Abstract
Mexico City is one of the most water-stressed cities in the world; poor quality water occurs in several parts of the City. The use of rainwater harvesting (RWH) as a source of drinking water is gaining acceptance in several contexts, but the quality of the water obtained through these systems has not been sufficiently studied. This manuscript presents the results of water quality tests from samples taken in each component of an RWH system, installed by Isla Urbana at the National Autonomous University of Mexico (UNAM), southern Mexico City. The RWH system culminates with a drinking fountain which supplies water for the students, and other members of the university community. Samples were retrieved from August 2014 to November 2015, approximately once per month. Results showed that with an adequate operation of the RWH system the major ions, fluoride, zinc, arsenic, lead, iron, copper, chromium, aluminum, nitrate, and total coliforms comply with national standards and international guidelines for drinking water. Thus, RWH constitutes a viable option for providing good quality water in a megacity that will become increasingly water-stressed due to climate change.
1. Introduction
As water scarcity has become an increasingly pressing issue worldwide, developing viable sustainable sources is now of the utmost importance. Rainwater harvesting (RWH) is a promising potential sustainable source for urban settings. In order to make the most efficient use of RWH, questions of economic viability, the quantity of water that can be harvested in a specific location, potential methods of implementation, and water quality need to be explored [1]. In this paper, we describe an RWH project in Mexico City—a city that, according to the Nature Conservancy’s 2015 list, is among the most water-stressed in the world [2]. Mexico City’s roughly 22 million inhabitants depend for their water on two main sources: primarily, an intensely overexploited aquifer, and, secondarily, on a highly energy-intensive system that pumps water 1100 m uphill over a 162 km distance [3]. Compounding the problem, extreme and uneven land subsidence, due to intensive extraction of groundwater, continually damages the grid, leading to an estimated 25% loss due to leaks, and an additional 14% that goes unmetered and is largely unaccounted for [4]. In addition, decades of urbanization of natural recharge areas has led to a rapidly deteriorating situation in which providing continuous access to water of acceptable quality is increasingly problematic. This does not even account for the effects of climate change, which is expected to intensify Mexico City’s hydrological cycle and decrease the availability of fresh water, while creating water scarcity in zones that deliver water to the city [5]. The rising population will only exacerbate these problems.
In addition, while some parts of the city receive abundant water of good quality, approximately 310,000 households lack access to running water within the home [6]. Poor water quality is an issue in several parts of the city, many of them located in low-income zones. As stated by Muller [7], it is incumbent upon us to take action to help poor urban communities become more resilient to the potential impacts of climate change. This action should take into account, and be founded upon, the close relationship between the hydrological cycle, urban ecosystems and society. Water management in a large urban area such as Mexico City is a complex issue that involves considering the existence of interactions between social, institutional, biophysical and urban factors [8]. Within this context, RWH as a source of domestic water constitutes one option to cope with water stress. Although multiple pilot programs are currently under way in Mexico City, the water quality, which can be obtained by RWH systems has not been sufficiently studied. Mexico City suffers problems with air pollution [9], a fact that leads many people to conclude that harvested rainwater must be of poor quality. Until now, however, studies in Mexico City have focused on the quality of direct rainfall, which can be very different from the quality of harvested rainwater. To date, and to the best of our knowledge, no detailed studies have been carried out looking specifically at the quality of water harvested with RWH systems.
This paper presents the results of near-monthly water quality tests taken between August 2014 and November 2015 from one RWH system at the National Autonomous University of Mexico (UNAM) main campus in southern Mexico City. The tests included a wide range of pollutants at different points along a professionally designed and well-maintained system.
2. Materials and Methods
2.1. Description of the RWH System
The RWH system used in this study is located at UNAM’s University City campus in southern Mexico City. It was designed and installed by Isla Urbana, a project dedicated to promote water sustainability and RWH in Mexico. The system (Figure 1) consists of the following features:
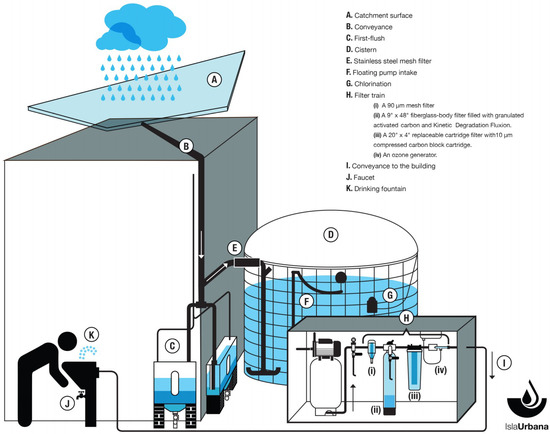
Figure 1.
Diagram of the rainwater harvesting (RWH) system.
- A. Catchment surface. This is a surface of tempered glass that covers the inner courtyard of the building. The roof is approximately 193.22 m2 and the water is channeled through a system of galvanized steel gutters.
- B. Conveyance. The water is transported through polypropylene pipes.
- C. First-flush. Two identical first-flush units developed by Isla Urbana called Tlaloques are used to divert the initial 400 L of precipitation during a rainfall event to wash the roof before water is allowed into the tank.
- D. Cistern. Made from a high-density polyethylene geomembrane contained in a galvanized steel mesh, the cistern has a 30,000 L capacity. The inlet of the cistern has a turbulence-reducing device fitted to reduce the disturbance of sedimented particles at the bottom by incoming water.
- E. Stainless steel mesh filter. Apertures are sized 1 × 2 mm to eliminate leaves and larger debris.
- F. Floating pump intake. Water is extracted from the tank through a pump connected to a floating intake inside the cistern, to draw water from a depth of about 25 cm below the surface level, avoiding the bottom of the tank, where sediments and other contaminants settle.
- G. Chlorination. Inside the cistern, a floating device with capacity for one tablet of 200 g, is used to dispense chlorine from tablets containing 65% of calcium hypochlorite, added every three months.
- H. Filter train. The cistern outflow passes through a filter train consisting of the following elements:
- (i)
- A 90 µm mesh filter.
- (ii)
- A 9″ × 48″ fiberglass body filter filled with granulated activated carbon and Kinetic Degradation Fluxion (KDF), an alloy of copper and zinc used in water purifiers.
- (iii)
- A 20″ × 4″ replaceable cartridge filter with a 10 µm compressed carbon block cartridge.
- (iv)
- An ozone generator.
- I. Conveyance to the building. The water continues from the filters to the drinking fountains, along two potable-grade polypropylene lines with fully thermo-fused joints and couplings to prevent leakage or contamination entry.
- J. Faucet.
- K. Drinking fountain. As a last stage of treatment, the drinking fountains have a filter train consisting of a 5-micron sediments filter and 3-micron compressed active carbon filter, impregnated with colloidal silver as a final disinfectant.
The system was designed with a treatment to guarantee water of drinkable quality. Additionally, to allow for a reduction in atmospheric pollution and rooftop deposits, no rainwater was harvested during the first two weeks of the rainy season (counted as starting when three or more rain events per week occurred for two consecutive weeks).
2.2. Sampling and Analysis
We compared the quality before and after the different stages of filtration (Figure 1). The water samples were taken in the cistern (point D), in the outflow of the mesh filter (point i within the filter train), after the filter train (point iv), from a faucet before the drinking fountain in the building (point J), and at the drinking fountain (point K).
Water samples were collected from August 2014 to November 2015, following methods established by the American Public Health Association (APHA) [10]. Samples from the cistern were taken with glass beakers previously acid washed and rinsed with deionized water, and at other points using polyethylene bottles washed with the same cleaning procedure. The samples were transported to the laboratory, arriving within 30 min of collection.
Temperature, conductivity and pH were measured in situ. Major ions were analyzed following standard methods [10] at the Geophysics Institute. Fluoride and chloride concentrations were determined with ion selective electrodes. Total dissolved solids were measured by gravimetrical analysis. SiO2 and Cr(VI) were analyzed colorimetrically (UV-visible Thermo Evolution 300). Copper, Cr total, Zn, and Fe were measured by flame atomic absorption spectrophotometry (Perkin Elmer AAnalyst 200), As by hydride generation, and Pb by graphite furnace. Nitrate was measured by UV spectrophotometry and checked with ion chromatography (Waters brand). Aluminum was determined by ICP-MS with a Thermo Scientific iCAP. Calibration curves were prepared with High Purity Standards solutions. Detection levels were: As (0.001 mg/L), Pb (0.0005 mg/L), Fe (0.05 mg/L), Zn (0.05 mg/L), Cu (0.05 mg/L), Ni (0.01 mg/L), Crt (0.05 mg/L), Cr(VI) (0.01 mg/L), SiO2 (3 mg/L), Al (0.0001 mg/L), Na+ (1 mg/L), K+ (0.5 mg/L), SO42− (4 mg/L), Ca2+ (0.8 mg/L), Mg2+ (0.8 mg/L), NO3− (0.9 mg/L), F− (0.01 mg/L), Cl− (0.04 mg/L).
Microbiological tests were conducted by the University’s General Direction of Healthcare following Mexican Official Methods by applying the 10-tube MPN method [10]. Free chlorine residual was estimated with a chlorine testing kit (chloroscope).
3. Results and Discussion
The main ions maintained concentrations below limits established by Mexican and International Drinking Water Standards and guidelines [11,12,13] all along the RWH system sampled points (Table 1). Magnesium and bicarbonate were the main ions, reaching maximum values of 4.90 mg/L and 68.20 mg/L respectively. Maximum concentrations of ions measured in the cistern (Table 2) decreased as HCO3−, NO3−, Na+, SO42−, Mg2+, K+, and F− (this does not apply to Ca2+ and Cl− since they were added in the cistern for disinfection). The average pH in the RWH was 6.35 (Table 1), with a minimum of 4.11 on September 2014 in the cistern and a maximum of 8.26 on August 2014 in the faucet. Conductivity presented also the maximum value on the same date, probably due to the initial wash off of the RWH system, and maintained values between 44 μS/cm and 101 μS/cm for the rest of the sampling period.

Table 1.
Physicochemical parameters, major and minor chemical species concentrations (average, maximum and minimum) measured in the RWH system from August 2014 to November 2015; Mexican and the United States Environmental Protection Agency (USEPA) Drinking Water Standards [11,12], and World Health Organization (WHO) [13] are included to compare with this study.

Table 2.
Physicochemical parameters, major and minor chemical species concentrations measured in the cistern from August 2014 to November 2015. Determinations in rainwater reported by García et al. [14] and Báez et al. [15] are included to compare with this study.
Concentrations of heavy metals, and nitrate at each sampling date and each sampling point are shown in Figure 2, Figure 3, Figure 4 and Figure 5. Total and hexavalent chromium were below detection levels in all samples. Copper had concentrations above detection levels in only seven samples from two sampling dates, reaching a maximum of 0.16 mg/L in August 2015 in the sample collected from the particle filter.
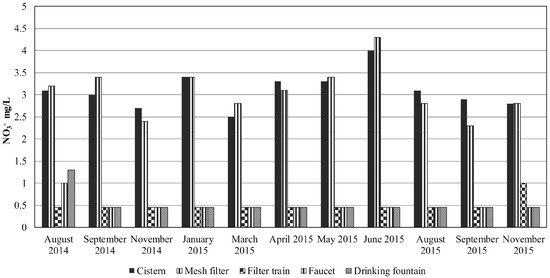
Figure 2.
Concentrations of nitrate (mg/L) in the RWH system during the sampled period.
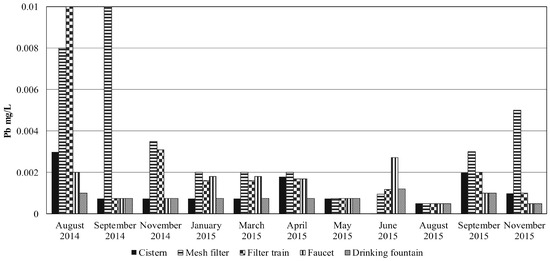
Figure 3.
Concentrations of lead (mg/L) in the RWH system during the sampled period.
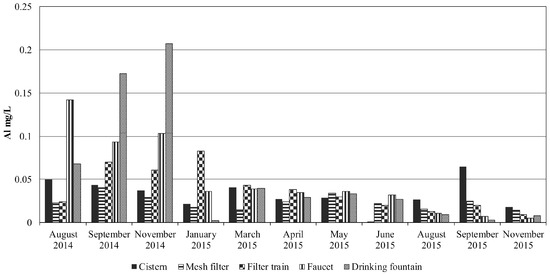
Figure 4.
Concentrations of aluminum (mg/L) in the RWH system during the sampled period.
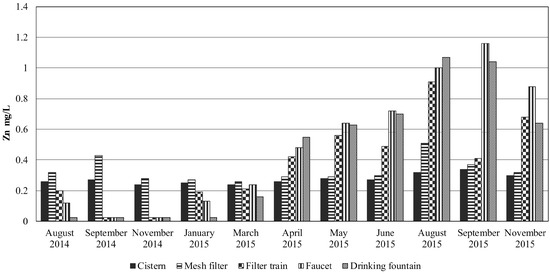
Figure 5.
Concentrations of zinc (mg/L) in the RWH system during the sampled period.
Fluoride is one of the analyzed chemical species, being present in concentrations above Mexican Drinking Water Standards (MDWS) [11] and WHO guidelines [13] in groundwater in many areas of the country. About 4 million people have been estimated to live in areas naturally enriched in fluoride [16,17]. Health effects have been reported in various states, including dental and skeletal fluorosis, and cognitive effects [18,19,20]. In contrast, in the RWH system, fluoride maintained concentrations below the MDWS [11] of 1.5 mg/L during the whole sampling period (Table 1). Collected rainwater in the cistern contained low fluoride levels (below 0.1 mg/L) during all sampling dates (Table 2), which were maintained with slight changes along the RWH system except in August 2014, when 0.21 mg/L in the drinking fountain were measured (Table 3), probably due to an initial wash off from the RWH materials, since the system was put in operation at the end of July and the first harvested water was collected in August.

Table 3.
Physicochemical parameters, major and minor chemical species concentrations measured in the drinking fountain from August 2014 to November 2015.
Arsenic is also one of the toxic elements widely distributed in Mexican aquifers, mostly because of water-rock interaction processes [17,21]. Arsenicosis related to consumption of polluted water has been detected in several areas [22,23,24,25]. In contrast, arsenic maintained very low levels in the RWH system during the studied period (Table 1) with a maximum concentration of 0.004 mg/L, well below the MDWS [11] (0.025 mg/L) and the WHO guideline [13] (0.010 mg/L).
Nitrate concentrations (Figure 2) ranged from less than 0.9 mg/L to 4.3 mg/L (corresponding to 0.2 to 0.97 mg/L N-NO3−), keeping values below the MDWS [11] and USEPA guideline [12] of 10 mg/L N-NO3−. Concentrations were similar to those reported by García et al. [14] in Mexico City rainwater (from 0.47 mg/L to 5.58 mg/L of nitrate). The cistern and the particle filter outflow contained the highest concentrations in all sampling dates; the filter train decreased nitrates to the detection level from September to November. A slight increase in nitrate concentration in the faucet and the drinking fountain in August 2014 may also have been due to the system wash off. Nitrate levels above the drinking water standard have been reported in surficial and groundwater sources in Mexico. Several sources were identified, such as fertilizers, wastewater, and manure [26,27,28]. Nitrate is also present in Mexico City groundwater [29,30]. The lack of sewerage systems in the recharge areas and leaks in the drainage pipes are possible causes of nitrate contamination south of Mexico City [31]. Concentrations up to 2.8 µg/m3 and 3.6 µg/m3 were reported in particulate matter (PM) 10 and PM 2.5 particles (water soluble fraction) collected in Mexico City related to traffic emissions [32]. Daily changes in nitrate atmospheric behavior related to its presence as NH4NO3, evaporation, and reaction of HNO3 with dust were accounted for by Molina et al. [33] and references therein. They also indicate that nitrates constitute a high portion of the NOx in the atmosphere of Mexico City. The highest nitrate concentrations in the RWH system were reached in June 2015 in the cistern and the particulate filter outflow. Similar concentrations measured in the cistern and the particulate filter show that it cannot remove nitrates. However, concentrations below the detection level measured at most dates in the samples obtained after the complete filter train, in the faucet and drinking fountain, showed the overall removal efficiency of the system.
Dust and metals have been found in PM samples collected in Mexico City, mainly associated with the industry and mobile sources [33]. Heavy metals (Fe, Zn, Mn, Pb, Hg, Sn, Cr, Ni, Ti, V, and Ag) are mainly attached to several nm particles [32] that may be carried by the rain and contribute to their presence in the harvested rainwater. However, concentrations of total Cr, Cr(VI), and Fe were below detection levels in the whole sampling period at all the sampling ports of the RWH system.
While Pb concentrations complied with the MDWS [11], it reached a concentration of 0.01 mg/L in the particle filter and filtration train in August and September 2014 (Figure 3). These maxima, as mentioned previously, may have been due to the system’s wash off. Nonetheless, all concentrations measured in the cistern were lower than the average of 0.00541 mg/L reported by García et al. [14] (Table 2).
The highest aluminum concentrations were measured in the drinking fountain in September and November 2014 (Figure 4), reaching the allowable limit established in MDWS (0.2 mg/L). This peak was due to an aluminum Venturi nozzle in the ozone injector, which was promptly replaced by a plastic equivalent. Since then, it maintained low values ranging from concentrations below detection level up to 0.065 mg/L in the cistern in September 2015 (Figure 4). Aluminum presence in the cistern may be due to the input from atmospheric dust. Aluminum in rainwater was related mainly to natural (geological) sources by García et al. [14]. Besides, the roof materials and the physicochemical characteristics of the rain may add metallic contaminants to the harvested rainwater [34].
Although zinc maintained concentrations below MDWS, an increase was observed, mainly from the outflow of the particle filter train and the next sampling ports, from April to September 2015 (Figure 5). This increase may have been due to dissolution from the KDF filter, galvanized gutters and pipes that increases at more acidic pH values (an inverse correlation R2 = 0.692 was determined for Zn vs. pH at the drinking fountain). Actually, pipes are reported as a source of zinc in drinking water by the WHO [13]. Zinc presence in the cistern may be produced from interaction of rainwater with the atmosphere, since this metal had the highest concentrations of the heavy metals measured in Mexico City (482 ng/m3) in the MILAGRO campaign [33]. However, WHO [13] has not established a guideline for this element in drinking water, stating that above 4 mg/L produces an astringent taste to the water.
Although sulfate is not as toxic as As or heavy metals, its maximum concentration is also established in MDWS as 400 mg/L. The WHO [35] recommends informing health authorities if drinking water contains in excess of 500 mg/L due to gastro-intestinal effects at high sulfate levels (laxative effects at 1000 mg/L). Sulfate in Mexico City´s atmosphere may be related to SO2 from industrial sources, although this source decreased with the replacement of fuel oil by natural gas in thermoelectric power plants [36]. Furthermore, emissions from the Popocatépetl volcano have also been identified as a SO2 source in Mexico City´s atmosphere [33] and may also contribute to sulfate in rainwater at some dates.
Microbiological analysis of the drinking water quality showed that in all but two cases, total coliforms at the drinking fountain were absent. In November 2014, results were 0.51/100 mL, and in January 2015, result was 0.051/100 mL. This was due to the building’s cleaning staff using a non-disposable towel to clean the drinking fountain. They were subsequently instructed to use disposable chlorine wipes, after which results returned to non-detectable levels. Free chlorine maintained values between 0.3 and 1.5 mg/L.
Concentrations of ions measured by García et al. [14] in rainwater collected at a site close to the RWH system in Mexico City from 2003 to 2004 are similar to those measured in the cistern (Table 2). Higher concentrations of calcium and chloride result from dissolution of calcium hypochlorite tablets added in the cistern for disinfection. Sulfate was the most abundant ion, followed by Ca2+, NH4+, H+, NO3−, Cl−, Na+, Mg2+, K+, and F− in rainwater collected in the megacity of Shenzhen in China [15]. Although sulfate was also the most abundant ion in Mexico City rainwater, other ions reported by García et al. [14] followed a different trend.
4. Conclusions
This study determined the viability of using rainwater as a source of drinking-quality water in Mexico City, using a system with various stages to capture, store, and treat rainwater.
Despite the presence of air pollution in Mexico City, and the skepticism this naturally causes about the suitability of rainwater for direct human use, this study demonstrates that an appropriately designed rainwater harvesting system can provide water of very high quality, which complies with Mexican and international water quality standards. Interestingly, the water in the tank that had not yet passed through filtration, and had only gone through a leaf screen, first flush system, and chlorination, also complied with drinking water standards for all tested parameters. The measured low levels of fluoride and arsenic are particularly relevant, demonstrating that RWH may be an option to avoid the exposure of the population to these harmful contaminants, which are present in various aquifers used for drinking water supply in Mexico.
Further research is needed to understand the degree to which these results, obtained from a single, carefully monitored and maintained system, apply in less controlled environments such as individual households.
Variations in roof type, system design, and geographic area could result in different water qualities. We recommend future research about water quality in RWH systems located in distinct geographic areas with contrasting air quality, rainfall patterns, climate, and including other parameters and contaminants such as turbidity, Hg, Cd and COV’s, and more extensively microbial testing. The results clearly suggest that, properly done, rainwater is a viable source of high-quality drinking water within the context of a megacity such as Mexico City.
RWH systems also contribute to increasing urban resilience to climate change in Mexico City.
Author Contributions
Conceptualization, M.Í.G., M.A.A.H., M.F.T.F. and E.L.C.; Funding acquisition, M.Í.G.; Investigation, M.Í.G., M.A.A.H. and M.F.T.F.; Methodology, M.Í.G., M.A.A.H. and E.L.C.; Project administration, M.Í.G. and M.F.T.F.; Supervision, M.Í.G. and M.F.T.F.; Visualization, E.L.C.; Writing—original draft, M.A.A.H., M.F.T.F. and E.L.C.; Writing—review and editing, M.Í.G., M.A.A.H., M.F.T.F. and E.L.C.
Funding
This research received no external funding.
Acknowledgments
We acknowledge the participation of Olivia Cruz, Alejandra Aguayo, and Nora Ceniceros in the Analytical Determinations. The General Direction of Healthcare of UNAM is acknowledged for microbiological tests and free chlorine determinations. We thank Renata Fenton for graphical support, and Monserrat Martínez for revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahman, A. Recent Advances in Modelling and Implementation of Rainwater Harvesting Systems towards Sustainable Development. Water 2017, 9, 595. [Google Scholar] [CrossRef]
- McDonald, R.I.; Weber, K.; Padowski, J.; Flörke, M.; Schneider, C.; Green, P.A.; Gleeson, T.; Eckman, S.; Lehner, B.; Balk, D.; et al. Water on an urban planet: Urbanization and the reach of urban water infrastructure. Glob. Environ. Chang. 2014, 27, 96–105. [Google Scholar] [CrossRef]
- CONAGUA (Comisión Nacional del Agua). Estadísticas del Agua en México, Edición 2016, 1st ed.; Secretaríade Medio Ambiente y Recursos Naturales: Ciudad de México, Mexico, 2016; pp. 118–121. [Google Scholar]
- Banco Mundial. Agua urbana en el Valle de México: ¿Un Camino verde para Mañana? 1st ed.; Banco Mundial: Washington, DC, USA, 2013; p. 92. [Google Scholar]
- Romero Lankao, P. Water in Mexico City: What will climate change bring to its history of water-related hazards and vulnerabilities? Environ. Urban. 2010, 22, 157–178. [Google Scholar] [CrossRef]
- INEGI (Instituto Nacional de Estadística y Geografía). Available online: http://www3.inegi.org.mx/sistemas/temas/default.aspx?s=est&c=21385 (accessed on 6 June 2018).
- Muller, M. Adapting to climate change: Water management for urban resilience. Environ. Urban. 2007, 19, 99–113. [Google Scholar] [CrossRef]
- Moreno-Mata, A.; Villasis-Keever, R.; Morató, J. Climatic change, management of water rain and flood risk in the metropolitan area of San Luis Potosí, México. In Urban Resilience for Risk and Adaptation Governance; Brunetta, G., Caldarice, O., Tollin, N., Rosas-Casals, M., Morato, J., Eds.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Molina, L.T.; Molina, M.J. Air Quality in the Mexico Megacity: An Integrated Assesment; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- APHA (American Public Health Association); AWWA (American Water Works Association); WEF (Water Environment Federation); Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; de Edición, N., Ed.; APHA; AWWA; WEF: Washington, DC, USA, 2005; ISBN 0-87553-047-8. [Google Scholar]
- Secretaría de Salud Modificación a la Norma Oficial Mexicana NOM-127-SSA1-1994 (2000). Salud Ambiental. Agua Para uso y Consumo Humano. Límites Permisibles de Calidad y Tratamientos a que se Debe Someter el Agua Para su Potabilización. Available online: http://www.salud.gob.mx/unidades/cdi/nom/m127ssa14.html (accessed on 7 June 2018).
- United States Environmental Protection Agency National Primary Drinking Water Regulations. Available online: https://www.epa.gov/sites/production/files/2016-06/documents/npwdr_complete_table.pdf (accessed on 7 June 2018).
- WHO (World Health Organization). Guidelines for Drinking-Water Quality. Available online: http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf (accessed on 8 June 2018).
- García, R.; Belmont, R.; Padilla, H.; Torres, M.C.; Báez, A. Trace metals and inorganic ion measurements in rain from Mexico City and a nearby rural area. Chem. Ecol. 2009, 25, 71–86. [Google Scholar] [CrossRef]
- Báez, A.; Belmont, R.; García, R.; Padilla, H.; Torres, M.C. Chemical composition of rainwater collected at a southwest site of Mexico City, Mexico. Atmos. Res. 2007, 86, 61–75. [Google Scholar] [CrossRef]
- Estupiñán-Day, S.V.; Heriberto; Duchon, K.; Soto Navarro, P.R.; Vega Gleason, S. Final report Task force meeting: Defluoridation systems for Latin America and the Caribbean. In Task Force Meeting Defluoridation Systems for Latin America and the Caribbean; Pan American Health Organization, Ed.; Pan American Health Organization: Washington, DC, USA, 2005; p. 81. [Google Scholar]
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Grimaldo, M.; Borja-Aburto, V.H.; Ramírez, A.L.; Ponce, M.; Rosas, M.; Díaz-Barriga, F. Endemic fluorosis in San Luis Potosi, Mexico. I. Identification of risk factors associated with human exposure to fluoride. Environ. Res. 1995, 68, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Herrera, M.; Martin-Dominguez, I.; Trejo-Vazquez, R.; Dozal, S. Well water fluoride, dental fluorosis, and bone fractures in the Guadiana Valley of Mexico. Fluoride 2001, 34, 139–149. [Google Scholar]
- Valdez Jimenez, L.; Lopez Guzman, O.D.; Cervantes Flores, M.; Costilla-Salazar, R.; Calderon Hernandez, J.; Alcaraz Contreras, Y.; Rocha-Amador, D.O. In utero exposure to fluoride and cognitive development delay in infants. Neurotoxicology 2017, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Morales-Arredondo, I.; Rodríguez, R.; Armienta, M.A.; Villanueva-Estrada, R.E. The origin of groundwater arsenic and fluorine in a volcanic sedimentary basin in central Mexico: A hydrochemistry hypothesis. Hydrogeology 2016, 24, 1029–1044. [Google Scholar] [CrossRef]
- Sampayo-Reyes, A.; Hernández, A.; El-Yamani, N.; López-Campos, C.; Mayet-Machado, E.; Rincón-Castañeda, C.B.; Limones-Aguilar, M.L.; López-Campos, J.E.; de León, M.B.; González-Hernández, S.; et al. Arsenic induces DNA damage in environmentally exposed Mexican children and adults. Influence of GSTO1 and AS3MT polymorphisms. Toxicol. Sci. 2010, 117, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.M.; Gutiérrez, M.; Alarcón-Herrera, M.T.; Villalba, M.D.L.; Deng, S. Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USA. Chemosphere 2011, 83, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Del Razo, L.M.; García-Vargas, G.G.; Valenzuela, O.L.; Castellanos, E.H.; Sánchez-Peña, L.C.; Currier, J.M.; Drobná, Z.; Loomis, D.; Stýblo, M. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: A cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ. Health 2011, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Currier, J.M.; Ishida, M.C.; González-Horta, C.; Sánchez-Ramírez, B.; Ballinas-Casarrubias, L.; Gutiérrez-Torres, D.S.; Cerón, R.H.; Morales, D.V.; Terrazas, F.A.B.; Del Razo, L.M.; et al. Associations between Arsenic Species in Exfoliated Urothelial Cells and Prevalence of Diabetes among Residents of Chihuahua, Mexico. Environ. Health Perspect. 2014, 122, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Canter, L.W. Nitrate in Groundwater; Lewis Publishers: Boca Raton, FL, USA, 1997. [Google Scholar]
- Muñoz, H.; Armienta, M.A.; Vera, A.; Ceniceros, N. Nitrato en el agua subterránea del Valle de Huamantla, Tlaxcala, México. Rev. Int. Contam. Ambient. 2004, 20, 91–97. [Google Scholar]
- Almasri, M.N. Nitrate contamination of groundwater: A conceptual management framework. Environ. Impact Assess. Rev. 2007, 27, 220–242. [Google Scholar] [CrossRef]
- Ryan, M.C. An investigation of inorganic nitrogen compounds in the groundwater in the Valley of Mexico. Geofis. Int. 1989, 28, 417–433. [Google Scholar]
- Edmunds, W.; Carrillo-Rivera, J.; Cardona, A. Geochemical evolution of groundwater beneath Mexico City. J. Hydrol. 2002, 258, 1–24. [Google Scholar] [CrossRef]
- Montiel-Palma, S.; Armienta Hernández, M.A.; Rodríguez Castillo, R.; Domínguez Mariani, E. Identificación de zonas de contaminación por nitratos en el agua subterránea de la zona sur de la Cuenca de México. Rev. Int. Contam. Ambient. 2014, 30, 149–165. [Google Scholar]
- Querol, X.P.; Pey, J.; Minguillón, M.C.; Pérez, N.; Alastuey, A.; Viana, M.; Moreno, T. PM Speciation and sources in Mexico during the MILAGRO-2006 Campaing. Atmos. Chem. Phys. 2008, 8, 111–128. [Google Scholar] [CrossRef]
- Molina, L.T.M.; Madronich, S.; Gaffney, J.S.; Apel, E.; de Foy, B.; Fast, J.; Ferrare, R.; Herndon, S.; Jimenez, J.L.; Lamb, B.; et al. An overview of the MILAGRO 2006 Campaign: Mexico City emissions and their transport and transformation. Atmos. Chem. Phys. 2010, 10, 8697–8760. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Sources of Pollution in Rooftop Rainwater Harvesting Systems and Their Control. Crit. Rev. Environ. Sci. Technol. 2011, 41, 2097–2167. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Sulfate in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/sulfate.pdf (accessed on 20 June 2018).
- Báez, A.P.; Belmont, R.D.; García, R.M.; Torres, M.C.B.; Padilla, H.G. Rainwater chemical composition at two sites in Central Mexico. Atmos. Res. 2006, 80, 67–85. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).