Simultaneous Removal of NOx and SO2 through a Simple Process Using a Composite Absorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Equipment
3. Results and Discussion
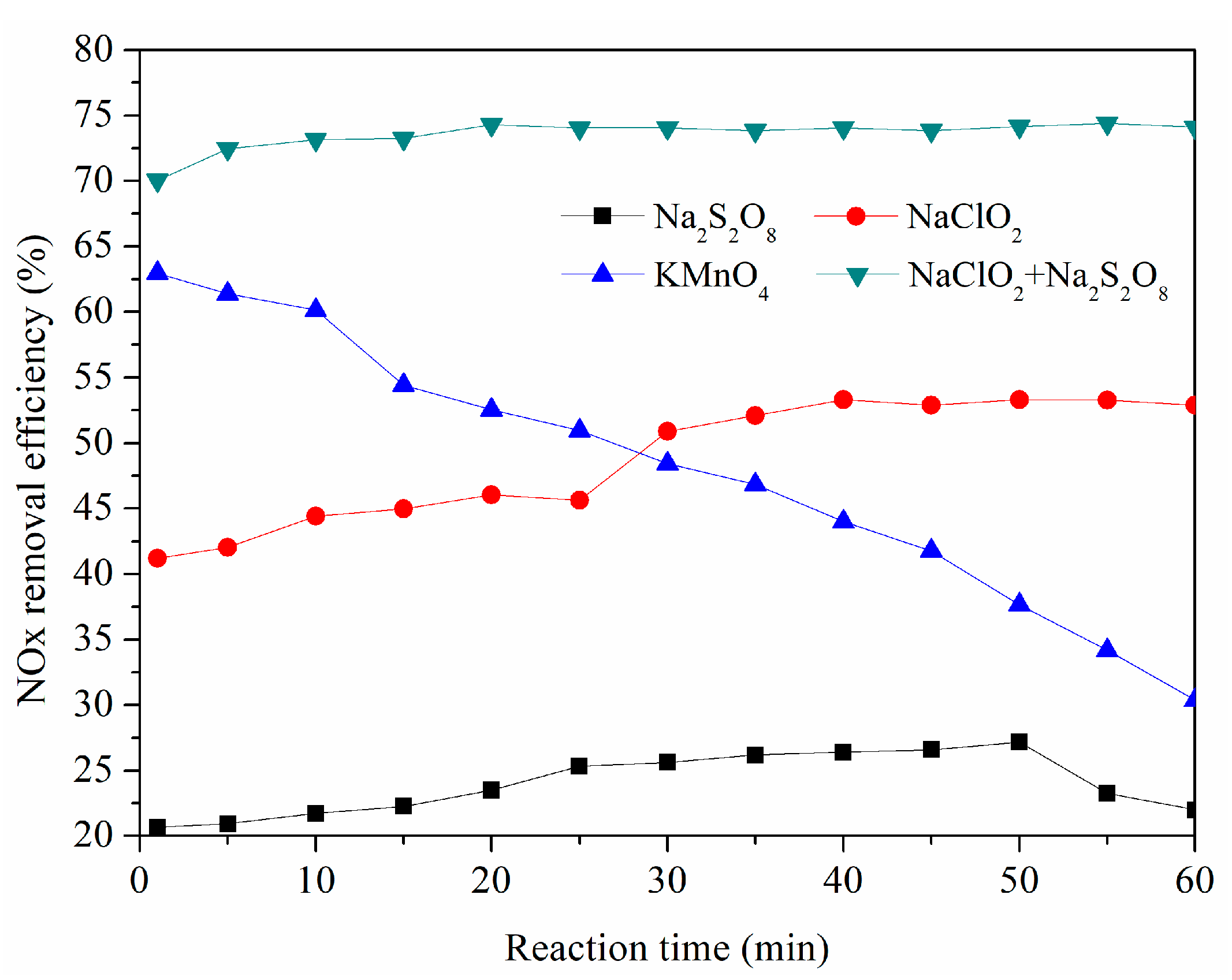
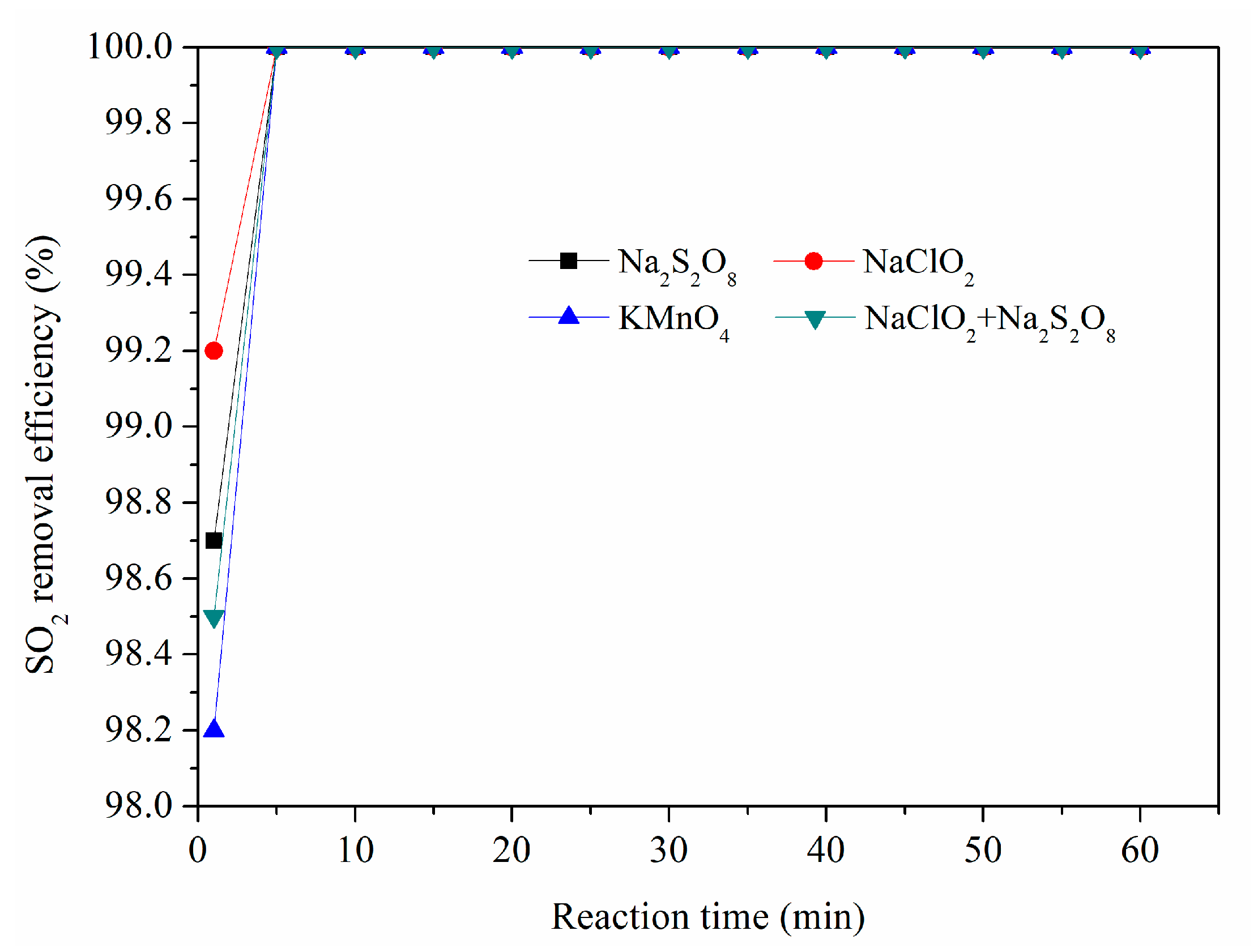
3.1. Simultaneous Removal of SO2 and NOx with Different Oxidant Solutions
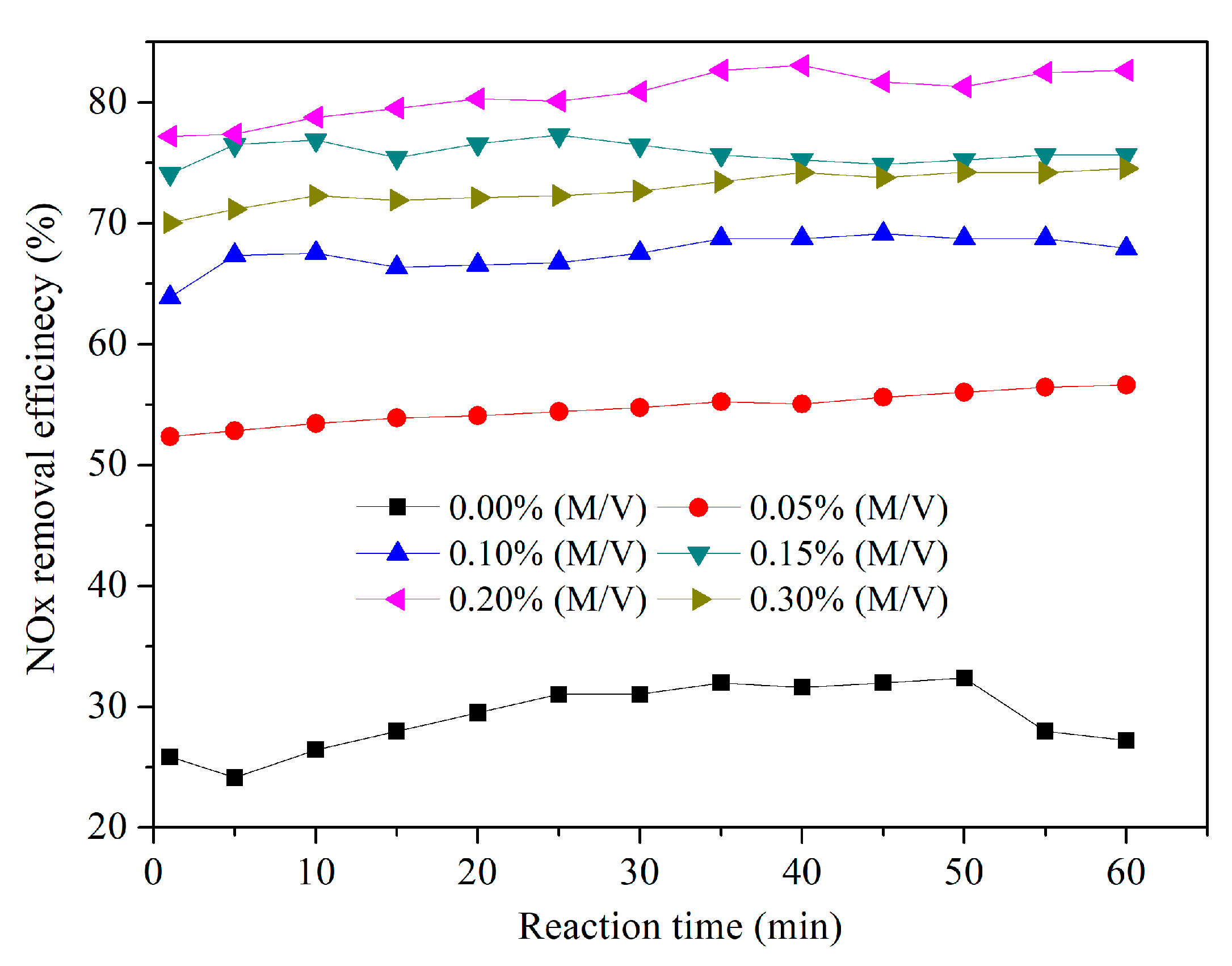
3.2. Effect of NaClO2 Concentration
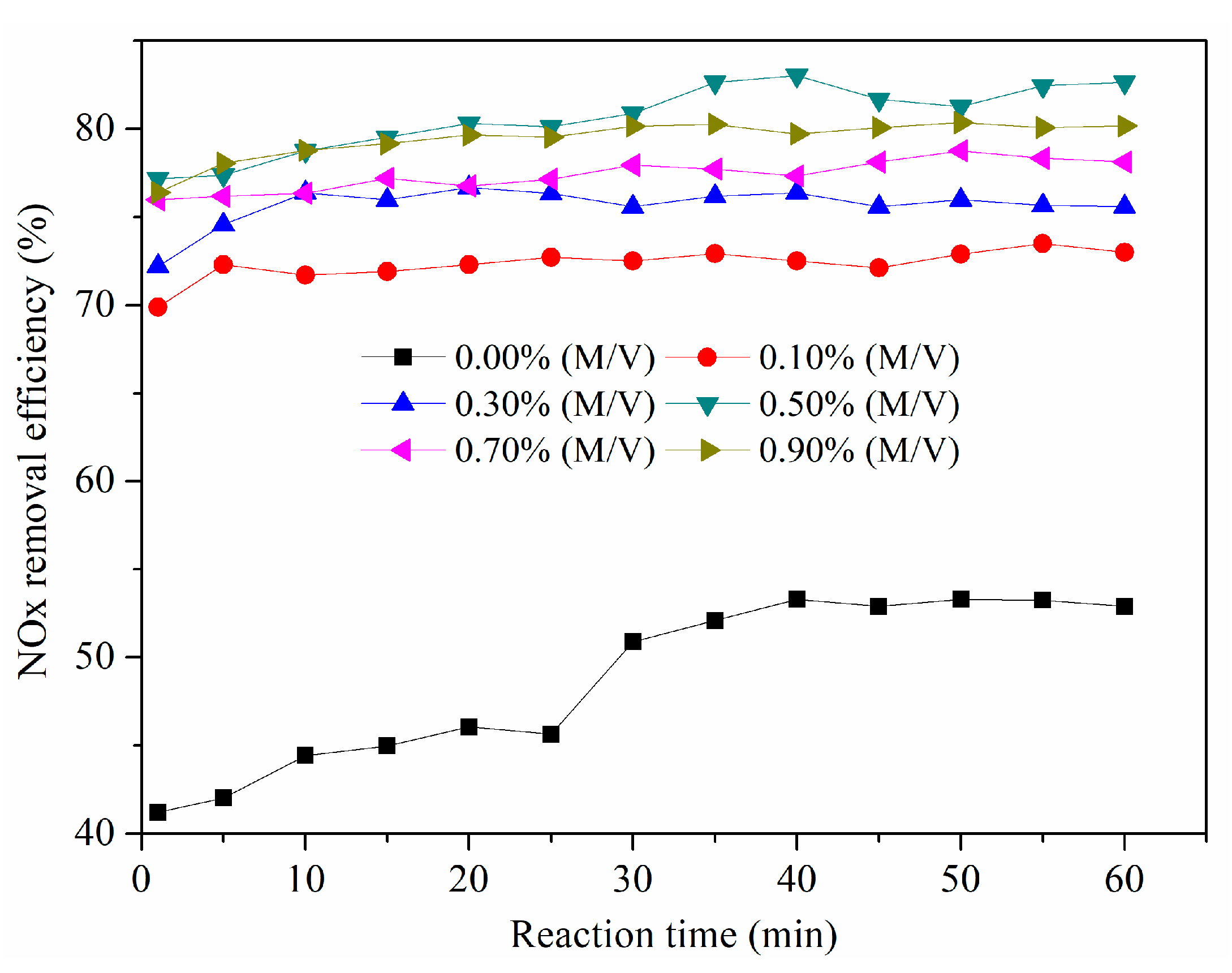
3.3. Effect of Na2S2O8 Concentration
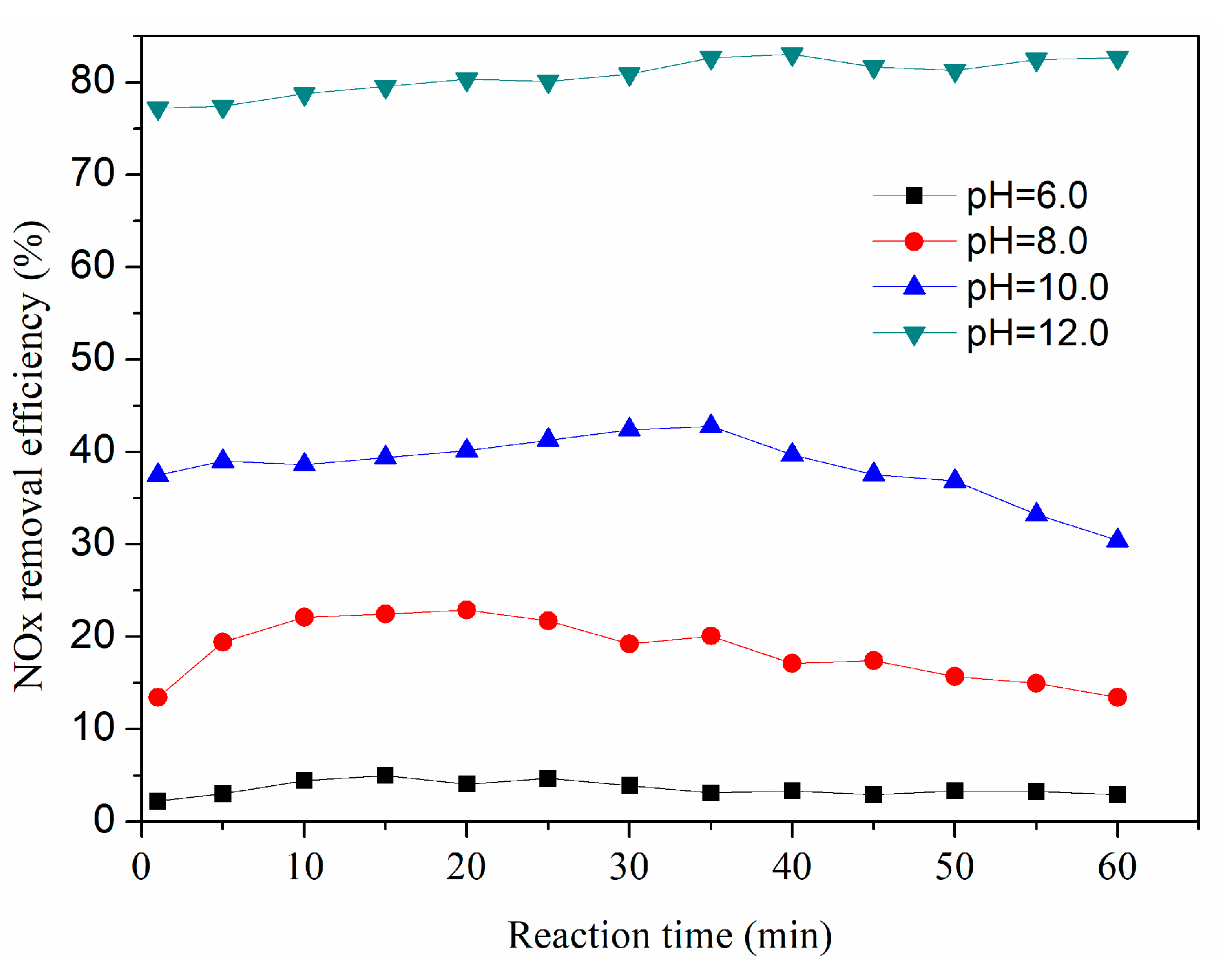
3.4. Effect of Initial pH
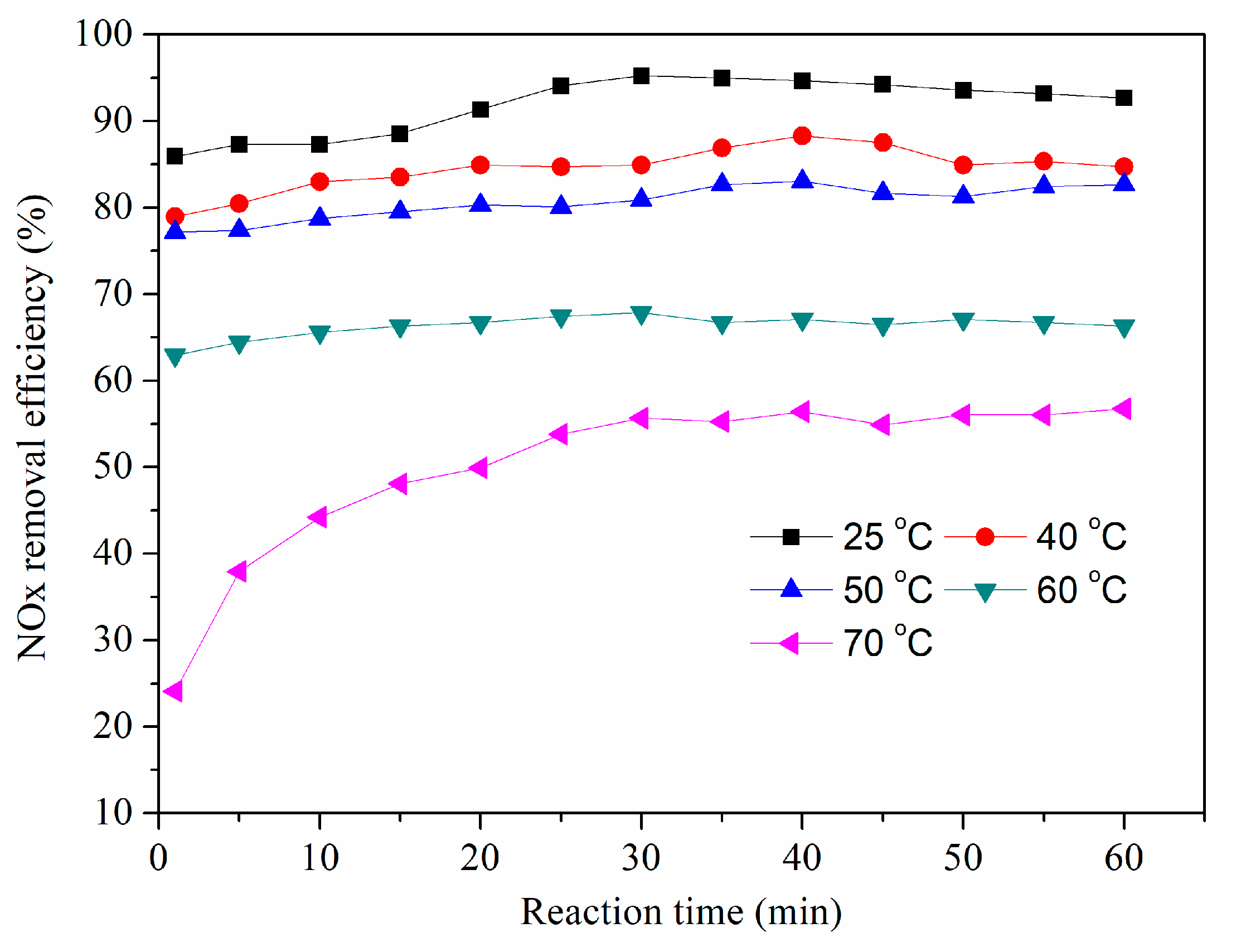
3.5. Effect of Solution Temperature
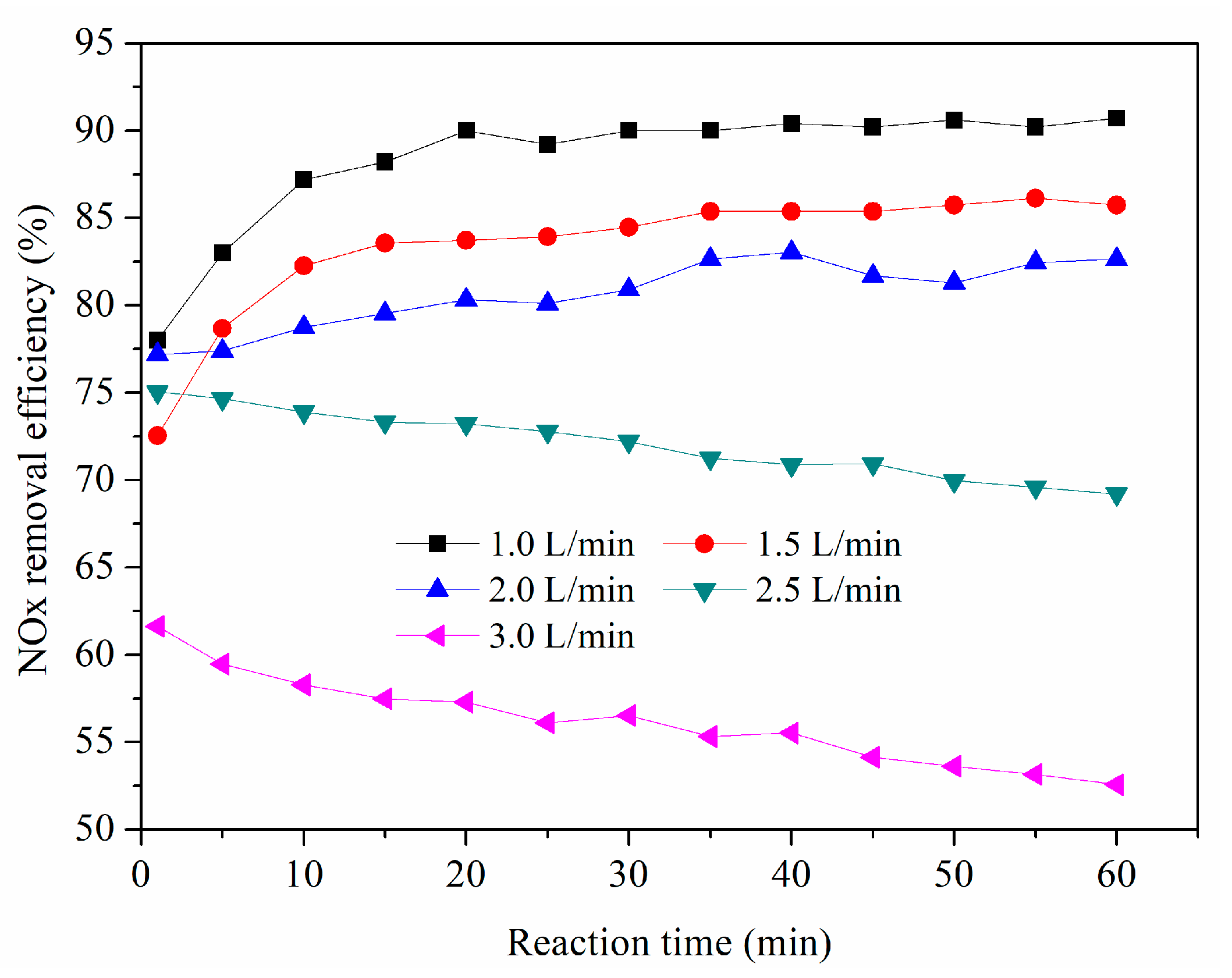
3.6. Effect of Gas Flow Rate
3.7. Effect of SO2 Concentration
3.8. Effect of NO Concentration
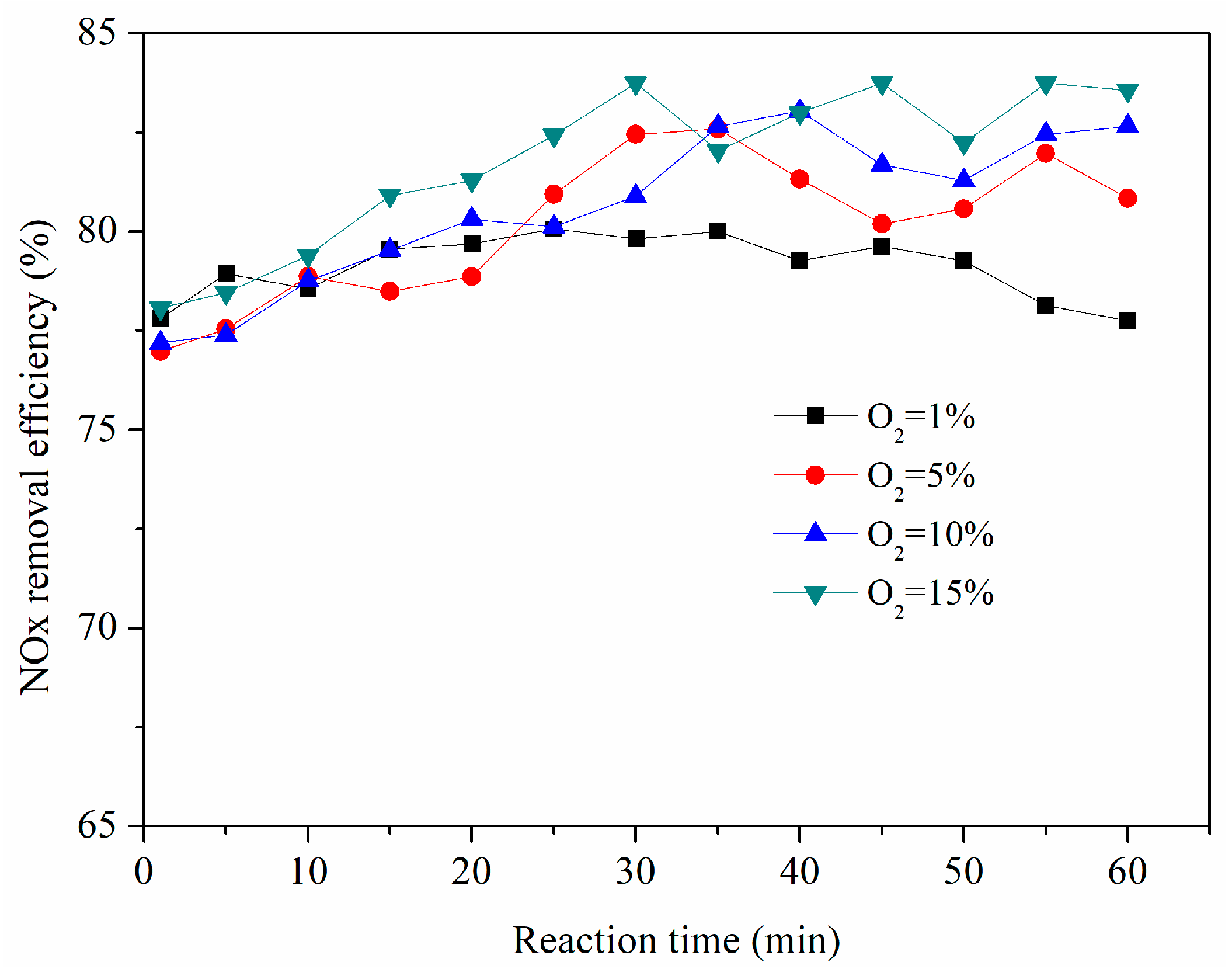
3.9. Effect of O2 Concentration
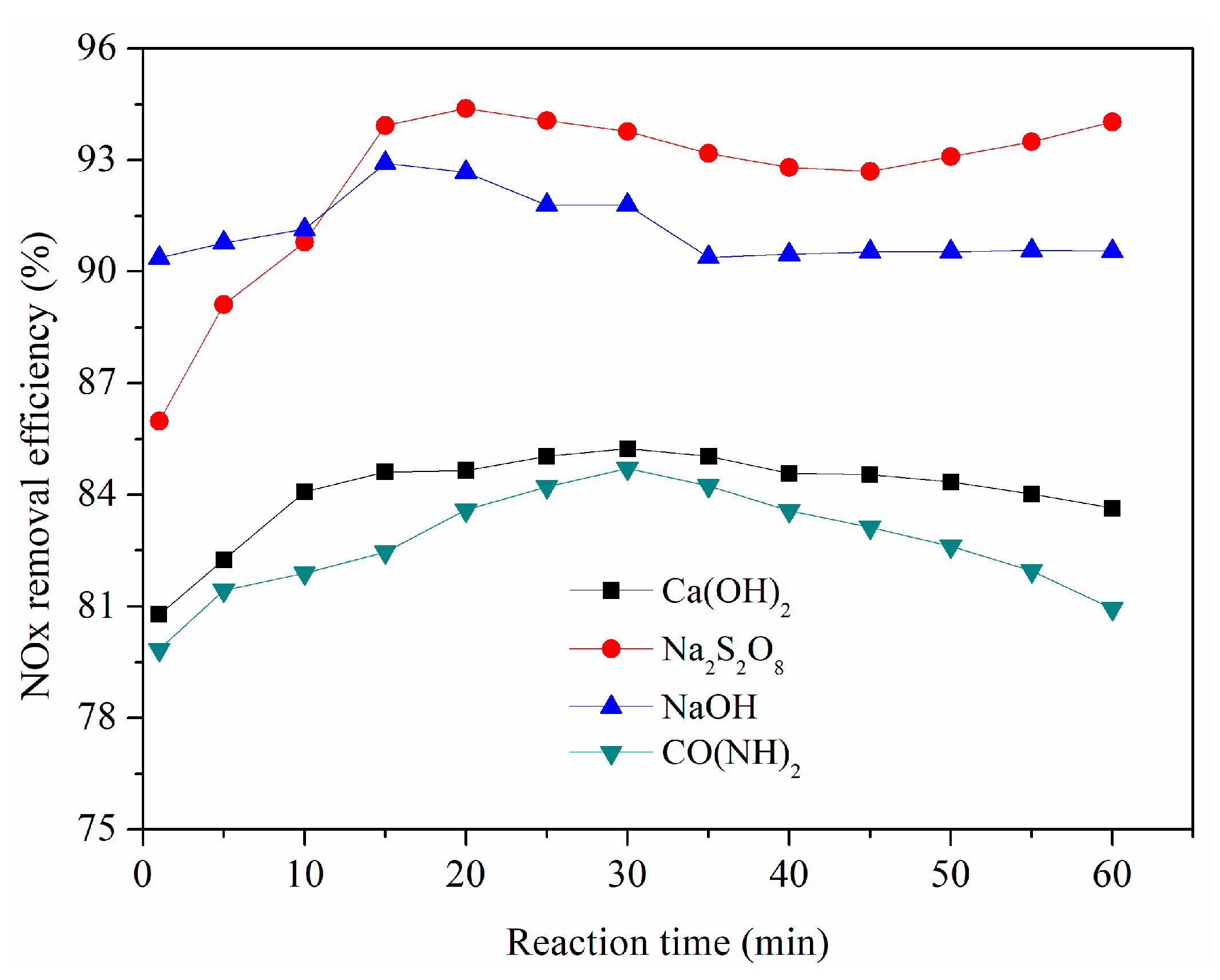
3.10. Tandem Double Column Absorption Experiments
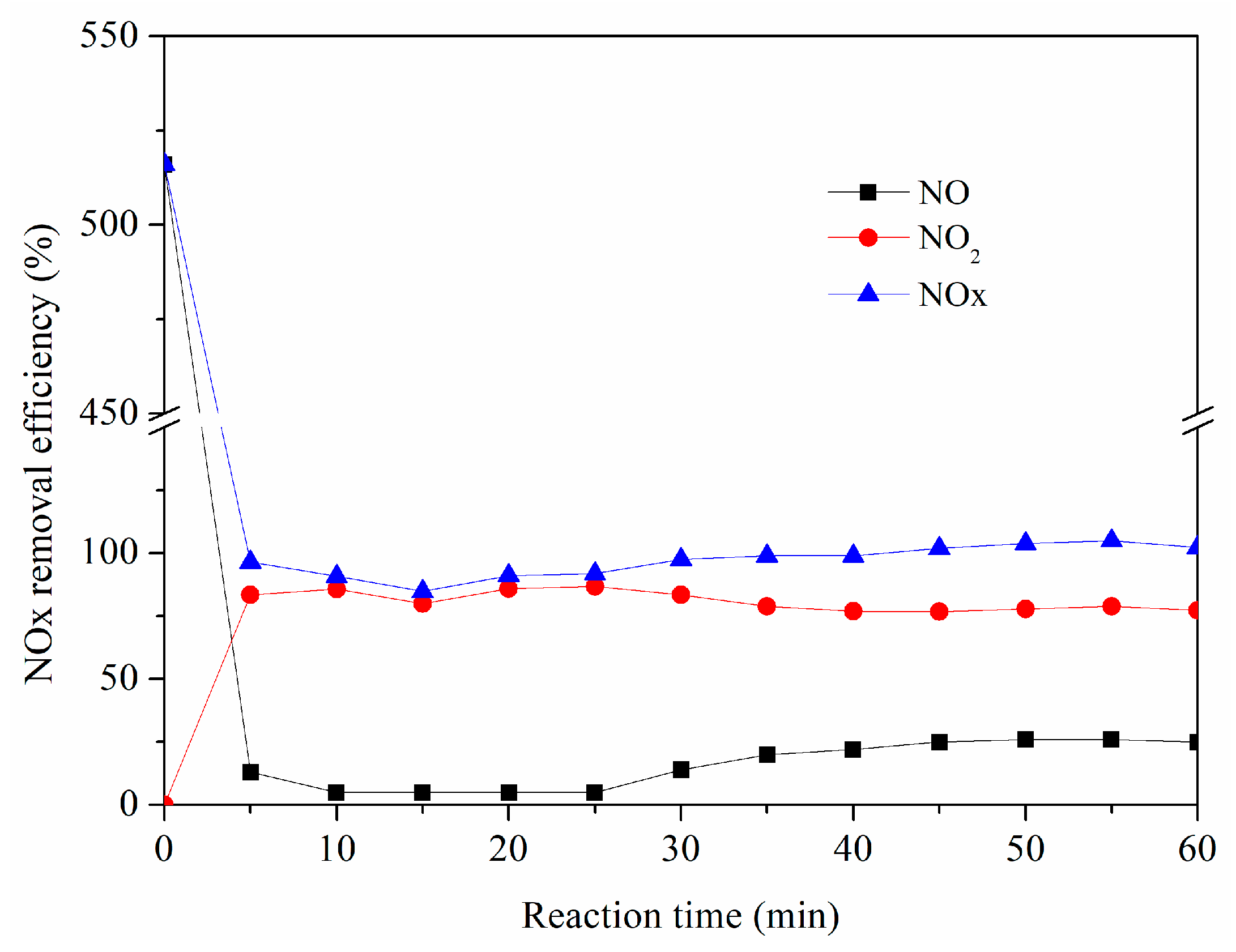
4. Product Analysis
5. Conclusions
- (1)
- NaClO2 in the solution played a more important role than Na2S2O8 for NOx removal. NaClO2 and Na2S2O8 concentrations, solution temperature, the initial pH of the solution, the gas flow rate, and SO2, NO, and O2 concentrations all had a certain impact on the NOx removal efficiency. Among them, solution temperature, the initial pH of the solution, and the oxidant concentrations had significant effects on the NOx removal efficiency.
- (2)
- Considering the NOx removal efficiency and its economic costs, the optimal conditions for NOx removal were determined to be when the solution temperature was 50 °C, the initial solution pH was 12, the gas flow rate was 2 L·min−1, and NaClO2 and Na2S2O8 concentrations were 0.2 wt.% and 0.5 wt.%, respectively. A NOx removal efficiency of more than 80% could be obtained at optimal conditions. When using a NaOH solution as an absorbent in the second absorber, the NOx removal efficiency could reach more than 90%.
- (3)
- A preliminary reaction mechanism for the simultaneous removal of NOx and SO2 was deduced, based on experimental results. The dual oxidant (NaClO2/Na2S2O8) solution can effectively remove multi-pollutants and, thus, it has the potential to be applied in the wet desulfurization and denitration process to realize the synergistic removal of multi-pollutants.
Author Contributions
Funding
Conflicts of Interest
References
- Li, K.W.; Chen, L.H.; White, S.J.; Han, K.; Lv, B.; Bao, K.J.; Wu, X.C.; Gao, X.; Azzi, M.; Cen, K.F. Effect of nitrogen oxides (NO and NO2) and toluene on SO2 photooxidation, nucleation and growth: A smog chamber study. Atmos. Res. 2017, 192, 38–47. [Google Scholar] [CrossRef]
- Yi, Z.; Hao, R.L.; Wang, T.H.; Yang, C.Y. Follow-up research for integrative process of pre-oxidation and post-absorption cleaning flue gas: Absorption of NO2, NO and SO2. Chem. Eng. J. 2015, 272, 55–65. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Deng, J.G.; Li, Z.; Li, Q.; Zhao, P.; Wang, L.G.; Sun, Y.Z.; Zheng, H.X.; Pan, L.; Zhao, S.; et al. Characteristics of NOx emission from Chinese coal-fired power plants equipped with new technologies. Atmos. Environ. 2016, 131, 164–170. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, Y.; Wang, Q.; Pan, J.F.; Zhang, J. Simultaneous removal of NO and SO2 using vacuum ultraviolet light (VUV)/heat/peroxymonosulfate (PMS). Chemosphere 2018, 190, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.X.; Zhao, B.T.; Deng, W.Y. Removal of NO by methane over iron in simulated flue gas with SO2. Fuel 2016, 170, 9–15. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Li, Y.R.; Zhu, T.Y.; Ye, M. Investigation of SO2 and NO adsorption species on activated carbon and the mechanism of NO promotion effect on SO2. Fuel 2015, 143, 536–542. [Google Scholar] [CrossRef]
- Ma, S.M.; Zhao, Y.C.; Yang, J.P.; Zhang, S.B.; Zhang, J.Y.; Zheng, C.G. Research progress of pollutants removal from coal-fired flue gas using non-thermal plasma. Renew. Sustain. Energy Rev. 2017, 67, 791–810. [Google Scholar] [CrossRef]
- Xia, D.H.; Hu, L.L.; He, C.; Pan, W.Q.; Yang, T.S.; Yang, Y.C.; Shu, D. Simultaneous photocatalytic elimination of gaseous NO and SO2 in a BiOI/Al2O3-padded trickling scrubber under visible light. Chem. Eng. J. 2015, 279, 929–938. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhang, Q.; Zhang, G.; Wang, Z.Y.; Zhu, P. Effects of sintering flue gas properties on simultaneous removal of SO2 and NO by ammonia-Fe(II)EDTA absorption. J. Energy Inst. 2017, 90, 522–527. [Google Scholar] [CrossRef]
- Raghunath, C.V.; Pandey, P.; Saini, R.; Mondal, M.K. Absorption of SO2 and NO through an integrative process with a cost-effective aqueous oxidant. Perspect. Sci. 2016, 8, 699–701. [Google Scholar] [CrossRef]
- Fang, P.; Cen, C.P.; Tang, Z.X.; Zhong, P.Y.; Chen, D.S.; Chen, Z.H. Simultaneous removal of SO2 and NOx by wet scrubbing using urea solution. Chem. Eng. J. 2011, 168, 52–59. [Google Scholar] [CrossRef]
- Fang, P.; Cen, C.P.; Wang, X.M.; Tang, Z.J.; Tang, Z.X.; Chen, D.S. Simultaneous removal of SO2, NO and Hg0 by wet scrubbing using urea+KMnO4 solution. Fuel Process. Technol. 2013, 106, 645–653. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, J.; Xie, F.; Wang, Q.; Pan, J.F.; Yin, Y.S. Study on enhancement mechanism of NO absorption in K2FeO4 solution basing on masstransfer-raction theory. Chem. Eng. Res. Des. 2016, 111, 196–203. [Google Scholar] [CrossRef]
- Hao, R.L.; Wang, X.H.; Mao, X.Z.; Tian, B.J.; Zhao, Y.; Yuan, B.; Tao, Z.C.; Shen, Y. An integrated dual-reactor system for simultaneous removal of SO2 and NO: Factors assessment, reaction mechanism and application prospect. Fuel 2018, 220, 240–247. [Google Scholar] [CrossRef]
- Mondal, M.K.; Chelluboyana, V.R. New experimental results of combined SO2 and NO removal from simulated gas stream by NaClO as low-cost absorbent. Chem. Eng. J. 2013, 217, 48–53. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, J.; Sheng, C.D.; Zhang, Y.C.; Zhao, L. Simultaneous removal of NO and SO2 from coal-fired flue gas by UV/H2O2 advanced oxidation process. Chem. Eng. J. 2010, 162, 1006–1011. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhou, J.H.; Zhu, Y.Q.; Wen, Z.C.; Liu, J.Z.; Cen, K.F. Simultaneous removal of NOx, SO2 and Hg in nitrogen flow in a narrow reactor by ozone injection: Experimental results. Fuel Process. Technol. 2007, 88, 817–823. [Google Scholar] [CrossRef]
- Xu, X.H.; Ye, Q.F.; Tang, T.M.; Wang, D.H. Hg0 oxidative absorption by K2S2O8 solution catalyzed by Ag+ and Cu2+. J. Hazard. Mater. 2008, 158, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Liu, Z.Y.; Wang, Y.; Yin, Y.S.; Pan, J.F.; Zhang, J.; Wang, Q. Simultaneous absorption of SO2 and NO from flue gas using ultrasound/Fe2+/heat coactivated persulfate system. J. Hazard. Mater. 2018, 342, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Tang, Z.J.; Chen, X.B.; Huang, J.H.; Chen, D.S.; Tang, Z.X.; Cen, C.P. Split, partial oxidation and mixed absorption: A novel process for synergistic removal of multiple pollutants from simulated flue gas. Ind. Eng. Chem. Res. 2017, 56, 5116–5126. [Google Scholar] [CrossRef]
- Hao, R.L.; Wang, X.H.; Mao, X.Z.; Tian, B.J.; Zhao, Y.; Yuan, B.; Tao, Z.C.; Shen, Y. Simultaneous desulfurization and denitrification through an integrative process utilizing NaClO2/Na2S2O8. Fuel Process. Technol. 2017, 159, 145–152. [Google Scholar] [CrossRef]
- Wang, Z.P.; Wang, Z.W.; Ye, Y.; Chen, N.; Li, H.W. Study on the removal of nitric oxide (NO) by dual oxidant (H2O2/S2O82−) system. Chem. Eng. Sci. 2016, 145, 133–140. [Google Scholar] [CrossRef]
- Khan, N.E.; Adewuyi, Y.G. Absorption and oxidation of nitric oxide (NO) by aqueous solutions of sodium persulfate in a bubble column reactor. Ind. Eng. Chem. Res. 2010, 49, 8749–8760. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Khan, M.A.; Sakyi, N.Y. Owusu, Ultrasound-Induced aqueous removal of nitric oxide from flue gases: Effects of sulfur dioxide, chloride, and chemical oxidant. J. Phys. Chem. A 2006, 110, 11098–11107. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Tratnyek, P.G.; Johnson, R.O. Sulfate radical-based degradation of polychlorinated biphenyls: Effects of chloride ion and reaction kinetics. J. Hazard. Mater. 2012, 227–228, 394–401. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Khan, M.A.; Sakyi, N.Y. Kinetics and modeling of the removal of nitric oxide by aqueous sodium persulfate simultaneously activated by temperature and Fe2+. Ind. Eng. Chem. Res. 2014, 53, 828–839. [Google Scholar] [CrossRef]
- Johnson, R.L.; Tratnyek, P.G.; Johnson, R.O. Persulfate persistence under thermal activation conditions. Environ. Sci. Technol. 2008, 42, 9350–9356. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.; Luo, Y.B.; Yu, P.; Cai, B.; Tan, H.Z. Removal of NO from flue gas by wet scrubbing with NaClO2/(NH2)2CO solutions. J. Ind. Eng. Chem. 2009, 15, 16–22. [Google Scholar] [CrossRef]
- Deshwal, B.R.; Jin, D.S.; Lee, S.H.; Moon, S.H.; Jung, J.H.; Lee, H.K. Removal of NO from flue gas by aqueous chlorine-dioxide scrubbing solution in a lab-scale bubbling reactor. J. Hazard. Mater. 2008, 150, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Q. Removal of elemental mercury from flue gas by thermally activated ammonium persulfate in a bubble column reactor. Environ. Sci. Technol. 2014, 48, 12181–12189. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, Y.G.; Sakyi, N.Y. Simultaneous absorption and oxidation of nitric oxide and sulfur dioxide by aqueous solutions of sodium persulfate activated by temperature. Ind. Eng. Chem. Res. 2013, 52, 11702–11711. [Google Scholar] [CrossRef]
- Lin, S.F.; Zhang, B.H.; Wei, Z.H.; Yang, L.; Ma, X.X. Initial study on simultaneous absorption and oxidation of NO and SO2 from coal-fired flue gas by aqueous solutions of sodium persulfate. Appl. Chem. Ind. 2015, 44, 1021–1025. (In Chinese) [Google Scholar] [CrossRef]
- Park, H.W.; Choi, S.; Park, D.W. Simultaneous treatment of NO and SO2 with aqueous NaClO2 solution in a wet scrubber combined with a plasma electrostatic precipitator. J. Hazard. Mater. 2015, 285, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Tang, Z.J.; Chen, X.B.; Huang, J.H.; Tang, Z.X.; Cen, C.P. Chloride ion removal from the wet flue gas desulfurization and denitrification wastewater using Friedel’s salt precipitation method. J. Chem. 2018, 2018, 5461060. [Google Scholar] [CrossRef]



| No. | Experiment | Experimental Conditions |
|---|---|---|
| 1 | Removal of SO2 and NO with different oxidant solutions | Single absorber, Q = 2 L/min, [NO] = 500 ppm, [SO2] = 1000 ppm, O2 = 10%, Tabsorption = 50 °C |
| 2 | Effect of NaClO2 concentration | Single absorber, Q = 2 L/min, [NO] = 500 ppm, [SO2] = 1000 ppm, O2 = 10%, Tabsorption = 50 °C, [Na2S2O8] = 0.5 wt.%, pH = 12 |
| 3 | Effect of Na2S2O8 concentration | Single absorber, Q = 2 L/min, [NO] = 500 ppm, [SO2] = 1000 ppm, O2 = 10%, Tabsorption = 50 °C, [NaClO2] = 0.2 wt.%, pH = 12 |
| 4 | Effect of initial pH | Single absorber, Q = 2 L/min, [NO] = 500 ppm, [SO2] = 1000 ppm, O2 = 10%, Tabsorption = 50 °C, [Na2S2O8] = 0.5 wt.%, [NaClO2] = 0.2 wt.% |
| 5 | Effect of solution temperature | Single absorber, Q = 2 L/min, [NO] = 500 ppm, [SO2] = 1000 ppm, O2 = 10%, [Na2S2O8] = 0.5 wt.%, [NaClO2] = 0.2 wt.%, pH = 12 |
| 6 | Effect of gas flow rate | Single absorber, [NO] = 500 ppm, [SO2] = 1000 ppm, O2 = 10%, Tabsorption = 50 °C, [Na2S2O8] = 0.5 wt.%, [NaClO2] = 0.2 wt.%, pH = 12 |
| 7 | Effect of SO2 concentration | Single absorber, Q = 2 L/min, [NO] = 500 ppm, O2 = 10%, Tabsorption = 50 °C, [Na2S2O8] = 0.5 wt.%, [NaClO2] = 0.2 wt.%, pH = 12 |
| 8 | Effect of NO concentration | Single absorber, Q = 2 L/min, [SO2] = 1000 ppm, O2 = 10%, Tabsorption = 50 °C, [Na2S2O8] = 0.5 wt.%, [NaClO2] = 0.2 wt.%, pH = 12 |
| 9 | Effect of O2 concentration | Single absorber, Q = 2 L/min, [NO] = 500 ppm, [SO2] = 1000 ppm, Tabsorption = 50 °C, [Na2S2O8] = 0.5 wt.%, [NaClO2] = 0.2 wt.%, pH = 12 |
| 10 | Tandem double column absorption experiments | Q = 2 L/min, [NO] = 500 ppm, [SO2] = 1000 ppm, O2 = 10%; Tabsorption = 50 °C, [Na2S2O8] = 0.5 wt.%, [NaClO2] = 0.2 wt.%, pH = 12 (the first absorber); Tabsorption = 25 °C, [reagent] = 5 wt.% (the second absorber) |
| 11 | Product analysis | Single absorber, Q = 2 L/min, [NO] = 500 ppm, [SO2] = 1000 ppm, O2 = 10%, Tabsorption = 50 °C, [Na2S2O8] = 0.5 wt.%, [NaClO2] = 0.2 wt.%, pH = 12 |
| NO2− | NO3− | Cl− | ClO2− | ClO3− | |
|---|---|---|---|---|---|
| Initial | — | — | 95.888 | 901.050 | 119.975 |
| End | — | 142.58 | 145.515 | 845.950 | 85.425 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, P.; Tang, Z.; Chen, X.; Zhong, P.; Huang, J.; Tang, Z.; Cen, C. Simultaneous Removal of NOx and SO2 through a Simple Process Using a Composite Absorbent. Sustainability 2018, 10, 4350. https://doi.org/10.3390/su10124350
Fang P, Tang Z, Chen X, Zhong P, Huang J, Tang Z, Cen C. Simultaneous Removal of NOx and SO2 through a Simple Process Using a Composite Absorbent. Sustainability. 2018; 10(12):4350. https://doi.org/10.3390/su10124350
Chicago/Turabian StyleFang, Ping, Zijun Tang, Xiongbo Chen, Peiyi Zhong, Jianhang Huang, Zhixiong Tang, and Chaoping Cen. 2018. "Simultaneous Removal of NOx and SO2 through a Simple Process Using a Composite Absorbent" Sustainability 10, no. 12: 4350. https://doi.org/10.3390/su10124350
APA StyleFang, P., Tang, Z., Chen, X., Zhong, P., Huang, J., Tang, Z., & Cen, C. (2018). Simultaneous Removal of NOx and SO2 through a Simple Process Using a Composite Absorbent. Sustainability, 10(12), 4350. https://doi.org/10.3390/su10124350





