Interaction of Heavy Metals (Cadmium and Selenium) in an Experimental Study on Goldfish: Hematobiochemical Changes and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
- Control Group: No treatment.
- cad Group (Cadmium exposure): Exposed to cadmium chloride (Sigma-Aldrich, St. Louis, MO, USA Company) at 2.8 mg/L (LD50 cadmium 11.2 mg/L in goldfish individuals) [14].
- sel Group (Selenium exposure): Exposed to sodium selenite (Sigma-Aldrich, USA) at 2 mg/L [15].
- cad+sel Group: Exposed simultaneously to cadmium chloride (2.8 mg/L) and sodium selenite (2 mg/L).
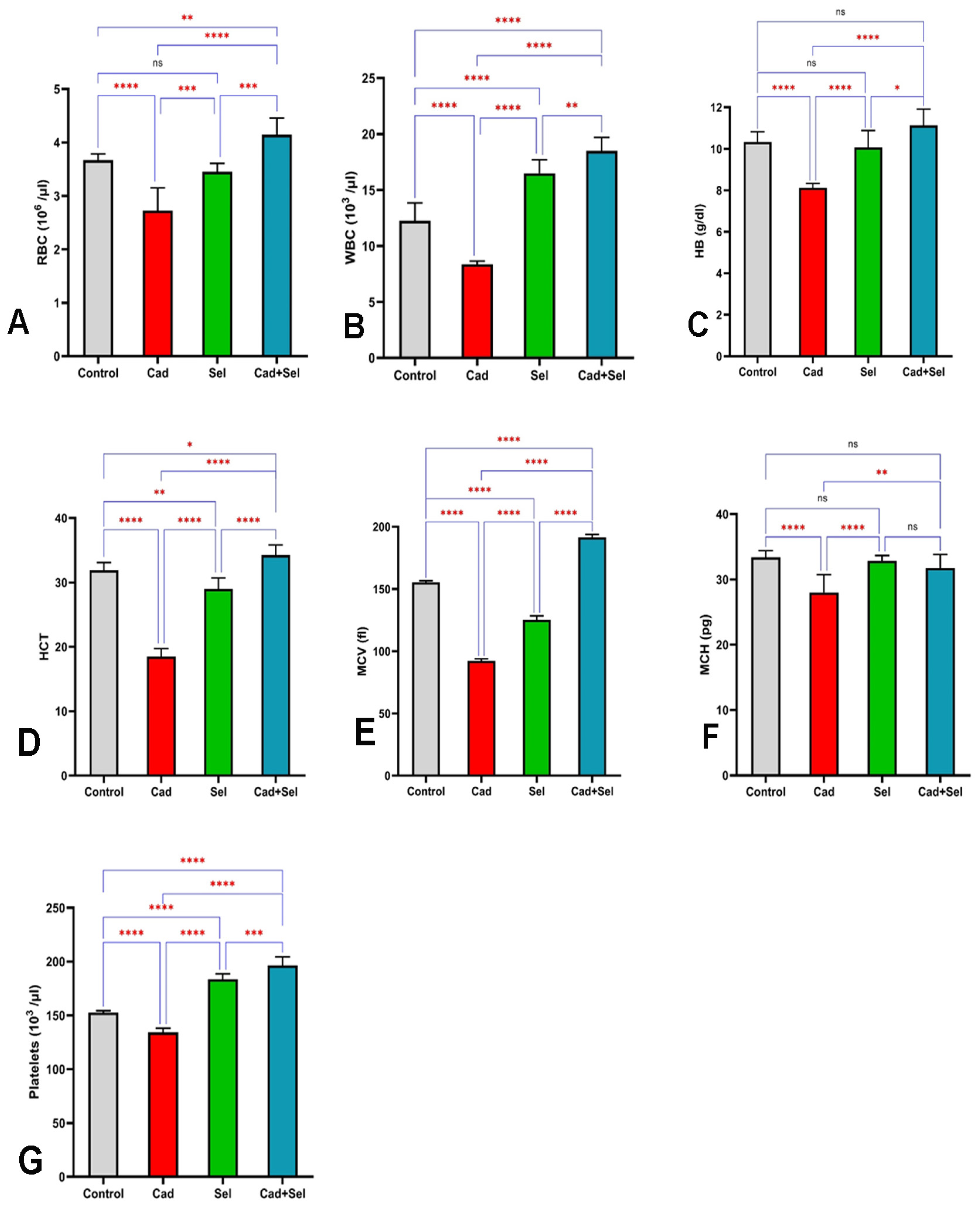
2.2. Hematological Analysis
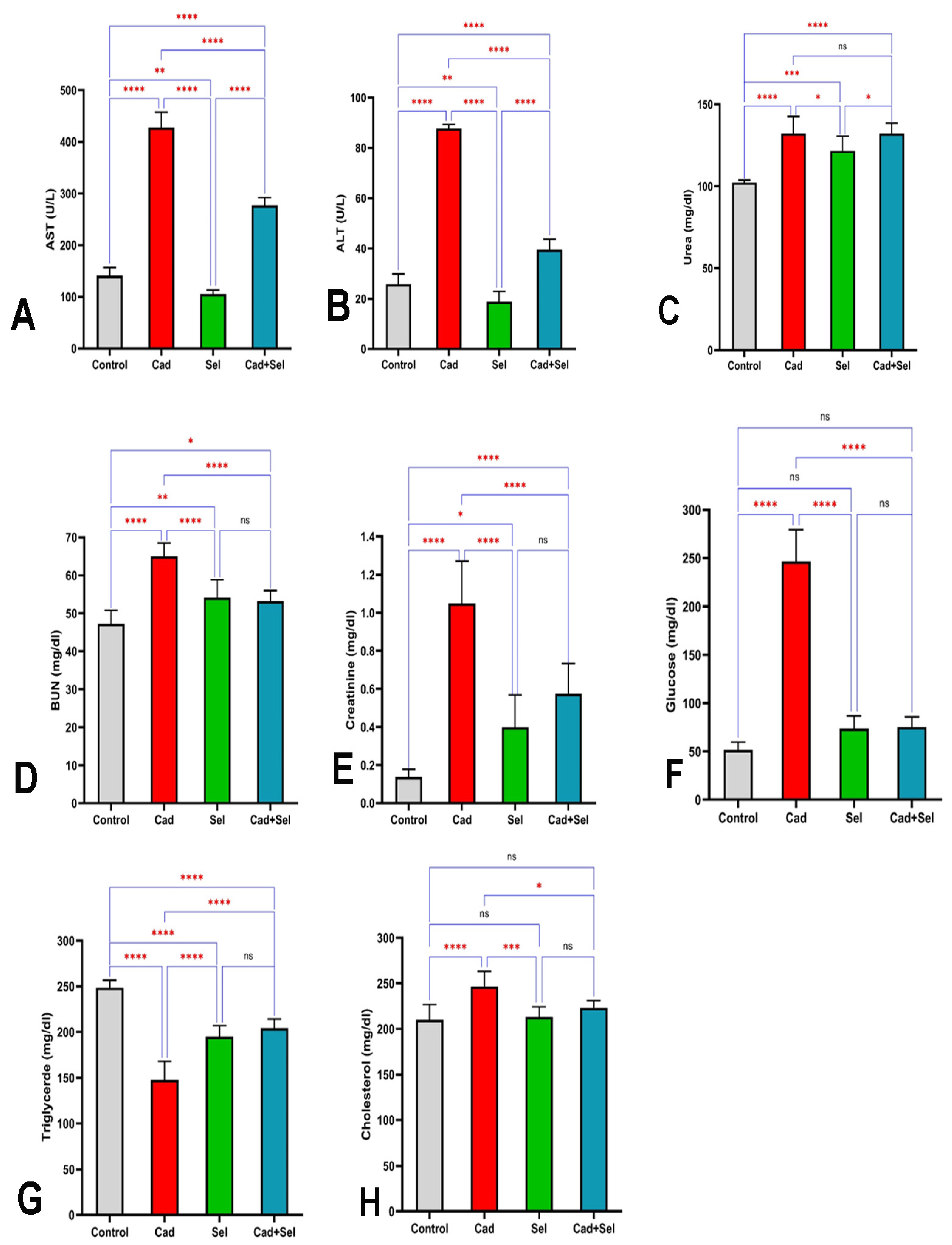
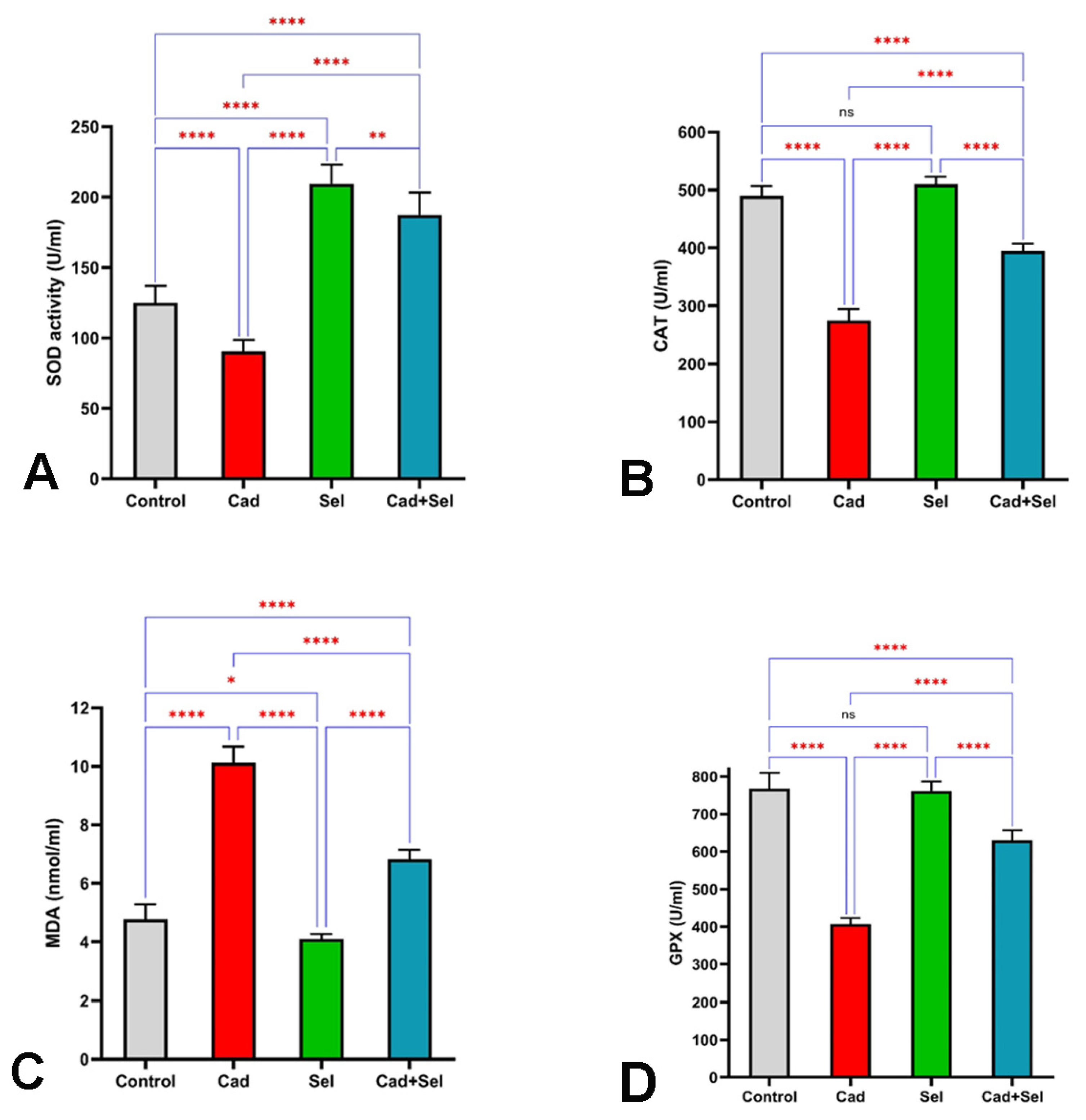
2.3. Biochemical Analysis
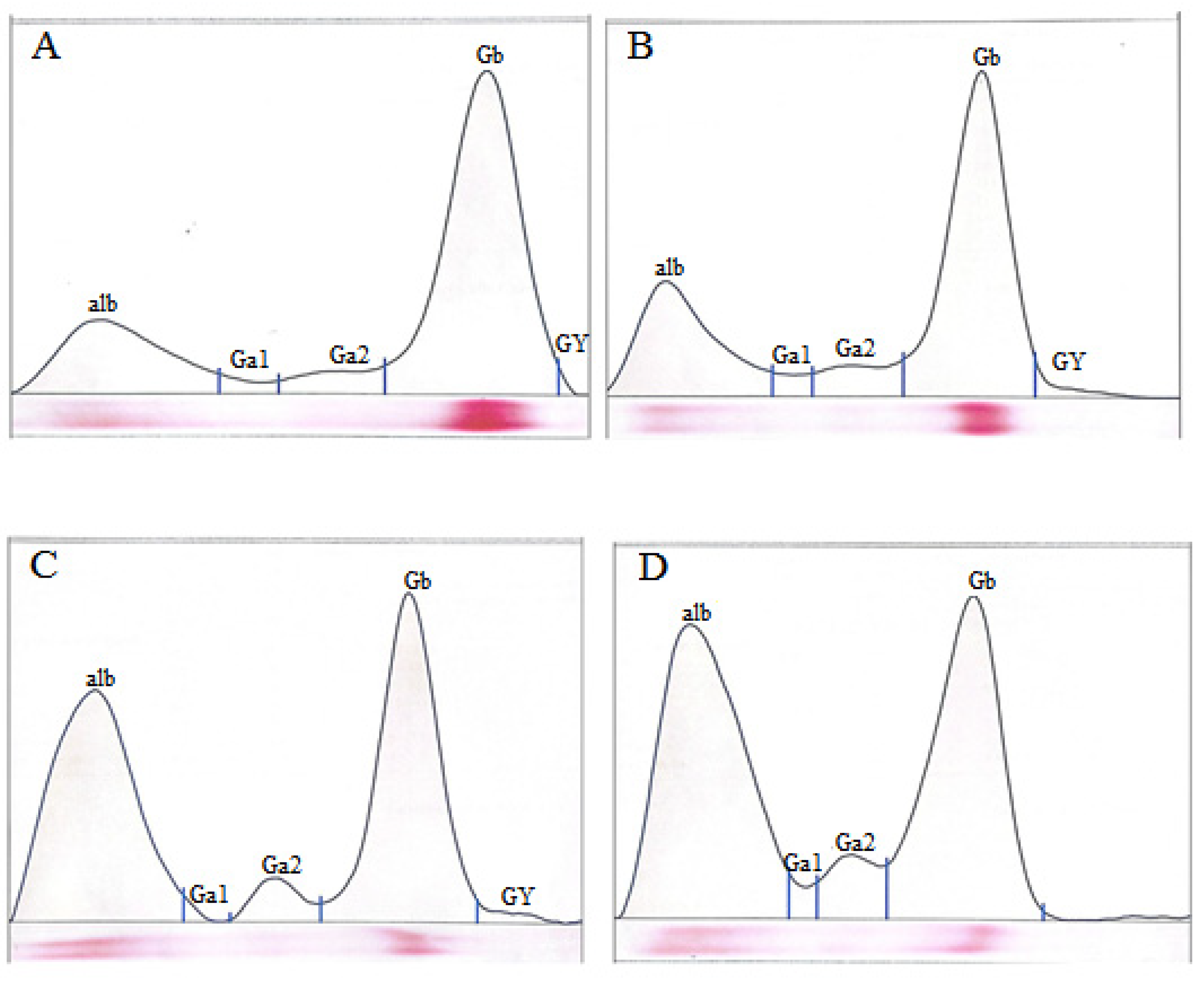
2.4. Serum Protein Electrophoresis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MT | Metallothionein |
| CRH | Corticotropin-releasing hormone |
| LPO | Lipid peroxidation |
| Ht | Hematocrit |
References
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Ali, Z.; Sher, N.; Muhammad, I.; Nayab, G.E.; Alouffi, A.; Almutairi, M.M.; Khan, I.; Ali, A. The combined effect of cadmium and copper induces bioaccumulation, and toxicity and disrupts the antioxidant enzymatic activities of goldfish (Carassius auratus). Toxicol. Rep. 2025, 14, 101972. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on cadmium exposure in the population and intervention strategies against cadmium toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef]
- Teschke, R. Copper, iron, cadmium, and arsenic, all generated in the universe: Elucidating their environmental impact risk on human health including clinical liver injury. Int. J. Mol. Sci. 2024, 25, 6662. [Google Scholar] [CrossRef]
- Unsal, V.; Dalkıran, T.; Çiçek, M.; Kölükçü, E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: A review. Adv. Pharm. Bull. 2020, 10, 184. [Google Scholar] [CrossRef]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef]
- Parui, R.; Nongthombam, G.S.; Hossain, M.; Adil, L.R.; Gogoi, R.; Bhowmik, S.; Barman, D.; Iyer, P.K. Impact of heavy metals on human health. In Remediation of Heavy Metals: Sustainable Technologies and Recent Advances; Wiley: Hoboken, NJ, USA, 2024; pp. 47–81. [Google Scholar]
- Uddin, M.H.; Ritu, J.R.; Putnala, S.K.; Rachamalla, M.; Chivers, D.P.; Niyogi, S. Selenium toxicity in fishes: A current perspective. Chemosphere 2024, 364, 143214. [Google Scholar] [CrossRef]
- Li, Z.-M.; Wang, X.-L.; Jin, X.-M.; Huang, J.-Q.; Wang, L.-S. The effect of selenium on antioxidant system in aquaculture animals. Front. Physiol. 2023, 14, 1153511. [Google Scholar] [CrossRef]
- Minich, W.B. Selenium metabolism and biosynthesis of selenoproteins in the human body. Biochemistry 2022, 87, S168–S177. [Google Scholar] [CrossRef] [PubMed]
- Antia, M.; Ezejiofor, A.N.; Orish, C.N.; Ugwu, T.; Cirovic, A.; Cirovic, A.; Ajibo, D.N.; Orisakwe, O.E. Zn and Se supplementation abrogated metals-(metaloids) mixture mediated ocular-thymus toxicity via modulation of oxido-inflammatory and antiapoptotic mechanisms in female Sprague Dawley rats. Eur. J. Clin. Exp. Med. 2024, 22, 870–884. [Google Scholar] [CrossRef]
- Sumana, S.L.; Chen, H.; Shui, Y.; Zhang, C.; Yu, F.; Zhu, J.; Su, S. Effect of dietary selenium on the growth and immune systems of fish. Animals 2023, 13, 2978. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The role of selenium in arsenic and cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Han, Q.; Yao, Y.; Xing, H.; Teng, X. Immunosuppression, oxidative stress, and glycometabolism disorder caused by cadmium in common carp (Cyprinus carpio L.): Application of transcriptome analysis in risk assessment of environmental contaminant cadmium. J. Hazard. Mater. 2019, 366, 386–394. [Google Scholar] [CrossRef]
- Borgia, V.; Thatheyus, A.; Murugesan, A. Impact of electroplating industry effluent on the electrophoretic protein pattern of serum in the freshwater fish Cyprinus carpio. Indian. J. Biochem. Biophys. 2019, 56, 460–465. [Google Scholar]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Lee, J.-W.; Jo, A.-H.; Choi, Y.J.; Choi, C.Y.; Kang, J.-C.; Kim, J.-H. Toxic Effects of Cadmium Exposure on Hematological and Plasma Biochemical Parameters in Fish: A Review. Toxics 2024, 12, 699. [Google Scholar] [CrossRef]
- Shahjahan, M.; Islam, M.J.; Hossain, M.T.; Mishu, M.A.; Hasan, J.; Brown, C. Blood biomarkers as diagnostic tools: An overview of climate-driven stress responses in fish. Sci. Total Environ. 2022, 843, 156910. [Google Scholar] [CrossRef]
- Gong, P.; Chen, F.; Liu, X.; Gong, X.; Wang, J.; Ma, Y. Protective effect of caffeic acid phenethyl ester against cadmium-induced renal damage in mice. J. Toxicol. Sci. 2012, 37, 415–425. [Google Scholar] [CrossRef]
- Arroyo, V.; Flores, K.; Ortiz, L.; Gómez-Quiroz, L.; Gutiérrez-Ruiz, M. Liver and cadmium toxicity. J. Drug Metab. Toxicol. S 2012, 5, 1–7. [Google Scholar]
- de la Iglesia, F.A.; Haskins, J.R.; Feuer, G. Hepatotoxicity of cardiovascular and antidiabetic drugs. In Drug-Induced Liver Disease; CRC Press: Boca Raton, FL, USA, 2003; pp. 549–552. [Google Scholar]
- Zhang, C.; Ge, J.; Lv, M.; Zhang, Q.; Talukder, M.; Li, J.-L. Selenium prevent cadmium-induced hepatotoxicity through modulation of endoplasmic reticulum-resident selenoproteins and attenuation of endoplasmic reticulum stress. Environ. Pollut. 2020, 260, 113873. [Google Scholar] [CrossRef] [PubMed]
- Gribling, A.; Wurth, L.; Verheggen, C.; Leichter, M.; Krol, A.; Bertrand, E.; Allmang, C. Selenoprotein mRNA cap hypermethylation and translation initiation. Микрoэлементы в медицине 2013, 14, 44–68. [Google Scholar]
- Chaudière, J. Biological and catalytic properties of selenoproteins. Int. J. Mol. Sci. 2023, 24, 10109. [Google Scholar] [CrossRef]
- Bulteau, A.-L.; Chavatte, L. Update on selenoprotein biosynthesis. Antioxid. Redox Signal. 2015, 23, 775–794. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, V.; Kumar, A.; Misra, A.N. Mechanisms of metalloid uptake, transport, toxicity, and tolerance in plants. In Emerging Trends of Plant Physiology for Sustainable Crop Production Apple; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 167–221. [Google Scholar]
- Ajayi, N.D.; Ajayi, S.A.; Oladoyinbo, O.B.; Olaniyi, O.O. A review of literature on transferrin: Deciphering its complex mechanism in cellular iron regulation and clinical implications. Asian J. Res. Infect. Dis. 2024, 15, 9–23. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yang, S.-G.; Jung, M.-M.; Kim, B.-S.; Yun, S.G.; Choi, C.Y. Effects of waterborne selenium on toxic and physiological stress response in goldfish, Carassius auratus. Mol. Cell. Toxicol. 2015, 11, 35–46. [Google Scholar] [CrossRef]
- Pi, J.; Li, X.; Zhang, T.; Li, D. Effects of acute exposure to sublethal waterborne cadmium on energy homeostasis in silver carp (Hypophthalmichthys molitrix). Bull. Environ. Contam. Toxicol. 2016, 97, 497–503. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology–a review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef]
- Thamizhazhagan, V.; Baranitharan, M.; Sridhar, N.; Senthilmurugan, S. Hematopathology and histolopathology alteration exposure periods of C4H10NO3PS in freshwater Channa pinctata. Asian J. Adv. Res. 2020, 3, 22–33. [Google Scholar]
- Li, X.; Liu, Q.; Pan, Y.; Chen, S.; Zhao, Y.; Hu, Y. New insights into the role of dietary triglyceride absorption in obesity and metabolic diseases. Front. Pharmacol. 2023, 14, 1097835. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, F.M.; Nasr, H.M. Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J. Trace Elem. Med. Biol. 2014, 28, 89–93. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashtjin, Y.A.; Raeeszadeh, M.; Khanghah, A.P. Interaction of Heavy Metals (Cadmium and Selenium) in an Experimental Study on Goldfish: Hematobiochemical Changes and Oxidative Stress. J. Xenobiot. 2025, 15, 57. https://doi.org/10.3390/jox15020057
Hashtjin YA, Raeeszadeh M, Khanghah AP. Interaction of Heavy Metals (Cadmium and Selenium) in an Experimental Study on Goldfish: Hematobiochemical Changes and Oxidative Stress. Journal of Xenobiotics. 2025; 15(2):57. https://doi.org/10.3390/jox15020057
Chicago/Turabian StyleHashtjin, Yasaman Aghaei, Mahdieh Raeeszadeh, and Ali Parsa Khanghah. 2025. "Interaction of Heavy Metals (Cadmium and Selenium) in an Experimental Study on Goldfish: Hematobiochemical Changes and Oxidative Stress" Journal of Xenobiotics 15, no. 2: 57. https://doi.org/10.3390/jox15020057
APA StyleHashtjin, Y. A., Raeeszadeh, M., & Khanghah, A. P. (2025). Interaction of Heavy Metals (Cadmium and Selenium) in an Experimental Study on Goldfish: Hematobiochemical Changes and Oxidative Stress. Journal of Xenobiotics, 15(2), 57. https://doi.org/10.3390/jox15020057






