Resistance and Biodegradation of Triclosan and Propylparaben by Isolated Bacteria from Greywater
Abstract
:1. Introduction
1.1. Resistance to Triclosan and Propylparaben
1.2. Mechanisms of Co-Selection
2. Materials and Methods
2.1. Exposure Experiment
2.2. Antimicrobial Susceptibility Testing
2.3. Triclosan and Propylparaben MIC Testing
2.4. Detection of ARGs
2.5. Metabolic Activity of the Microbial Isolates
2.6. Biodegradation Experiment
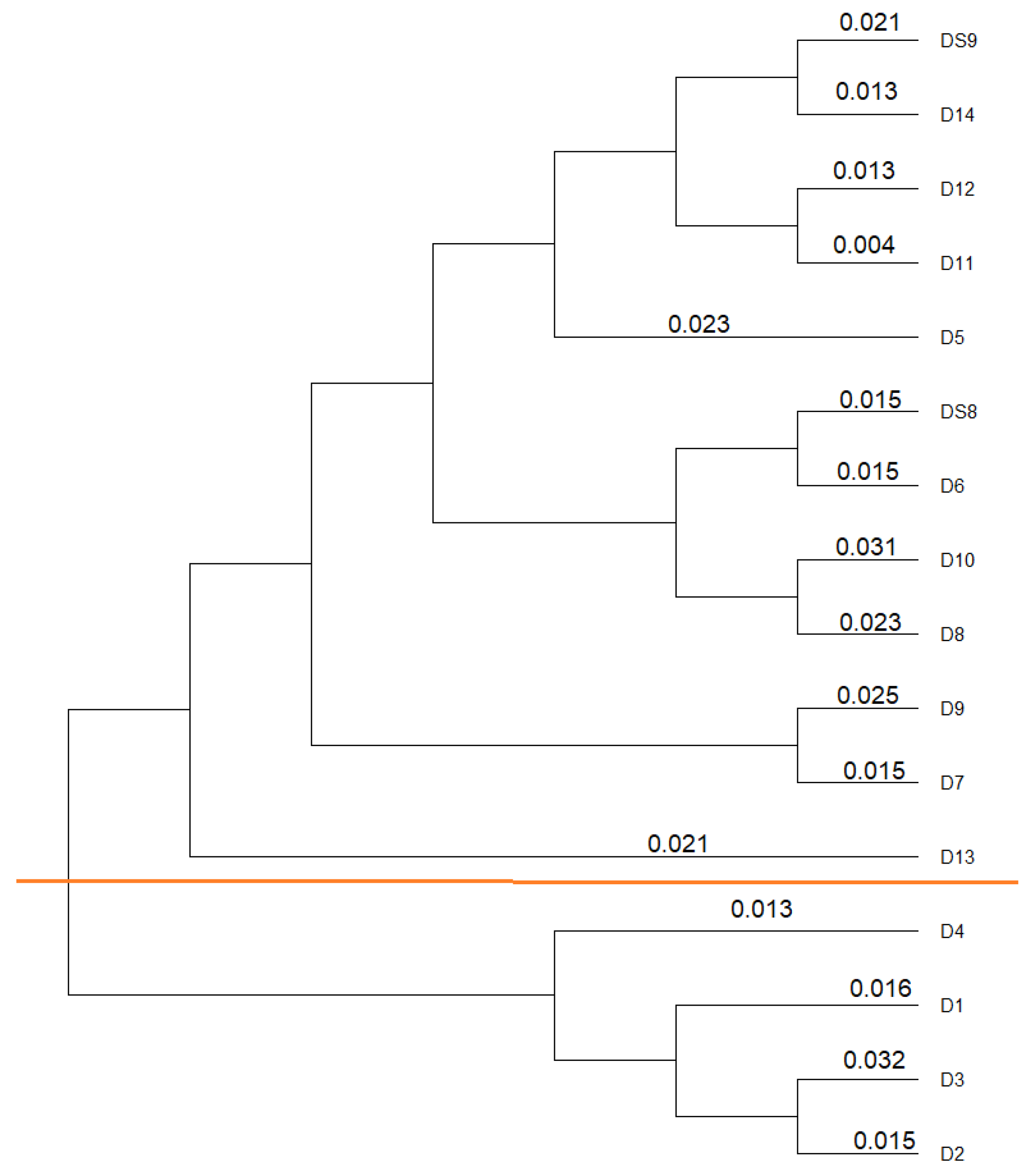
2.7. Strains Similarity Analysis
2.8. Statistical Analysis
3. Results
3.1. Comparing Actual Resistance (Etest) with ARG Existence
3.2. Similarity Analysis of the Isolates
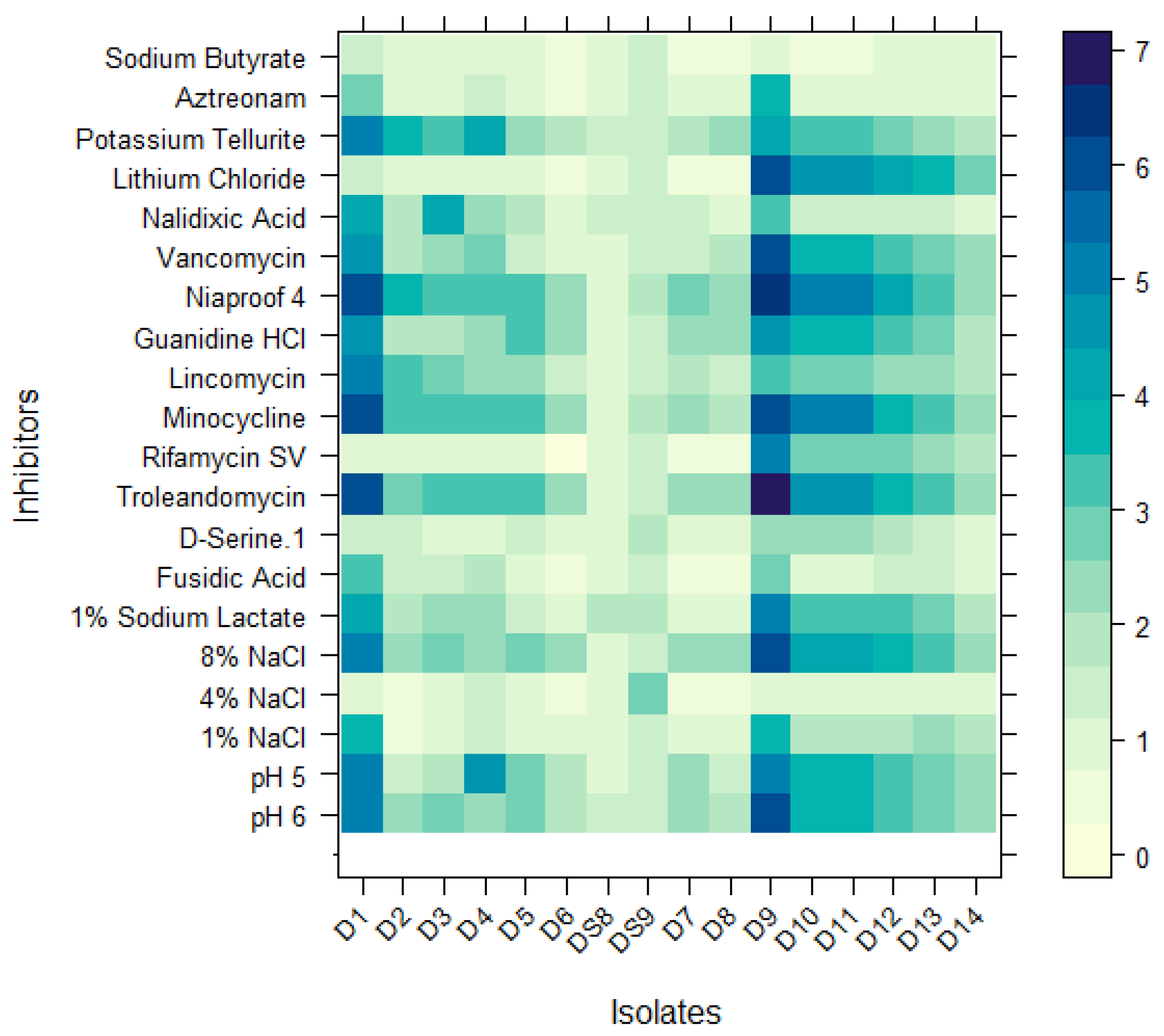
3.3. Characterization of Isolates Using a Carbon Source Utilization Pattern
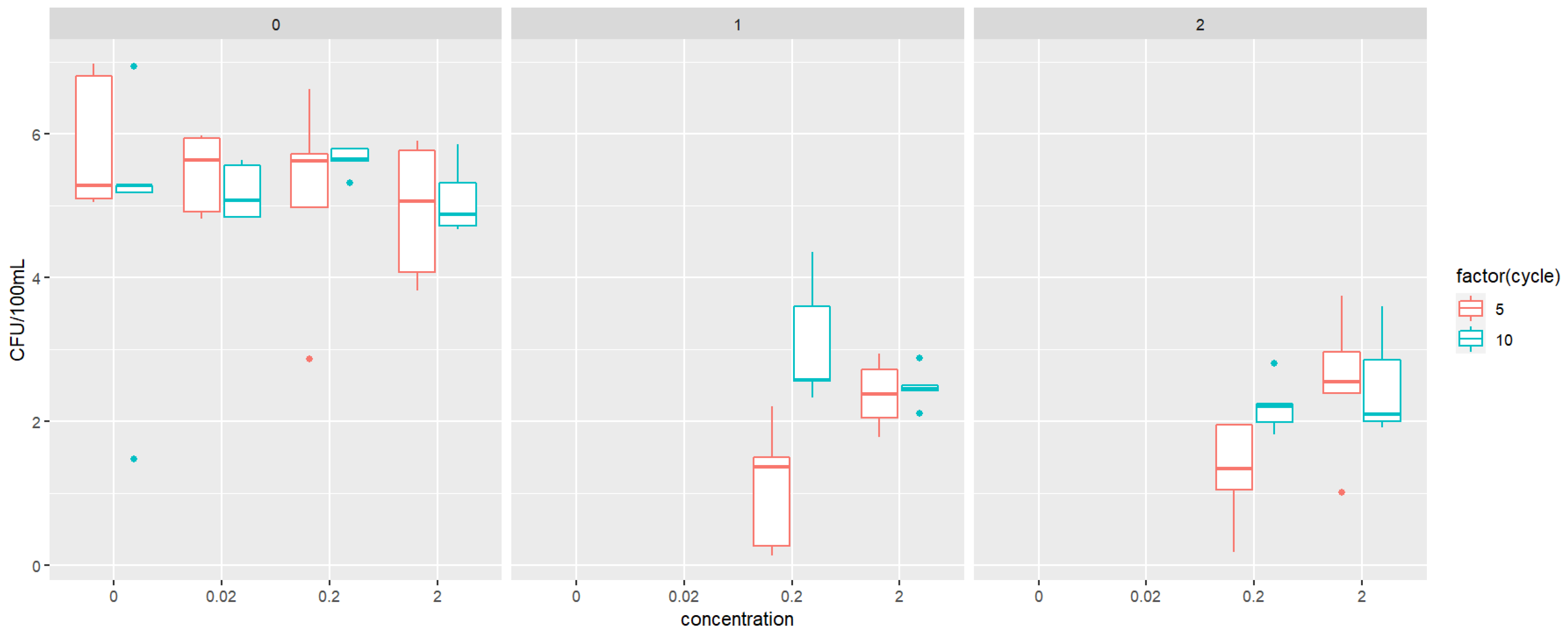
3.4. Resistance and Biodegradation of Triclosan and Propylparaben
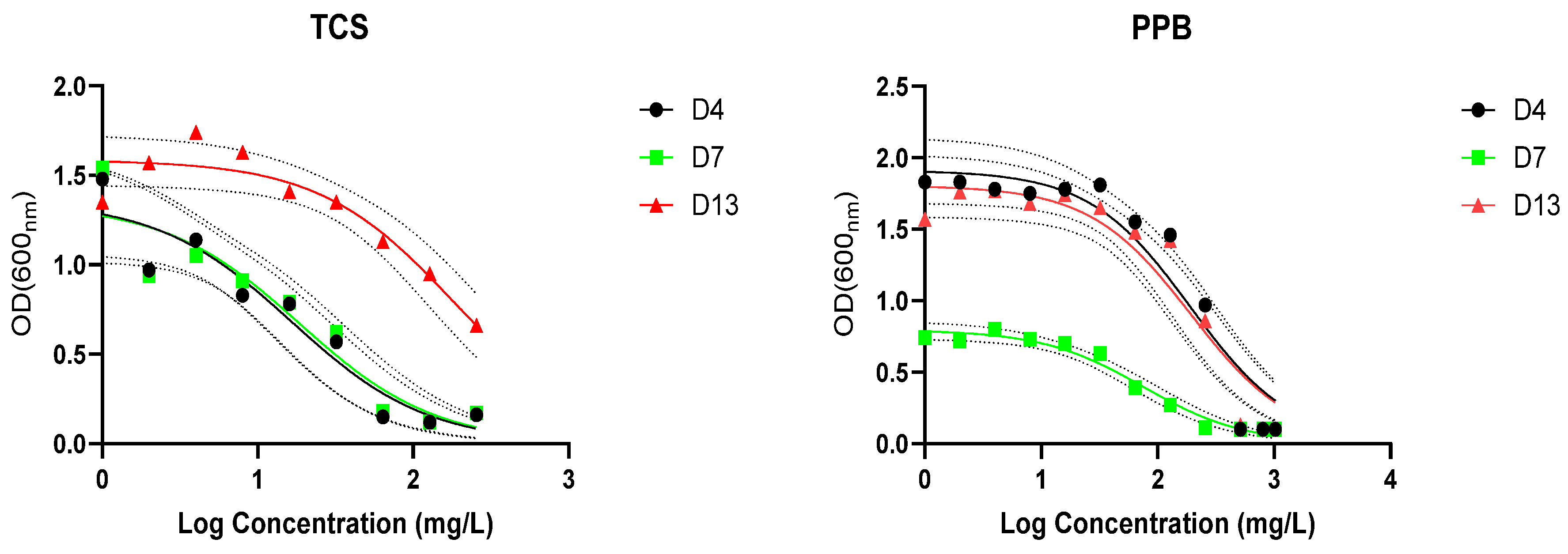
3.5. Biodegradation of Triclosan and Propylparaben
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMP | Emerging micropollutant |
| TCS | Triclosan |
| PPB | Propylparaben |
| MIC | Minimum inhibitory concentration |
| HGT | Horizontal gene transfer |
| OD | Optical density |
| PCR | Polymerase chain reaction |
References
- James, D.; Surendran, S.; Ifelebuegu, A.; Ganjian, E.; Kinuthia, J. Grey Water Reclamation for Urban Non-Potable Reuse-Challenges and Solutions. 2016. Available online: https://www.researchgate.net/publication/315705246 (accessed on 16 August 2023).
- Giuliano, C.A.; Rybak, M.J. Efficacy of Triclosan as an Antimicrobial Hand Soap and Its Potential Impact on Antimicrobial Resistance: A Focused Review; Pharmacotherapy Publications Inc.: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom. 2015, 16, 964. [Google Scholar] [CrossRef]
- Goudarzi, M.; Navidinia, M. Overview Perspective of Bacterial Strategies of Resistance to Biocides and Antibiotics; Kowsar Medical Institute: Tehran, Iran, 2019. [Google Scholar] [CrossRef]
- Romero, J.L.; Burgos, M.J.G.; Pérez-Pulido, R.; Gálvez, A.; Lucas, R. Resistance to Antibiotics, Biocides, Preservatives and Metals in Bacteria Isolated from Seafoods: Co-Selection of Strains Resistant or Tolerant to Different Classes of Compounds. Front. Microbiol. 2017, 8, 1650. [Google Scholar] [CrossRef] [PubMed]
- Alfiya, Y.; Dubowski, Y.; Friedler, E. Diurnal patterns of micropollutants concentrations in domestic greywater. Urban Water J. 2018, 15, 399–406. [Google Scholar] [CrossRef]
- Leal, L.H.; Vieno, N.; Temmink, H.; Zeeman, G.; Buisman, C.J.N. Occurrence of xenobiotics in gray water and removal in three biological treatment systems. Environ. Sci. Technol. 2010, 44, 6835–6842. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, T.; Zhang, Y.; Stein, O.R.; Arias, C.A.; Brix, H.; Carvalho, P.N. Effects of constructed wetland design on ibuprofen removal—A mesocosm scale study. Sci. Total Environ. 2017, 609, 38–45. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Pharmaceutical biodegradation under three anaerobic redox conditions evaluated by chemical and toxicological analyses. Sci. Total Environ. 2018, 618, 658–664. [Google Scholar] [CrossRef]
- Glover, C.M.; Liu, Y.; Liu, J. Assessing the Risk from Trace Organic Contaminants Released via Greywater Irrigation to the Aquatic Environment; Elsevier Ltd.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Cameron, A.; Barbieri, R.; Read, R.; Church, D.; Adator, E.H.; Zaheer, R.; McAllister, T.A. Functional screening for triclosan resistance in a wastewater metagenome and isolates of Escherichia coli and Enterococcus spp. From a large Canadian healthcare region. PLoS ONE 2019, 14, e0211144. [Google Scholar] [CrossRef]
- Kumar, K.S.; Priya, S.M.; Peck, A.M.; Sajwan, K.S. Mass loadings of triclosan and triclocarbon from four wastewater treatment plants to three rivers and landfill in Savannah, Georgia, USA. Arch. Environ. Contam. Toxicol. 2010, 58, 275–285. [Google Scholar] [CrossRef]
- Singer, H.; Müller, S.; Tixier, C.; Pillonel, L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ. Sci. Technol. 2002, 36, 4998–5004. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of triclosan, triclocarban, and their transformation products in wastewater under nitrifying conditions. J. Water Process Eng. 2019, 28, 144–151. [Google Scholar] [CrossRef]
- Birošová, L.; Mikulášová, M. Development of triclosan and antibiotic resistance in Salmonella enterica serovar Typhimurium. J. Med. Microbiol. 2009, 58, 436–441. [Google Scholar] [CrossRef]
- Halden, R.U. On the need and speed of regulating triclosan and triclocarban in the United States. Environ. Sci. Technol. 2014, 48, 3603–3611. [Google Scholar] [CrossRef]
- Schweizer, H.P. Triclosan: A widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 2001, 202, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fahimipour, A.K.; Ben Maamar, S.; McFarland, A.G.; Blaustein, R.A.; Chen, J.; Glawe, A.J.; Kline, J.; Green, J.L.; Halden, R.U.; Van Den Wymelenberg, K.; et al. Antimicrobial Chemicals Associate with Microbial Function and Antibiotic Resistance Indoors. mSystems 2018, 3. [Google Scholar] [CrossRef]
- Lu, J.; Jin, M.; Nguyen, S.H.; Mao, L.; Li, J.; Coin, L.J.; Yuan, Z.; Guo, J. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018, 118, 257–265. [Google Scholar] [CrossRef]
- Wang, D.; Ning, Q.; Deng, Z.; Zhang, M.; You, J. Role of Environmental Stresses in Elevating Resistance Mutations in Bacteria: Phenomena and Mechanisms; Elsevier Ltd.: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Rana, P.; Ghouse, S.M.; Akunuri, R.; Madhavi, Y.V.; Chopra, S.; Nanduri, S. FabI (Enoyl Acyl Carrier Protein Reductase)—A Potential Broad Spectrum Therapeutic Target and Its Inhibitors; Elsevier Masson s.r.l.: Paris, France, 2020. [Google Scholar] [CrossRef]
- Zhu, L.; Bi, H.; Ma, J.; Hu, Z.; Zhang, W.; Cronan, J.E.; Wang, H. The two functional enoyl-acyl carrier protein reductases of Enterococcus faecalis do not mediate triclosan resistance. mBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, Y.; Sun, J.; Li, J.; Bai, J.; Zhang, C. Chronic Exposure to an Environmentally Relevant Triclosan Concentration Induces Persistent Triclosan Resistance but Reversible Antibiotic Tolerance in Escherichia coli. Environ. Sci. Technol. 2019, 53, 3277–3286. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J. The impact of triclosan on the spread of antibiotic resistance in the environment. Front. Microbiol. 2014, 5, 780. [Google Scholar] [CrossRef]
- Westfall, C.; Flores-Mireles, A.L.; Robinson, J.I.; Lynch, A.J.L.; Hultgren, S.; Henderson, J.P.; Levin, P.A. The widely used antimicrobial triclosan induces high levels of antibiotic tolerance in vitro and reduces antibiotic efficacy up to 100-fold in vivo. Antimicrob. Agents Chemother. 2019, 63, e02312-18. [Google Scholar] [CrossRef]
- Sanchez, P.; Moreno, E.; Martinez, J.L. The biocide triclosan selects Stenotrophomonas maltophilia mutants that overproduce the SmeDEF multidrug efflux pump. Antimicrob. Agents Chemother. 2005, 49, 781–782. [Google Scholar] [CrossRef]
- Karatzas, K.A.G.; Webber, M.A.; Jorgensen, F.; Woodward, M.J.; Piddock, L.J.V.; Humphrey, T.J. Prolonged treatment of Salmonella enterica serovar Typhimurium with commercial disinfectants selects for multiple antibiotic resistance, increased efflux and reduced invasiveness. J. Antimicrob. Chemother. 2007, 60, 947–955. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Li, J.; Mao, L.; Nguyen, S.H.; Duarte, T.; Coin, L.; Bond, P.; Yuan, Z.; Guo, J. Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera. Environ. Int. 2018, 121, 1217–1226. [Google Scholar] [CrossRef]
- Valkova, N.; Lépine, F.; Valeanu, L.; Dupont, M.; Labrie, L.; Bisaillon, J.-G.; Beaudet, R.; Shareck, F.; Villemur, R. Hydrolysis of 4-Hydroxybenzoic Acid Esters (Parabens) and Their Aerobic Transformation into Phenol by the Resistant Enterobacter cloacae Strain EM. Appl. Environ. Microbiol. 2001, 67, 2404–2409. [Google Scholar] [CrossRef]
- Selvaraj, K.K.; Sivakumar, S.; Sampath, S.; Shanmugam, G.; Sundaresan, U.; Ramaswamy, B.R. Paraben resistance in bacteria from sewage treatment plant effluents in India. Water Sci. Technol. 2013, 68, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety Assessment of Esters of p-Hydroxybenzoic Acid (Parabens); Elsevier Ltd.: Amsterdam, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy Around Parabens: Alternative Strategies for Preservative use in Cosmetics and Personal Care Products; Academic Press Inc.: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Bredin, J.; Davin-Régli, A.; Pagès, J.M. Propyl paraben induces potassium efflux in Escherichia coli. J. Antimicrob. Chemother. 2005, 55, 1013–1015. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef]
- Lazar, V.; Oprea, E.; Ditu, L.M. Resistance, Tolerance, Virulence and Bacterial Pathogen Fitness—Current State and Envisioned Solutions for the Near Future. Pathogens 2023, 12, 746. [Google Scholar] [CrossRef]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G.; et al. Mechanism of antibacterial resistance, strategies and next-generation antimicrobials to contain antimicrobial resistance: A review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef]
- Björkman, J.; Andersson, D.I. The cost of antibiotic resistance from a bacterial perspective. Drug Resist. Updates 2000, 3, 237–245. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety SCCS. Opinion on Triclosan Antimicrobial Resistance. 2010. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_023.pdf. (accessed on 10 November 2024).
- Itzhari, D.; Shuai, W.; Hartmann, E.M.; Ronen, Z. Heterogeneous Antibiotic Resistance Gene Removal Impedes Evaluation of Constructed Wetlands for Effective Greywater Treatment. Antibiotics 2024, 13, 315. [Google Scholar] [CrossRef]
- Shuai, W.; Itzhari, D.; Ronen, Z.; Hartmann, E.M. Mitigation of antimicrobial resistance genes in greywater treated at household level. Sci. Total Environ. 2023, 890, 164136. [Google Scholar] [CrossRef]
- Henderson, M.; Ergas, S.J.; Ghebremichael, K.; Gross, A.; Ronen, Z. Occurrence of Antibiotic-Resistant Genes and Bacteria in Household Greywater Treated in Constructed Wetlands. Water 2022, 14, 758. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic action and resistance: Updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Front. Pharmacol. 2023, 14, 1305294. [Google Scholar] [CrossRef]
- Heydariyan, Z.; Soofivand, F.; Dawi, E.A.; Al-Kahdum, S.A.A.; Hameed, N.M.; Salavati-Niasari, M. A Comprehensive Review: Different Approaches for Encountering of Bacterial Infection of Dental Implants and Improving Their Properties; Editions de Sante: Paris, France, 2023. [Google Scholar] [CrossRef]
- Sköld, O. Sulfonamide resistance: Mechanisms and trends. Drug Resist. Updates 2000, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.; Salisbury, B.H.; Zito, P. Ciprofloxacin; StatPearls Publishing: Tampa, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535454/ (accessed on 7 November 2024).
- Bereda, G. Clinical Pharmacology of Ampicillin. J. Pharm. Res. Rep. 2022, 3, 1–3. [Google Scholar] [CrossRef]
- Karaman, R. From Conventional Prodrugs to Prodrugs Designed by Molecular Orbital Methods. Front. Comput. Chem. 2015, 2, 187–249. [Google Scholar]
- Itzhari, D.; Ronen, Z. The Emergence of Antibiotics Resistance Genes, Bacteria, and Micropollutants in Grey Wastewater. Appl. Sci. 2023, 13, 2322. [Google Scholar] [CrossRef]
- Singh, M.P. Application of Biolog FF MicroPlate for substrate utilization and metabolite profiling of closely related fungi. J. Microbiol. Methods 2009, 77, 102–108. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. Past: Paleontological Statistics Software Package for Education and Data Analysis. 2001. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 25 November 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Scholars Portal: Toronto, ON, USA, 2019. [Google Scholar]
- Welsch, T.T.; Gillock, E.T. Triclosan-resistant bacteria isolated from feedlot and residential soils. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2011, 46, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [PubMed]
- Spiers, A.J.; Buckling, A.; Rainey, P.B. The causes of Pseudomonas diversity. Microbiology 2000, 146, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Meade, M.J.; Waddell, R.L.; Callahan, T.M. Soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp. denitrificans inactivate triclosan in liquid and solid substrates. FEMS Microbiol. Lett. 2001, 204, 45–48. [Google Scholar] [CrossRef]
- Turner, T.L.; Wunderink, R.; Ozer, E.; Hauser, A. First report of Pseudomonas nitroreducens cultured from the lungs of a patient with pneumonia. BMJ Case Rep. 2021, 14, e241327. [Google Scholar] [CrossRef]
- CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2023. [Google Scholar]
- Wayne, P.; CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2020. [Google Scholar]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-inactivating enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Tachon, S.; Michelon, D.; Chambellon, E.; Cantonnet, M.; Mezange, C.; Henno, L.; Cachon, R.; Yvon, M. Experimental conditions affect the site of tetrazolium violet reduction in the electron transport chain of Lactococcus lactis. Microbiology 2009, 155, 2941–2948. [Google Scholar] [CrossRef]
- Stefanowicz, A.M. The Biolog Plates Technique as a Tool in Ecological Studies of Microbial Communities. Pol. J. Environ. Stud. 2006, 15, 669–676. Available online: https://www.researchgate.net/publication/259921125 (accessed on 28 January 2025).
- Koburger, T.; Hübner, N.O.; Braun, M.; Siebert, J.; Kramer, A. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother. 2010, 65, 1712–1719. [Google Scholar] [CrossRef]
- Loughlin, M.F.; Jones, M.V.; Lambert, P.A. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J. Antimicrob. Chemother. 2002, 49, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Barabasz, W.; Pikulicka, A.; Wzorek, Z.; Nowak, A.K. Ecotoxicological aspects of the use of parabens in the production of cosmetics. Czas. Tech. 2019, 12, 99–124. [Google Scholar] [CrossRef]
- Pereira, A.R.; Simões, M.; Gomes, I.B. Parabens as Environmental Contaminants of Aquatic Systems Affecting Water Quality and Microbial Dynamics; Elsevier B.V.: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Reis, A.C.; Kolvenbach, B.A.; Nunes, O.C.; Corvini, P.F.X. Biodegradation of Antibiotics: The New Resistance Determinants—Part I; Elsevier B.V.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Tian, H.; Ma, Y.J.; Li, W.Y.; Wang, J.W. Efficient degradation of triclosan by an endophytic fungus Penicillium oxalicum B4. Environ. Sci. Pollut. Res. 2018, 25, 8963–8975. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, H.; Jiang, Z.; Jiang, J.; Lu, Z. Degradation of Triclosan in the Water Environment by Microorganisms: A Review. Microorganisms 2022, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, Q.; Wang, Y.W.; Deng, C.X.; Yu, C.P. Comparative studies of aerobic and anaerobic biodegradation of methylparaben and propylparaben in activated sludge. Ecotoxicol. Environ. Saf. 2017, 138, 25–31. [Google Scholar] [CrossRef]
- Kagle, J.; Porter, A.W.; Murdoch, R.W.; Rivera-Cancel, G.; Hay, A.G. Chapter 3 Biodegradation of Pharmaceutical and Personal Care Products. Adv. Appl. Microbiol. 2009, 67, 65–108. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Thi, L.-A.P.; Chandana, P.S.; Do, H.-T.; Pham, T.-H.; Lee, T.; Nguyen, T.D.; Le Phuoc, C.; Huong, P.T. The degradation of paraben preservatives: Recent progress and sustainable approaches toward photocatalysis. Chemosphere 2021, 276, 130163. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Tu, Y.; Yang, Z. Biodegradation of four selected parabens with aerobic activated sludge and their transesterification product. Ecotoxicol. Environ. Saf. 2018, 156, 48–55. [Google Scholar] [CrossRef]
- Leoni, B.; Patelli, M.; Soler, V.; Nava, V. Ammonium transformation in 14 lakes along a trophic gradient. Water 2018, 10, 265. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Evaluation of the occurrence and biodegradation of parabens and halogenated by-products in wastewater by accurate-mass liquid chromatography-quadrupole-time-of-flight-mass spectrometry (LC-QTOF-MS). Water Res. 2011, 45, 6770–6780. [Google Scholar] [CrossRef]
- James, M.O.; Marth, C.J.; Rowland-Faux, L. Slow O-demethylation of methyl triclosan to triclosan, which is rapidly glucuronidated and sulfonated in channel catfish liver and intestine. Aquat. Toxicol. 2012, 124–125, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Shareef, A.; Kookana, R.; Gaylard, S.; Hoare, S.; Kildea, T. The distribution of triclosan and methyl-triclosan in marine sediments of Barker Inlet, South Australia. J. Environ. Monit. 2011, 13, 801–806. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, T.; Zhou, Y.; Liu, Z.-F.; Zhang, Y.; Bian, Y.; Feng, X.-S. Triclosan and Related Compounds in the Environment: Recent Updates on Sources, Fates, Distribution, Analytical Extraction, Analysis, and Removal Techniques; Elsevier B.V.: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Fu, Q.; Liao, C.; Du, X.; Schlenk, D.; Gan, J. Back Conversion from Product to Parent: Methyl Triclosan to Triclosan in Plants. Environ. Sci. Technol. Lett. 2018, 5, 181–185. [Google Scholar] [CrossRef]
- Abbott, T.; Kor-Bicakci, G.; Islam, M.S.; Eskicioglu, C. A review on the fate of legacy and alternative antimicrobials and their metabolites during wastewater and sludge treatment. Int. J. Mol. Sci. 2020, 21, 9241. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, J.; Ma, J.; Cronan, J.E.; Wang, H. Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan-resistant enoyl-acyl carrier protein reductase. Antimicrob. Agents Chemother. 2010, 54, 689–698. [Google Scholar] [CrossRef]
- Akrami, F.; Rajabnia, M.; Pournajaf, A. Resistance integrons; A mini review. Casp. J. Intern. Med. 2019, 10, 370–376. [Google Scholar]
- Leclercq, R.; Courvalin, P. Resistance to macrolides and related antibiotics in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2002, 46, 2727–2734. [Google Scholar] [CrossRef]
- Ehlers, M.M.; Veldsman, C.; Makgotlho, E.P.; Dove, M.G.; Hoosen, A.A.; Kock, M.M. Detection of blaSHV, blaTEM and blaCTX-M antibiotic resistance genes in randomly selected bacterial pathogens from the Steve Biko Academic Hospital. FEMS Immunol. Med. Microbiol. 2009, 56, 191–196. [Google Scholar] [CrossRef]
- Maillard, J.Y. Impact of benzalkonium chloride, benzethonium chloride and chloroxylenol on bacterial antimicrobial resistance. J. Appl. Microbiol. 2022, 133, 3322–3346. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Głaz, P.; Rosińska, A.; Woźniak, S.; Boguszewska-Czubara, A.; Biernasiuk, A.; Matosiuk, D. Effect of Commonly Used Cosmetic Preservatives on Healthy Human Skin Cells. Cells 2023, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Thenault, V.; Abdo, P.; Margulis, K.; Magdassi, S.; Prokopovich, P. Antimicrobial activity of bone cements embedded with organic nanoparticles. Int. J. Nanomed. 2015, 10, 6317–6329. [Google Scholar]
- del Valle, P.; García-Armesto, M.R.; de Arriaga, D.; González-Donquiles, C.; Rodríquez-Fernández, P.; Rúa, J. Antimicrobial activity of kaempferol and resveratrol in binary combinations with parabens or propyl gallate against Enterococcus faecalis. Food Control 2016, 61, 213–220. [Google Scholar] [CrossRef]
- Suller, M.T.E.; Russel, A.D. Triclosan and antibiotic resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 46, 11–18. [Google Scholar] [CrossRef]
- Cookson, B.D.; Farrelly, H.; Stapleton, P.; Garvey, R.P.J.; Price, M.R. Transferable Resistance to Triclosan in MRSA; Oxford Blackwell Scientific: Oxford, UK, 1991. [Google Scholar]
- Assadian, O.; Wehse, K.; Hubner, N.O.; Koburger, T.; Bagel, S.; Jethon, F.; Kramer, A. Minimum inhibitory (MIC) and minimum microbicidal concentration (MMC) of polihexanide and triclosan against antibiotic sensitive and resistant Staphylococcus aureus and Escherichia coli strains. GMS Krankenhhyg Interdiszip. 2011, 6, Doc06. [Google Scholar] [CrossRef]
- Aiello, A.E.; Marshall, B.; Levy, S.B.; Della-Latta, P.; Larson, E. Relationship between triclosan and susceptibilities of bacteria isolated from hands in the community. Antimicrob. Agents Chemother. 2004, 48, 2973–2979. [Google Scholar] [CrossRef]





| Mechanisms of Antibacterial Resistance | Type of Resistance | Co-Selection |
|---|---|---|
| Target site modifications | Acquired | No |
| Reduced permeability of the outer membrane | Natural | Yes |
| Increased efflux pumps | Natural | Yes |
| Enzymatic modification | Natural/acquired | No |
| Antibiotic | Mechanisms of Action |
|---|---|
| TC = tetracycline | inhibits protein biosynthesis by targeting the ribosomal 30S subunits [44] |
| GM = gentamicin | inhibits protein synthesis and binds irreversibly to ribosomal 30S subunits [45] |
| TS = trimethoprim/sulfonamide | inhibits folic acid metabolism [44] by targeting the enzyme dihydropteroate synthase (DHPS) [46] |
| CL = ciprofloxacin | inhibits DNA replication by inhibiting bacterial DNA topoisomerase and DNA gyrase [47] |
| AM = ampicillin | inhibits bacterial cell wall synthesis by binding one or more of the binding proteins [48] |
| AC = amoxicillin | inhibits the biosynthesis and repair of the bacterial wall [49] |
| Isolate Details | Identification | PCR (Presence or Absence) | Etest (MIC µg/mL) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Cycle | Chemical | Conc. (mg/L) | Most Similar Accession Number | fabV | tetG | qnrS | intI | blaCTX | ermB | sul1 | Fab I | TC | GM | T/S | CI | AM | AC | |
| D1 | 10 | TCS | 2 | Achromobacter insolitus | CP026973.1 | x | x | x | x | x | 0.5 [S] | 12 [I] | - [*] | 0.5 [S] | 1.5 [S] | 1.5 [S] | |||
| D2 | 10 | TCS | 2 | Achromobacter insolitus | CP026973.1 | x | x | x | x | x | 0.5 [S] | 12 [I] | - [*] | 0.75 [S] | 1.5 [S] | 1.5 [S] | |||
| D3 | 10 | TCS | 0.2 | Achromobacter insolitus | CP026973.1 | x | x | x | x | 0.5 [S] | 8 [I] | - [*] | 0.5 [S] | 1 [S] | 1 [S] | ||||
| D4 | 10 | TCS | 2 | Achromobacter insolitus | CP026973.1 | x | x | x | x | x | 0.38 [S] | 12 [I] | - [*] | 1 [S] | 1.5 [S] | 1.5 [S] | |||
| D5 | 10 | TCS | 0.2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | 4 [S] | 0.75 [S] | - [*] | 0.094 [S] | - [*] | - [*] | |||||
| D6 | 10 | TCS | 2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | x | x | x | 24 [R] | 48 [R] | - [*] | 1.5 [I] | 1.5 [S] | 1.5 [S] | ||
| DS8 | 5 | TCS | 2 | Pseudomonas sp. AU2510 | AY486376.1 | x | x | x | x | x | x | 3 [S] | 16 [R] | - [*] | 1.5 [I] | 256 [S] | - [*] | ||
| DS9 | 5 | TCS | 2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | x | x | 0.75 [S] | 12 [I] | - [*] | 0.75 [S] | 6 [S] | 12 [S] | |||
| D7 | 10 | PPB | 2 | Pseudomonas sp. BT5A | LC097206.1 | x | x | x | x | x | x | 24 [R] | 96 [R] | - [*] | 6 [R] | 1.0 [S] | 1.5 [S] | ||
| D8 | 10 | PPB | 2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | x | x | x | 24 [R] | 12 [I] | - [*] | 1.0 [S] | 1.5 [S] | 1.5 [S] | ||
| D9 | 10 | PPB | 2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | x | x | x | 4 [S] | 0.75 [S] | - [*] | 0.094 [S] | - [*] | - [*] | ||
| D10 | 10 | PPB | 2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | x | x | 3 [S] | 0.75 [S] | - [*] | 0.064 [S] | - [*] | 96 [R] | |||
| D11 | 10 | PPB | 0.2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | x | x | x | x | x | 4 [S] | 0.5 [S] | - [*] | 0.064 [S] | 3 [S] | 24 [I] |
| D12 | 5 | PPB | 2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | x | 3 [S] | 0.5 [S] | 4 [R] | 0.094 [S] | 256 [R] | 64 [R] | ||||
| D13 | 5 | PPB | 2 | Pseudomonas sp. BT5A | LC097206.1 | x | x | x | 6 [I] | 1 [S] | 6 [R] | 0.064 [S] | 256 [R] | 64 [R] | |||||
| D14 | 5 | PPB | 2 | Pseudomonas nitroreducens | CP049140.1 | x | x | x | x | x | 1.0 [S] | 8 [I] | 12 [R] | 0.75 [S] | - [*] | 8 [S] | |||
| TCS | PPB | |||||
|---|---|---|---|---|---|---|
| Strain | IC50 (mg/L) | R2 | MIC (mg/L) | IC50 (mg/L) | R2 | MIC (mg/L) |
| D4 | 16.61 | 0.918 | 64.0 | 191.0 | 0.919 | 512 |
| D7 | 18.63 | 0.894 | 128.0 | 75.0 | 0.974 | 512 |
| D13 | 182.1 | 0.0891 | >256 | 193.4 | 0.918 | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itzhari, D.; Nzeh, J.; Ronen, Z. Resistance and Biodegradation of Triclosan and Propylparaben by Isolated Bacteria from Greywater. J. Xenobiot. 2025, 15, 56. https://doi.org/10.3390/jox15020056
Itzhari D, Nzeh J, Ronen Z. Resistance and Biodegradation of Triclosan and Propylparaben by Isolated Bacteria from Greywater. Journal of Xenobiotics. 2025; 15(2):56. https://doi.org/10.3390/jox15020056
Chicago/Turabian StyleItzhari, Daniella, Joseph Nzeh, and Zeev Ronen. 2025. "Resistance and Biodegradation of Triclosan and Propylparaben by Isolated Bacteria from Greywater" Journal of Xenobiotics 15, no. 2: 56. https://doi.org/10.3390/jox15020056
APA StyleItzhari, D., Nzeh, J., & Ronen, Z. (2025). Resistance and Biodegradation of Triclosan and Propylparaben by Isolated Bacteria from Greywater. Journal of Xenobiotics, 15(2), 56. https://doi.org/10.3390/jox15020056








