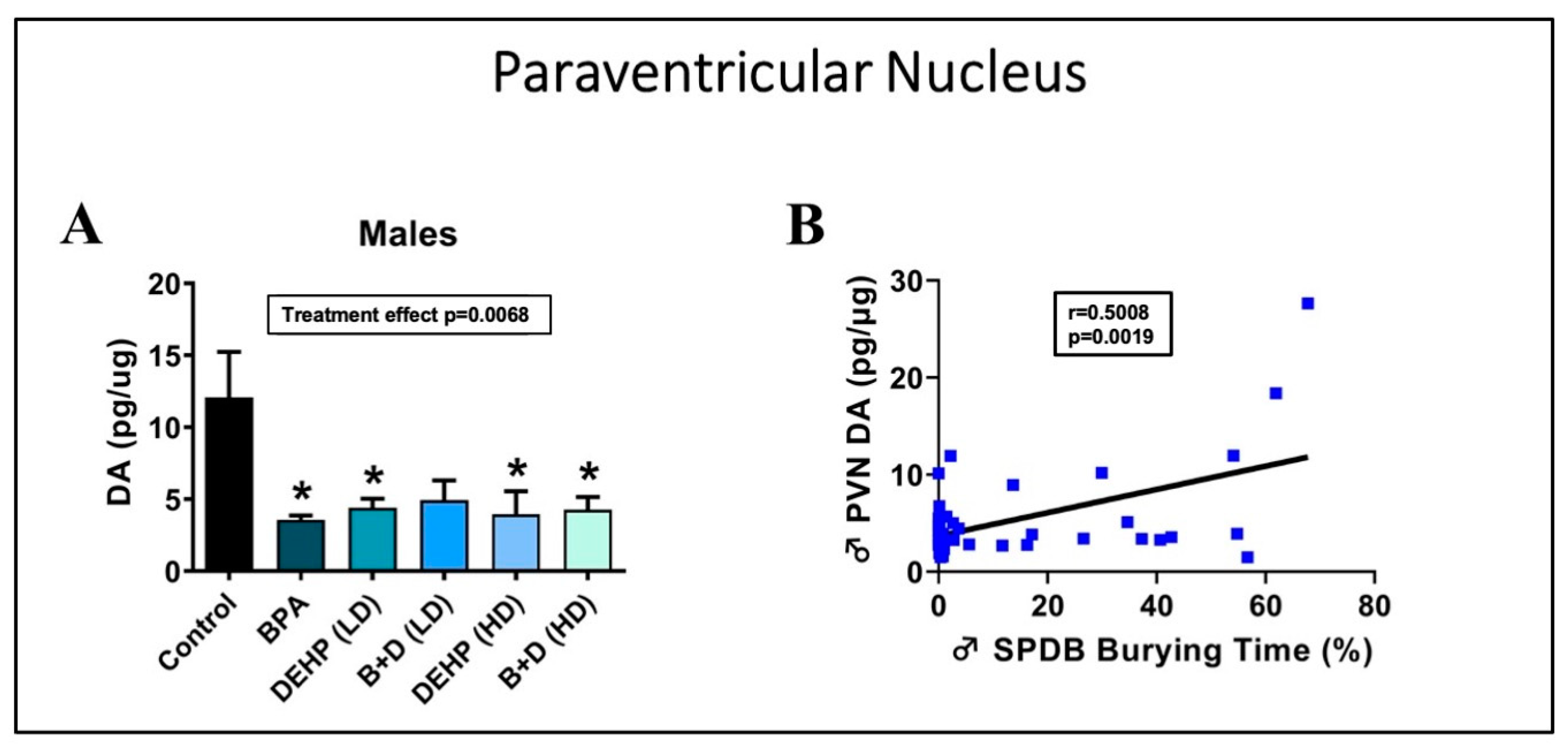
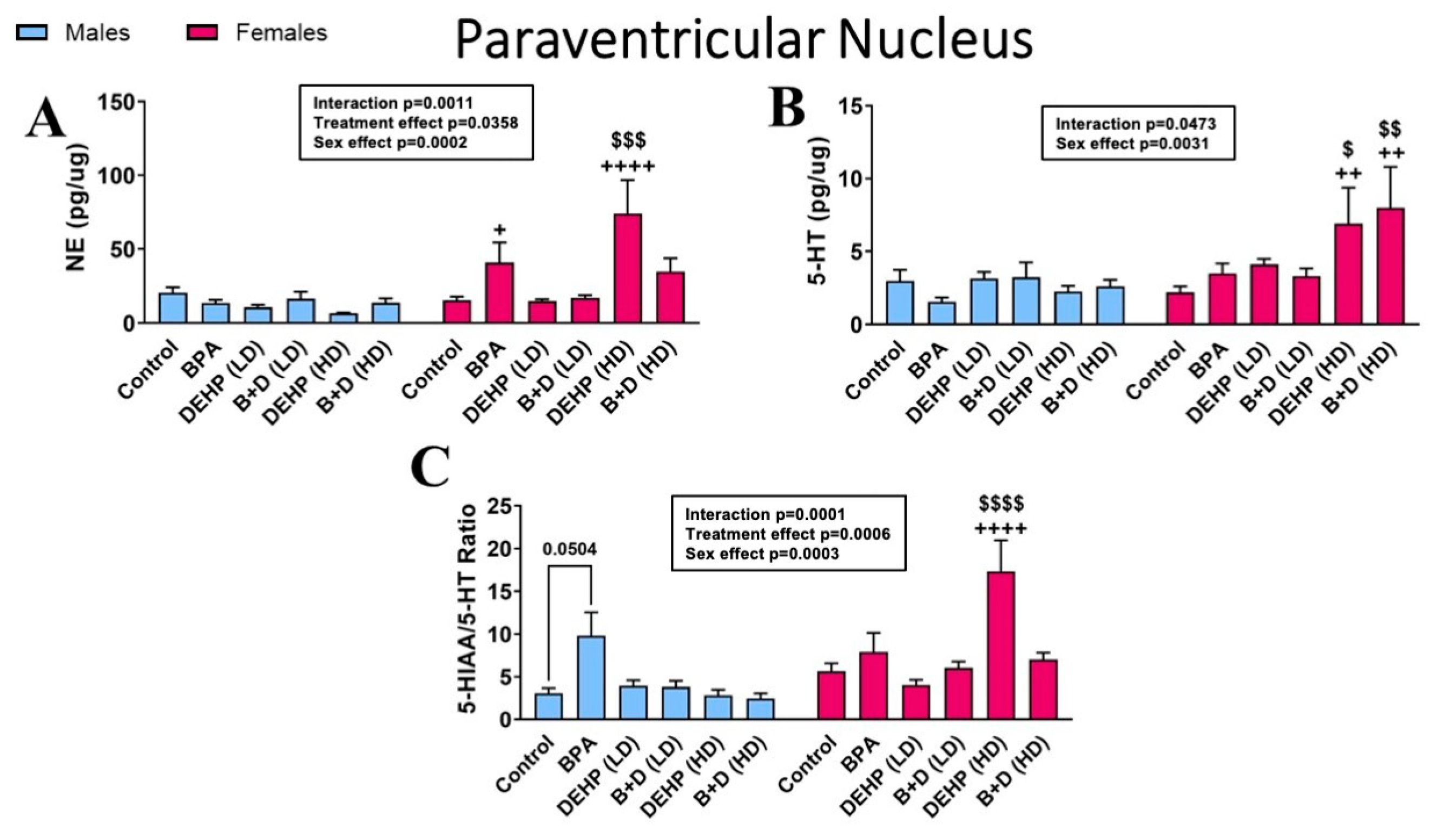
3.2. Paraventricular Nucleus of the Hypothalamus
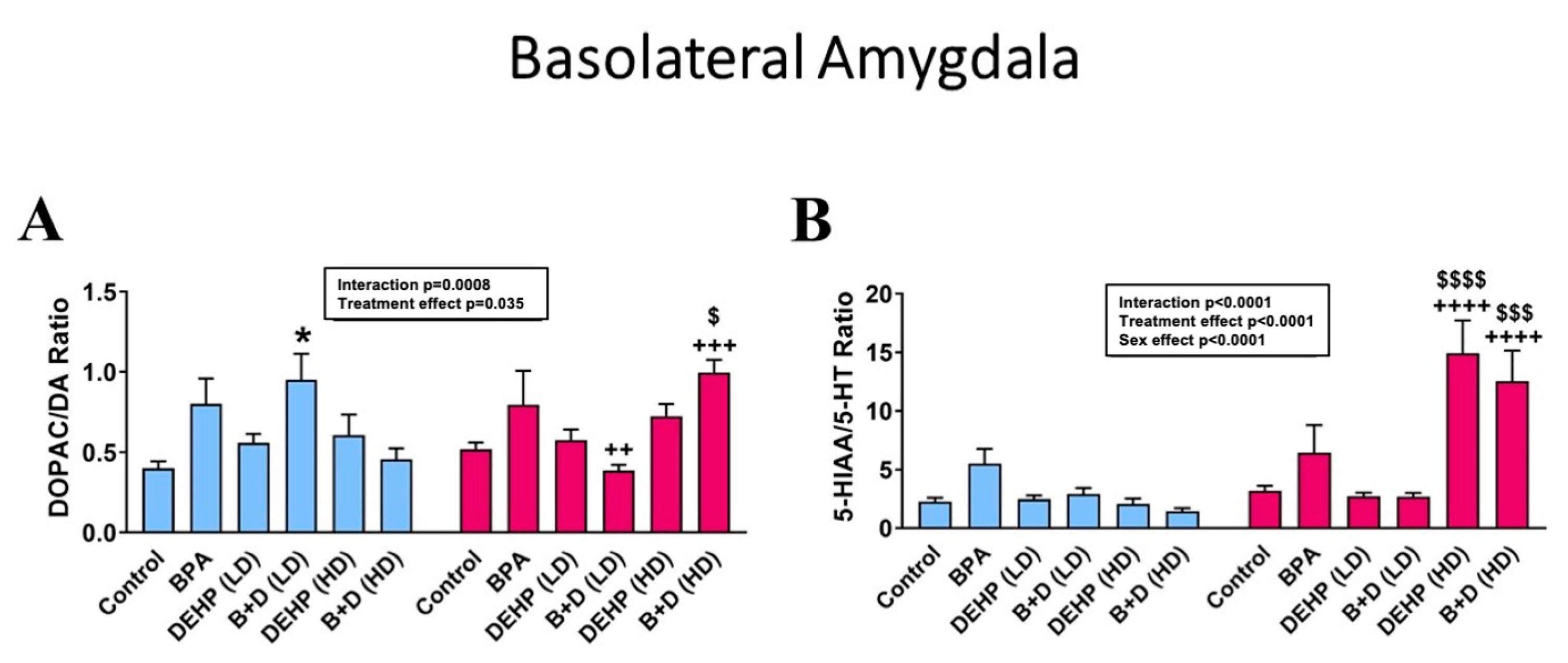
3.3. Basolateral Amygdala
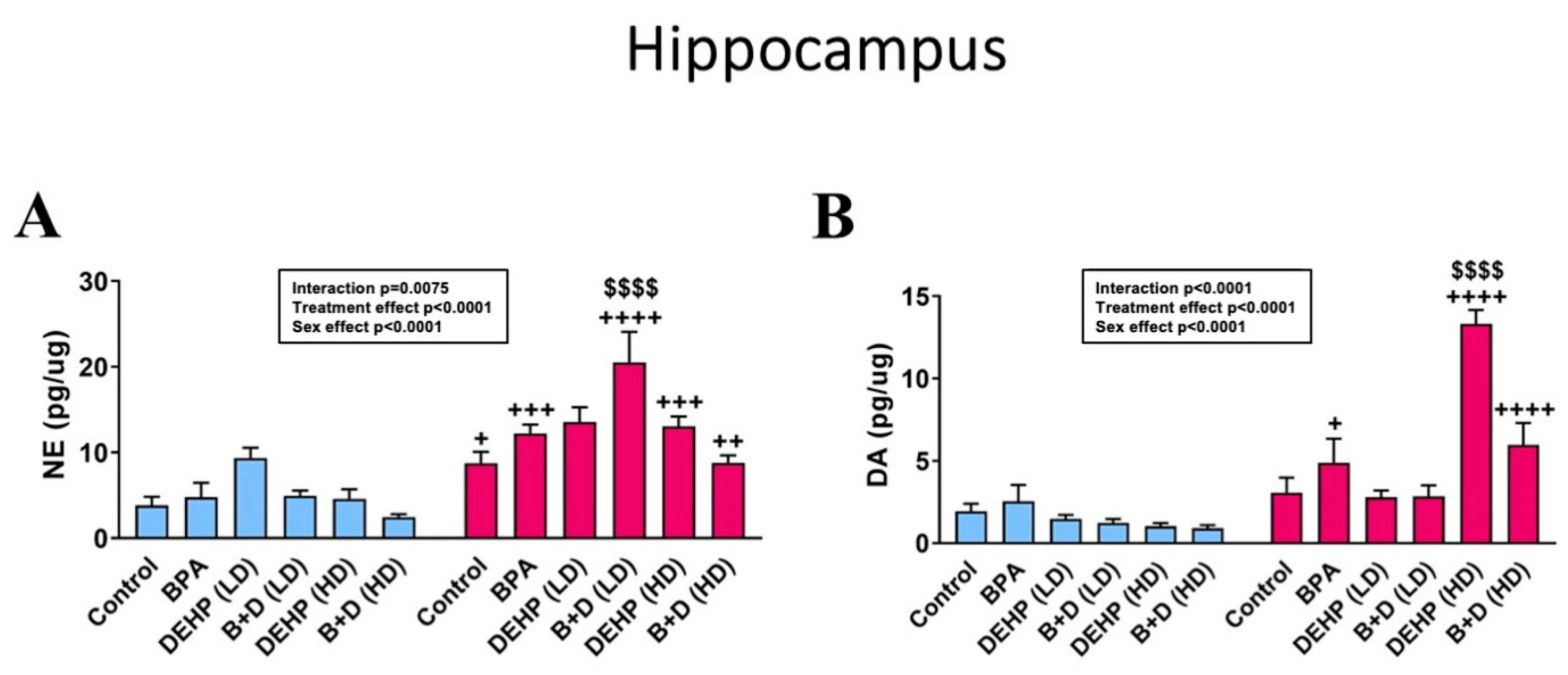
3.4. Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duenas-Moreno, J.; Mora, A.; Cervantes-Aviles, P.; Mahlknecht, J. Groundwater contamination pathways of phthalates and bisphenol A: Origin, characteristics, transport, and fate—A review. Environ. Int. 2022, 170, 107550. [Google Scholar] [CrossRef] [PubMed]
- Kajta, M.; Wójtowicz, A.K. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol. Rep. 2013, 65, 1632–1639. (In English) [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; Pérez-Lobato, R.; Olea, N.; Fernández, M.F. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology 2015, 49, 174–184. (In English) [Google Scholar] [CrossRef] [PubMed]
- Altemus, M.; Sarvaiya, N.; Epperson, C.N. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014, 35, 320–330. (In English) [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.L.; Baio, J.; Van Naarden Braun, K.; Bilder, D.; Charles, J.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016, 65, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ramtekkar, U.P.; Reiersen, A.M.; Todorov, A.A.; Todd, R.D. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: Implications for DSM-V and ICD-11. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 217–228.e3. (In English) [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Henare, K.; Thorstensen, E.B.; Ponnampalam, A.P.; Mitchell, M.D. Transfer of bisphenol A across the human placenta. Am. J. Obstet. Gynecol. 2010, 202, 393.e1–393.e7. (In English) [Google Scholar] [CrossRef] [PubMed]
- Singh, A.R.; Lawrence, W.H.; Autian, J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J. Pharm. Sci. 1975, 64, 1347–1350. (In English) [Google Scholar] [CrossRef]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. (In English) [Google Scholar] [CrossRef]
- Nesan, D.; Kurrasch, D.M. Gestational Exposure to Common Endocrine Disrupting Chemicals and Their Impact on Neurodevelopment and Behavior. Annu. Rev. Physiol. 2020, 82, 177–202. (In English) [Google Scholar] [CrossRef]
- Rebuli, M.E.; Patisaul, H.B. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J. Steroid Biochem. Mol. Biol. 2016, 160, 148–159. (In English) [Google Scholar] [CrossRef]
- Biemann, R.; Fischer, B.; Santos, A.N. Adipogenic effects of a combination of the endocrine-disrupting compounds bisphenol A, diethylhexylphthalate, and tributyltin. Obes. Facts. 2014, 7, 48–56. (In English) [Google Scholar] [CrossRef]
- Suteau, V.; Briet, C.; Lebeault, M.; Gourdin, L.; Henrion, D.; Rodien, P.; Munier, M. Human amniotic fluid-based exposure levels of phthalates and bisphenol A mixture reduce INSL3/RXFP2 signaling. Environ. Int. 2020, 138, 105585. (In English) [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. (In English) [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Guo, W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018, 9, 2201. (In English) [Google Scholar] [CrossRef]
- Ruhé, H.G.; Mason, N.S.; Schene, A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol. Psychiatry 2007, 12, 331–359. (In English) [Google Scholar] [CrossRef]
- Honma, T.; Miyagawa, M.; Suda, M.; Wang, R.S.; Kobayashi, K.; Sekiguchi, S. Effects of perinatal exposure to bisphenol A on brain neurotransmitters in female rat offspring. Ind. Health 2006, 44, 510–524. (In English) [Google Scholar] [CrossRef]
- Nakamura, K.; Itoh, K.; Yoshimoto, K.; Sugimoto, T.; Fushiki, S. Prenatal and lactational exposure to low-doses of bisphenol A alters brain monoamine concentration in adult mice. Neurosci Lett. 2010, 484, 66–70. (In English) [Google Scholar] [CrossRef]
- Ogi, H.; Itoh, K.; Ikegaya, H.; Fushiki, S. Alterations of neurotransmitter norepinephrine and gamma-aminobutyric acid correlate with murine behavioral perturbations related to bisphenol A exposure. Brain Dev. 2015, 37, 739–746. (In English) [Google Scholar] [CrossRef]
- Castro, B.; Sánchez, P.; Miranda, M.T.; Torres, J.M.; Ortega, E. Identification of dopamine- and serotonin-related genes modulated by bisphenol A in the prefrontal cortex of male rats. Chemosphere 2015, 139, 235–239. (In English) [Google Scholar] [CrossRef]
- Xin, F.; Fischer, E.; Krapp, C.; Krizman, E.N.; Lan, Y.; Mesaros, C.; Snyder, N.W.; Bansal, A.; Robinson, M.B.; Simmons, R.A.; et al. Mice exposed to bisphenol A exhibit depressive-like behavior with neurotransmitter and neuroactive steroid dysfunction. Horm. Behav. 2018, 102, 93–104. (In English) [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, J.; Wu, L.; Lu, H.; Wang, Z.; Yu, P.; Xiao, H.; Gao, R.; Yu, J. Perinatal exposure to bisphenol A causes a disturbance of neurotransmitter metabolic pathways in female mouse offspring: A focus on the tryptophan and dopamine pathways. Chemosphere 2020, 254, 126715. (In English) [Google Scholar] [CrossRef]
- Ponzi, D.; Gioiosa, L.; Parmigiani, S.; Palanza, P. Effects of Prenatal Exposure to a Low-Dose of Bisphenol A on Sex Differences in Emotional Behavior and Central Alpha(2)-Adrenergic Receptor Binding. Int. J. Mol. Sci. 2020, 21, 3269. (In English) [Google Scholar] [CrossRef] [PubMed]
- Tando, S.; Itoh, K.; Yaoi, T.; Ogi, H.; Goto, S.; Mori, M.; Fushiki, S. Bisphenol A exposure disrupts the development of the locus coeruleus-noradrenergic system in mice. Neuropathology 2014, 34, 527–534. (In English) [Google Scholar] [CrossRef]
- Hatcher, K.M.; Willing, J.; Chiang, C.; Rattan, S.; Flaws, J.A.; Mahoney, M.M. Exposure to di-(2-ethylhexyl) phthalate transgenerationally alters anxiety-like behavior and amygdala gene expression in adult male and female mice. Physiol. Behav. 2019, 207, 7–14. (In English) [Google Scholar] [CrossRef] [PubMed]
- Yirun, A.; Ozkemahli, G.; Balci, A.; Erkekoglu, P.; Zeybek, N.D.; Yersal, N.; Kocer-Gumusel, B. Neuroendocrine disruption by bisphenol A and/or di(2-ethylhexyl) phthalate after prenatal, early postnatal and lactational exposure. Environ. Sci. Pollut. Res. Int. 2021, 28, 26961–26974. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kaimal, A.; Hooversmith, J.M.; Cherry, A.D.; Garrity, J.T.; Al Mansi, M.H.; Martin, N.M.; Buechter, H.; Holmes, P.V.; MohanKumar, P.S.; MohanKumar, S.M.J. Prenatal exposure to bisphenol A and/or diethylhexyl phthalate alters stress responses in rat offspring in a sex- and dose-dependent manner. Front. Toxicol. 2023, 5, 1264238. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Bisphenol A Action Plan (CASRN 80-05-7); 2010. Available online: https://iris.epa.gov/static/pdfs/0356_summary.pdf (accessed on 16 March 2020).
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food Exposure and Impact on Human Health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Lakind, J.S.; Naiman, D.Q. Bisphenol A (BPA) daily intakes in the United States: Estimates from the 2003-2004 NHANES urinary BPA data. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 608–615. (In English) [Google Scholar] [CrossRef] [PubMed]
- Blystone, C.R.; Kissling, G.E.; Bishop, J.B.; Chapin, R.E.; Wolfe, G.W.; Foster, P.M. Determination of the di-(2-ethylhexyl) phthalate NOAEL for reproductive development in the rat: Importance of the retention of extra animals to adulthood. Toxicol. Sci. 2010, 116, 640–646. (In English) [Google Scholar] [CrossRef]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Brüning, T. Assessing exposure to phthalates—The human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. (In English) [Google Scholar] [CrossRef]
- Environmental Protection Agency. Integrated Risk Information System (IRIS) Chemical Assessment Summary: Di(2-ethylhexyl)phthalate (DEHP); CASRN 117-81-7. 1987. Available online: https://iris.epa.gov/static/pdfs/0014_summary.pdf (accessed on 16 March 2020).
- Simone, J.; Bogue, E.A.; Bhatti, D.L.; Day, L.E.; Farr, N.A.; Grossman, A.M.; Holmes, P.V. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology 2015, 62, 265–278. (In English) [Google Scholar] [CrossRef]
- Palkovits, M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973, 59, 449–450. (In English) [Google Scholar] [CrossRef]
- Balasubramanian, P.; Subramanian, M.; Nunez, J.L.; Mohankumar, S.M.; Mohankumar, P.S. Chronic estradiol treatment decreases brain derived neurotrophic factor (BDNF) expression and monoamine levels in the amygdala–implications for behavioral disorders. Behav. Brain Res. 2014, 261, 127–133. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. (In English) [Google Scholar] [CrossRef]
- Malikowska-Racia, N.; Sałat, K.; Nowaczyk, A.; Fijałkowski, Ł.; Popik, P. Dopamine D2/D3 receptor agonists attenuate PTSD-like symptoms in mice exposed to single prolonged stress. Neuropharmacology 2019, 155, 1–9. (In English) [Google Scholar] [CrossRef] [PubMed]
- Linnet, J. The anticipatory dopamine response in addiction: A common neurobiological underpinning of gambling disorder and substance use disorder? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 98, 109802. (In English) [Google Scholar] [CrossRef]
- Vaessen, T.; Hernaus, D.; Myin-Germeys, I.; van Amelsvoort, T. The dopaminergic response to acute stress in health and psychopathology: A systematic review. Neurosci. Biobehav. Rev. 2015, 56, 241–251. [Google Scholar] [CrossRef]
- Boyce, P.J.; Finlay, J.M. Neonatal depletion of cortical dopamine: Effects on dopamine turnover and motor behavior in juvenile and adult rats. Brain Res. Dev. Brain Res. 2005, 156, 167–175. (In English) [Google Scholar] [CrossRef]
- George, T.P.; Verrico, C.D.; Roth, R.H. Effects of repeated nicotine pre-treatment on mesoprefrontal dopaminergic and behavioral responses to acute footshock stress. Brain Res. 1998, 801, 36–49. (In English) [Google Scholar] [CrossRef]
- Dunn, A.J.; Berridge, C.W. Corticotropin-releasing factor administration elicits a stress-like activation of cerebral catecholaminergic systems. Pharmacol. Biochem. Behav. 1987, 27, 685–691. (In English) [Google Scholar] [CrossRef] [PubMed]
- Olguín, H.J.; Guzmán, D.C.; García, E.H.; Mejía, G.B. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 9730467. (In English) [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. (In English) [Google Scholar] [CrossRef] [PubMed]
- Feenstra, M.G.; Kalsbeek, A.; van Galen, H. Neonatal lesions of the ventral tegmental area affect monoaminergic responses to stress in the medial prefrontal cortex and other dopamine projection areas in adulthood. Brain Res. 1992, 596, 169–182. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Feenstra, M.G.; van Galen, H.; Uylings, H.B. Monoamine and metabolite levels in the prefrontal cortex and the mesolimbic forebrain following neonatal lesions of the ventral tegmental area. Brain Res. 1989, 479, 339–343. (In English) [Google Scholar] [CrossRef]
- Molina-Holgado, E.; Dewar, K.M.; Grondin, L.; van Gelder, N.M.; Reader, T.A. Amino acid levels and gamma-aminobutyric acidA receptors in rat neostriatum, cortex, and thalamus after neonatal 6-hydroxydopamine lesion. J. Neurochem. 1993, 60, 936–945. (In English) [Google Scholar] [CrossRef] [PubMed]
- Luu, B.E.; Green, S.R.; Childers, C.L.; Holahan, M.R.; Storey, K.B. The roles of hippocampal microRNAs in response to acute postnatal exposure to di(2-ethylhexyl) phthalate in female and male rats. Neurotoxicology 2017, 59, 98–104. (In English) [Google Scholar] [CrossRef]
- Smith, C.A.; Farmer, K.; Lee, H.; Holahan, M.R.; Smith, J.C. Altered Hippocampal Lipid Profile Following Acute Postnatal Exposure to Di(2-Ethylhexyl) Phthalate in Rats. Int. J. Environ. Res. Public Health 2015, 12, 13542–13559. (In English) [Google Scholar] [CrossRef] [PubMed]
- Heidbreder, C.A.; Weiss, I.; Domeney, A.; Pryce, C.; Homberg, J.; Hedou, G.; Feldon, J.; Moran, M.; Nelson, P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 2000, 100, 749–768. (In English) [Google Scholar] [CrossRef]
- Sullivan, R.M.; Szechtman, H. Asymmetrical influence of mesocortical dopamine depletion on stress ulcer development and subcortical dopamine systems in rats: Implications for psychopathology. Neuroscience 1995, 65, 757–766. (In English) [Google Scholar] [CrossRef]
- Abercrombie, E.D.; Keller, R.W., Jr.; Zigmond, M.J. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: Pharmacological and behavioral studies. Neuroscience 1988, 27, 897–904. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hansen, N. The Longevity of Hippocampus-Dependent Memory Is Orchestrated by the Locus Coeruleus-Noradrenergic System. Neural Plast. 2017, 2017, 2727602. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lovick, T.A.; Zangrossi, H., Jr. Effect of Estrous Cycle on Behavior of Females in Rodent Tests of Anxiety. Front. Psychiatry 2021, 12, 711065. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.M.; Hernandez-Saavedra, D.; Boas, S.M.; Pan, Y.X.; Juraska, J.M. Perinatal High-Fat Diet and Bisphenol A: Effects on Behavior and Gene Expression in the Medial Prefrontal Cortex. Dev. Neurosci. 2019, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Al-Harbi, N.O.; Ahmad, S.F.; Alhazzani, K.; Attia, S.M.; Alsanea, S.; Alhoshani, A.; Mahmood, H.M.; Alfardan, A.S.; Bakheet, S.A. Exposure to the plasticizer, Di-(2-ethylhexyl) phthalate during juvenile period exacerbates autism-like behavior in adult BTBR T + tf/J mice due to DNA hypomethylation and enhanced inflammation in brain and systemic immune cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110249. [Google Scholar] [CrossRef] [PubMed]
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 2015, 74, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; McArthur, S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 2010, 62, 155–198. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.A.; Toledo, J.; Sosa, L.d.V.; Peinetti, N.; Torres, A.I.; De Paul, A.L.; Gutiérrez, S. The phthalate DEHP modulates the estrogen receptors alpha and beta increasing lactotroph cell population in female pituitary glands. Chemosphere 2020, 258, 127304. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Mele, E.; Marino, M.; Viggiano, A.; Nori, S.L.; Meccariello, R. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. Int. J. Mol. Sci. 2021, 22, 972. [Google Scholar] [CrossRef]
- Ernst, J.; Grabiec, U.; Falk, K.; Dehghani, F.; Schaedlich, K. The endocrine disruptor DEHP and the ECS: Analysis of a possible crosstalk. Endocr. Connect. 2020, 9, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Loganathan, N.; Belsham, D.D. Bisphenol A induces Pomc gene expression through neuroinflammatory and PPARgamma nuclear receptor-mediated mechanisms in POMC-expressing hypothalamic neuronal models. Mol. Cell. Endocrinol. 2019, 479, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.K.; Sitarz-Glownia, A.M.; Wnuk, A.; Kajta, M.; Szychowski, K.A. Involvement of the peroxisome proliferator-activated receptor gamma (Ppargamma) and matrix metalloproteinases-2 and -9 (Mmp-2 and -9) in the mechanism of action of di(2-ethylhexyl)phthalate (DEHP) in cultured mouse brain astrocytes and neurons. Toxicol. Vitr. 2023, 92, 105639. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Morita, M.; Sugimoto, M.; Imanishi, S.; Manabe, N. Effects of in utero exposure to bisphenol A on mRNA expression of arylhydrocarbon and retinoid receptors in murine embryos. J. Reprod. Dev. 2005, 51, 315–324. [Google Scholar] [CrossRef]
- Wojtowicz, A.K.; Sitarz-Glownia, A.M.; Szczesna, M.; Szychowski, K.A. The Action of Di-(2-Ethylhexyl) Phthalate (DEHP) in Mouse Cerebral Cells Involves an Impairment in Aryl Hydrocarbon Receptor (AhR) Signaling. Neurotox. Res. 2019, 35, 183–195. [Google Scholar] [CrossRef]
| Treatment | Dose | Number of Animals Per Group |
|---|---|---|
| Control | 20 μL Phosphate Buffered Saline (PBS) | 7 |
| BPA | 5 μg/kg BW/day | 7 |
| DEHP low dose (LD) | 5 μg/kg BW/day | 6 |
| DEHP high dose (HD) | 7.5 mg/kg BW/day | 6 |
| BPA + LD-DEHP | 5 μg/kg/day of BPA + 5 μg/kg/day of DEHP | 6 |
| BPA + HD-DEHP | 5 μg/kg/day of BPA + 7.5 mg/kg/day of DEHP | 7 |
| Neurotransmitter | Control | BPA (5 μg) | DEHP (5 μg) | BPA + 5 μg DEHP | DEHP (7.5 mg) | BPA + 7.5 mg DEHP | Sex Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | ||
| Medial Prefrontal Cortex | |||||||||||||
| DOPAC (pg/μg) | 2.0 ± 0.5 | 1.9 ± 0.2 | 4.7 ± 1.7 + | 2.3 ± 0.3 + | 1.6 ± 0.1 | 2.0 ± 0.2 | 1.7 ± 0.3 | 2.1 ± 0.7 | 1.2 ± 0.4 ++ | 4.5 ± 0.6 ++ | 2.4 ± 0.7 | 2.9 ± 0.5 | NS |
| 5-HIAA (pg/μg) | 7.1 ± 1.8 | 8.0 ± 1.9 | 10.5 ± 2.2 | 32.0 ± 17.1 | 7.5 ± 0.6 | 11.3 ± 0.7 | 7.1 ± 0.9 | 13.8 ± 1.8 | 6.7 ± 3.0 ++++ | 109.2 ± 28.0 $$$$,++++ | 4.1 ± 0.3 + | 37.1 ± 13.2 + | p < 0.0001 |
| Paraventricular Nucleus | |||||||||||||
| DA (pg/μg) | 12.1 ± 3.2 | 5.8 ± 0.9 | 3.6 ± 0.3 | 12.7 ± 4.2 | 4.4 ± 0.6 | 7.2 ± 0.7 | 4.9 ± 1.4 | 6.2 ± 0.8 | 4.0 ± 1.6 ++ | 25.8 ± 5.9 ++ | 4.3 ± 0.9 ++++ | 31.2 ± 13.9 $$,++++ | p = 0.0008 |
| DOPAC (pg/μg) | 1.9 ± 0.2 | 1.4 ± 0.4 | 1.7 ± 0.5 | 2.9 ± 1.2 | 1.1 ± 0.1 | 1.3 ± 0.4 | 1.3 ± 0.2 | 1.1 ± 0.3 | 0.9 ± 0.1 ++++ | 6.0 ± 2.2 $$$,++++ | 1.5 ± 0.2 | 1.7 ± 0.5 | p = 0.0310 |
| 5-HIAA (pg/μg) | 8.7 ± 1.5 | 11.0 ± 1.8 | 11.8 ± 1.8 | 24.4 ± 6.2 | 11.3 ± 0.8 | 15.9 ± 1.4 | 10.4 ± 1.6 | 18.2 ± 1.0 | 4.1 ± 0.3 ++++ | 103.6 ± 42.4 $$$,++++ | 5.1 ± 0.4 ++ | 63.0 ± 30.2 ++ | p = 0.0006 |
| DOPAC/DA ratio | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.5 ± 0.1 + | 0.3 ± 0.1 + | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.4 ± 0.1 ++ | 0.1 ± 0.0 ++ | p = 0.0002 |
| Basolateral Amygdala | |||||||||||||
| NE (pg/μg) | 7.7 ± 2.2 + | 13.7 ± 1.8 + | 7.8 ± 1.8 ++++ | 22.0 ± 2.2 ++++ | 9.9 ± 0.5 + | 15.3 ± 1.2 + | 7.2 ± 0.7 +++ | 17.6 ± 2.3 +++ | 6.7 ± 1.3 ++++ | 20.6 ± 2.3 ++++ | 5.8 ± 1.0 +++ | 14.7 ± 2.7 +++ | p < 0.0001 |
| DA (pg/μg) | 8.7 ± 1.7 | 12.4 ± 1.6 | 6.2 ± 1.3 +++ | 16.2 ± 2.5+++ | 15.2 ± 2.5 | 15.1 ± 1.6 | 7.3 ± 1.0 ++ | 15.5 ± 1.6 ++ | 7.2 ± 1.9 +++ | 18.5 ± 0.8 +++ | 5.5 ± 1.4 ++ | 13.6 ± 3.3 ++ | p < 0.0001 |
| 5-HT (pg/μg) | 2.7 ± 0.4 | 3.9 ± 0.8 | 2.1 ± 0.4 ++ | 4.9 ± 1.0 ++ | 3.8 ± 0.4 | 5.0 ± 0.4 | 2.9 ± 0.6+ | 5.6 ± 0.9 + | 2.9 ± 0.7 | 3.5 ± 0.8 | 3.6 ± 0.6 | 3.0 ± 0.9 | p = 0.0015 |
| DOPAC (pg/μg) | 3.5 ± 0.8 | 6.3 ± 0.7 | 4.2 ± 0.8 ++ | 10.9 ± 1.9 ++ | 8.2 ± 1.1 | 9.0 ± 1.7 | 7.0 ± 1.8 | 6.0 ± 0.8 | 3.8 ± 1.0 ++++ | 13.6 ± 1.7 $,++++ | 2.2 ± 0.4 ++++ | 14.6 ± 4.1 $$,++++ | p < 0.0001 |
| 5-HIAA (pg/μg) | 5.9 ± 1.2 | 10.8 ± 1.3 | 12.4 ± 3.7 | 24.7 ± 7.1 | 8.7 ± 0.5 | 13.0 ± 0.9 | 7.4 ± 0.6 | 14.0 ± 1.5 | 8.2 ± 3.2 ++++ | 43.8 ± 5.1 $$$,++++ | 4.6 ± 0.6 ++++ | 39.3 ± 14.4 $$,++++ | p < 0.0001 |
| Hippocampus | |||||||||||||
| 5-HT (pg/μg) | 0.9 ± 0.2 + | 2.0 ± 0.6 + | 1.8 ± 0.5 | 1.8 ± 0.3 | 1.6 ± 0.2 | 2.4 ± 0.3 | 1.0 ± 0.2 ++ | 2.7 ± 0.6 ++ | 0.9 ± 0.2 | 1.4 ± 0.2 | 0.9 ± 0.3 | 1.3 ± 0.2 | p = 0.0009 |
| DOPAC (pg/μg) | 0.4 ± 0.1 + | 1.3 ± 0.4 + | 1.0 ± 0.5 | 0.9 ± 0.1 | 0.8 ± 0.2 | 1.1 ± 0.4 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.1 ++ | 1.5 ± 0.5 ++ | 0.5 ± 0.2 | 1.1 ± 0.2 | p = 0.0063 |
| 5-HIAA (pg/μg) | 3.8 ± 0.8 + | 8.1 ± 2.6 + | 6.5 ± 1.5 | 9.5 ± 1.3 | 7.3 ± 0.5 | 10.0 ± 0.6 | 5.4 ± 0.8 ++ | 11.8 ± 2.1 ++ | 4.1 ± 1.1 +++ | 13.0 ± 1.3 +++ | 2.3 ± 0.2 +++ | 10.7 ± 2.4 +++ | p < 0.0001 |
| DOPAC/DA ratio | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 + | 0.3 ± 0.1 + | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.5 ± 0.1 | 0.3 ± 0.1 | p = 0.0283 |
| 5-HIAA/5-HT ratio | 4.2 ± 0.8 | 4.0 ± 1.0 | 8.0 ± 2.5 | 7.4 ± 2.3 | 4.9 ± 0.4 | 4.5 ± 0.6 | 5.4 ± 0.6 | 4.9 ± 0.8 | 4.9 ± 0.9 | 10.5 ± 2.6 | 3.4 ± 0.8 | 9.1 ± 1.5 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaimal, A.; Hooversmith, J.M.; Mansi, M.H.A.; Holmes, P.V.; MohanKumar, P.S.; MohanKumar, S.M.J. Prenatal Exposure to Bisphenol A and/or Diethylhexyl Phthalate Impacts Brain Monoamine Levels in Rat Offspring. J. Xenobiot. 2024, 14, 1036-1050. https://doi.org/10.3390/jox14030058
Kaimal A, Hooversmith JM, Mansi MHA, Holmes PV, MohanKumar PS, MohanKumar SMJ. Prenatal Exposure to Bisphenol A and/or Diethylhexyl Phthalate Impacts Brain Monoamine Levels in Rat Offspring. Journal of Xenobiotics. 2024; 14(3):1036-1050. https://doi.org/10.3390/jox14030058
Chicago/Turabian StyleKaimal, Amrita, Jessica M. Hooversmith, Maryam H. Al Mansi, Philip V. Holmes, Puliyur S. MohanKumar, and Sheba M. J. MohanKumar. 2024. "Prenatal Exposure to Bisphenol A and/or Diethylhexyl Phthalate Impacts Brain Monoamine Levels in Rat Offspring" Journal of Xenobiotics 14, no. 3: 1036-1050. https://doi.org/10.3390/jox14030058
APA StyleKaimal, A., Hooversmith, J. M., Mansi, M. H. A., Holmes, P. V., MohanKumar, P. S., & MohanKumar, S. M. J. (2024). Prenatal Exposure to Bisphenol A and/or Diethylhexyl Phthalate Impacts Brain Monoamine Levels in Rat Offspring. Journal of Xenobiotics, 14(3), 1036-1050. https://doi.org/10.3390/jox14030058







