Struvite Phosphorus Recovery from Aerobically Digested Municipal Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Characterization
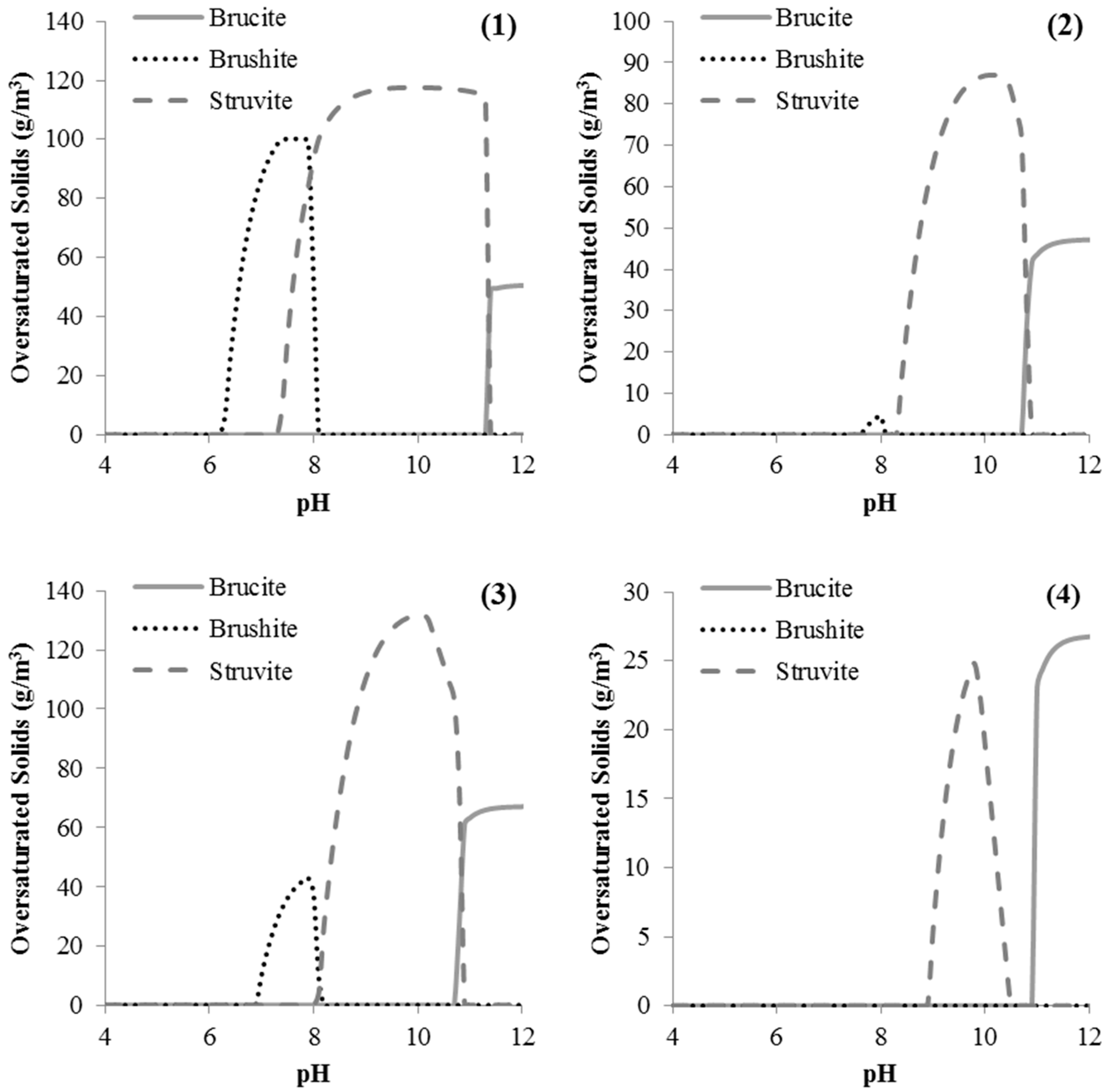
2.2. Model Predictions
2.3. Experimental Procedure
2.4. Statistical Analyses
3. Results
3.1. Filtrate Characterization
3.2. Effect of NaOH, Sparging and Mg on Struvite Precipitation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable use of phosphorus: A finite resource. Sci. Total Environ. 2013, 461–462, 799–803. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. United States Environmental Protection Agency Clean Watersheds Needs Survey 2012 Report to Congress; U.S. EPA: Washington, DC, USA, 2016; p. 41.
- Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F.L.; Abu-Orf, M.; Bowden, G.; Pfrang, W. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; Metcalf & Eddy, Ed.; McGraw-Hill Education: New York, NY, USA, 2014; ISBN 978-0-07-340118-8. [Google Scholar]
- Vaccari, D.A. Sustainability and the Phosphorus Cycle: Inputs, Outputs, Material Flow, and Engineering. Environ. Eng. Appl. Res. Pract. 2011, 12, 1–11. [Google Scholar]
- U.S. Department of Agriculture Economic Research Service (USDA ERS). Fertilizer Use and Price. Available online: https://www.ers.usda.gov/data-products/fertilizer-use-and-price/ (accessed on 13 June 2018).
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- FDEP. Biosolids in Florida: 2013 Summary; Domestic Wastewater Program; Florida Department of Environmental Protection: Tallahassee, FL, USA, 2014; p. 26.
- Davis, M.L. Water and Wastewater Engineering Design Principles and Practice; McGraw-Hill: New York, NY, USA, 2013; ISBN 978-0-07-171384-9. [Google Scholar]
- Nowak, O. Optimizing the Use of Sludge Treatment Facilities at Municipal WWTPs. J. Environ. Sci. Health Part A 2006, 41, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Jefferson, B.; Parsons, S.A. Agglomeration of struvite crystals. Water Res. 2007, 41, 419–425. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Rawn, A.M.; Banta, A.P.; Pomeroy, R. Multiple-stage sewage sludge digestion. Trans. Am. Soc. Civ. Eng. 1939, 104, 93–119. [Google Scholar]
- Venkatesan, A.K.; Hamdan, A.-H.M.; Chavez, V.M.; Brown, J.D.; Halden, R.U. Mass Balance Model for Sustainable Phosphorus Recovery in a US Wastewater Treatment Plant. J. Environ. Qual. 2014, 45, 84. [Google Scholar] [CrossRef]
- Battistoni, P.; Boccadoro, R.; Fatone, F.; Pavan, P. Auto-Nucleation and Crystal Growth of Struvite in a Demonstrative Fluidized Bed Reactor (FBR). Environ. Technol. 2005, 26, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Fattah, K.P.; Mavinic, D.S.; Koch, F.A.; Jacob, C. Determining the feasibility of phosphorus recovery as struvite from filter press centrate in a secondary wastewater treatment plant. J. Environ. Sci. Health Part A 2008, 43, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Latimer, R.; Nguyen, V.; Smeby, K.; Vadiveloo, E.; Pitt, P.; Sawyer, H.; Harris, R.; Porter, R.; Elmendorf, H.; Richards, T. Nutrient Recovery from Was Streams for Struvite Control and Recycle Load Reduction. Proc. Water Environ. Fed. 2012, 2012, 564–589. [Google Scholar] [CrossRef]
- U.S. EPA. POTW Sludge Sampling and Analysis Guidance Document; Office of Water Office of Wastewater Enforcement & Compliance: Washington DC, USA, 1989; ISBN Document No. PB93-227957.
- AWWA; American Public Health Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Water Works Assn: Washington, DC, USA, 2012; ISBN 978-0-87553-013-0. [Google Scholar]
- U.S. EPA. Methods for the Determination of Metals in Environmental Samples; U.S. Environmental Protection Agency: Washington, DC, USA, 1991.
- Gustafsson, J.P. Visual MINTEQ Ver 3.0; Department of Land and Water Resources Engineering, Royal Institute of Technology: Stockholm, Sweden, 2011. [Google Scholar]
- Ohlinger, K.N.; Young, T.M.; Schroeder, E.D. Predicting struvite formation in digestion. Water Res. 1998, 32, 3607–3614. [Google Scholar] [CrossRef]
- Hao, X.-D.; Wang, C.-C.; Lan, L.; van Loosdrecht, M.C.M. Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Musvoto, E.V.; Wentzel, M.C.; Ekama, G.A. Integrated chemical–physical processes modelling—II. simulating aeration treatment of anaerobic digester supernatants. Water Res. 2000, 34, 1868–1880. [Google Scholar] [CrossRef]
- Babić-Ivančić, V.; Kontrec, J.; Brečević, L.; Kralj, D. Kinetics of struvite to newberyite transformation in the precipitation system MgCl2–NH4H2PO4–NaOH–H2O. Water Res. 2006, 40, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Çelen, I.; Buchanan, J.R.; Burns, R.T.; Bruce Robinson, R.; Raj Raman, D. Using a chemical equilibrium model to predict amendments required to precipitate phosphorus as struvite in liquid swine manure. Water Res. 2007, 41, 1689–1696. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards; International Center for Diffraction Data: Swarthmore, PA, USA, 1980.
- Lew, B.; Phalah, S.; Sheindorf, C.; Kummel, M.; Rebhun, M.; Lahav, O. Favorable Operating Conditions for Obtaining High-Value Struvite Product from Sludge Dewatering Filtrate. Environ. Eng. Sci. 2010, 27, 733–741. [Google Scholar] [CrossRef]
- Korchef, A.; Saidou, H.; Amor, M.B. Phosphate recovery through struvite precipitation by CO2 removal: Effect of magnesium, phosphate and ammonium concentrations. J. Hazard. Mater. 2011, 186, 602–613. [Google Scholar] [CrossRef]
- Cohen, Y.; Kirchmann, H. Increasing the pH of Wastewater to High Levels with Different Gases—CO2 Stripping. Water Air Soil Pollut. 2004, 159, 265–275. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Electric Power Monthly; U.S. Energy Information Administration: Washington, DC, USA, 2017. Available online: https://www.eia.gov/electricity/monthly/ (accessed on 28 January 2018).
- Lahav, O.; Telzhensky, M.; Zewuhn, A.; Gendel, Y.; Gerth, J.; Calmano, W.; Birnhack, L. Struvite recovery from municipal-wastewater sludge centrifuge supernatant using seawater NF concentrate as a cheap Mg(II) source. Sep. Purif. Technol. 2013, 108, 103–110. [Google Scholar] [CrossRef]
- GLUMRB. Recommended Standards for Wastewater Facilities; Great Lakes Upper Mississippi River Board of State and Provincial Public Health and Environmental Mangers: Albany, NY, USA, 2014; p. 175. [Google Scholar]
- U.S. ACE. Civil Works Construction Cost Index System Manual; United States Army Corps of Engineers: Washington, DC, USA, 2003; p. 57.
- U.S. EPA. Construction Costs for Municipal Wastewater Treatment Plants: 1973–1982; United States Environmental Protection Agency, Office of Water Program Operations: Washington, DC, USA, 1983; p. 259.
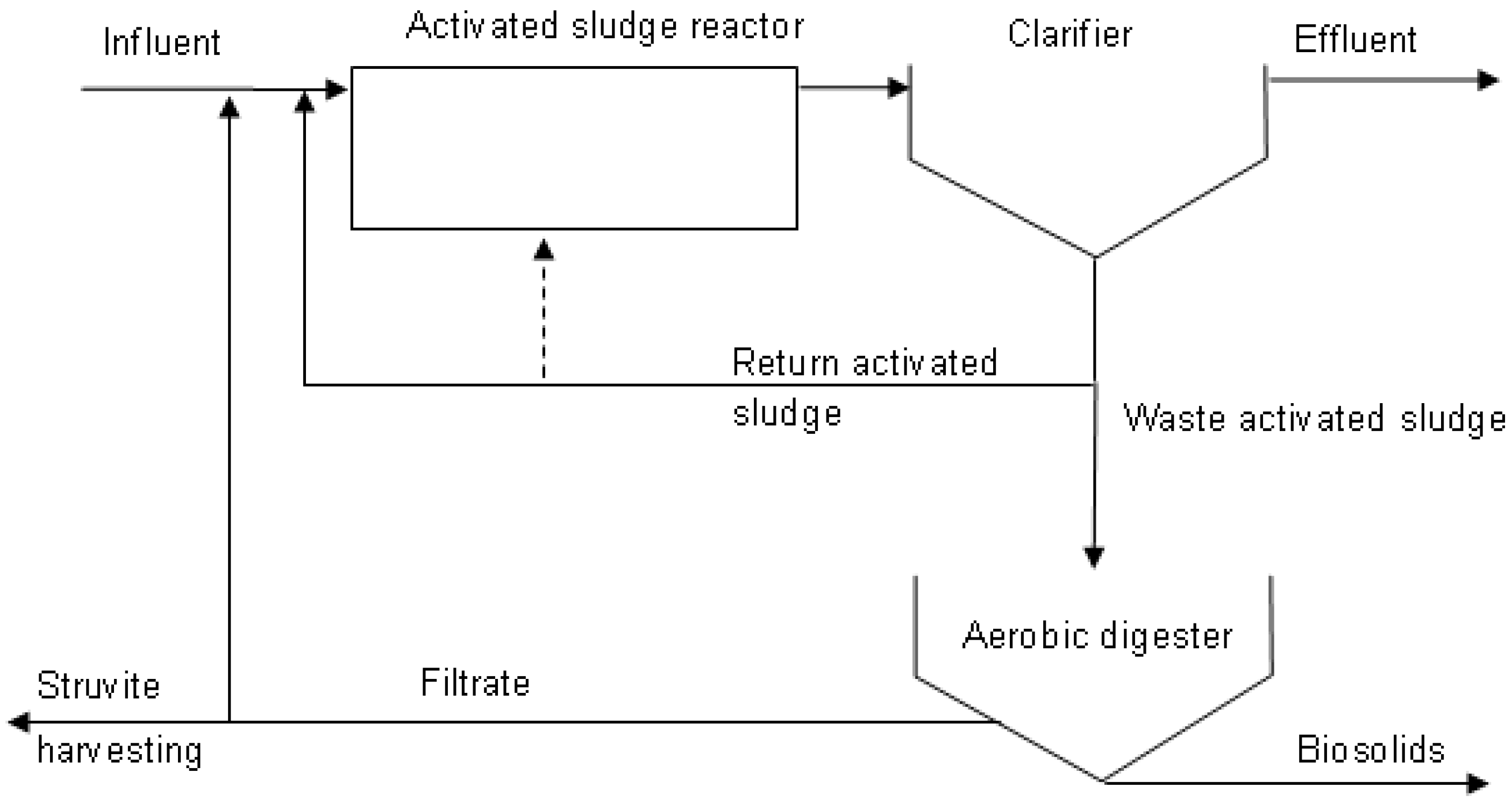




| Parameters | Wastewater Treatment Plant | |||
|---|---|---|---|---|
| 1 1 | 2 2 | 3 2 | 4 2 | |
| Population Served | 8313 | 3980 | 109 | 735 |
| Design Treatment Capacity (m3 d−1) | 2650 | 1893 | 946 | 371 |
| Average Treatment (m3 d−1) | 2445 | 976 | 518 | 278 |
| Influent P Load (kg d−1) | 17.6 | 7.0 | 3.7 | 2.0 |
| Effluent P Load (kg d−1) | 4.9 | 2.0 | 1.0 | 0.6 |
| Parameters 1 | Wastewater Treatment Plant | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| pH | 8.0 ± 0.4 | 7.8 ± 0.4 | 7.8 ± 0.4 | 8.1 ± 0.3 |
| ORP (mV) | 225 ± 37 | 271 ± 32 | 269 ± 42 | 227 ± 33 |
| Alkalinity (as CaCO3) (g/m3) | 1197 ± 24 | 359 ± 37 | 424 ± 90 | 515 ± 197 |
| NH4+ (g/m3) | 404 ± 112 | 89 ± 26 | 8.2 ± 2.1 | 9.6 ± 2.9 |
| PO43− (g/m3) | 400 ± 141 | 120 ± 33 | 128 ± 56 | 35 ± 5 |
| Mg2+ (g/m3) | 32 ± 4 | 20 ± 3 | 30 ± 4 | 11 ± 3 |
| Ca2+ (g/m3) | 41 ± 13 | 43 ± 1.7 | 48 ± 11 | 38 ± 12 |
| Fe3+ (g/m3) | 0.9 ± 0.7 | 0.4 ± 0.2 | 1.0 ± 0.7 | 2.0 ± 0.3 |
| Al3+ (g/m3) | 0.7 ± 0.5 | 0.7 ± 0.4 | 1.2 ± 0.7 | 1.2 ± 1.2 |
| Location | Filtrate pH | Struvite-P Recovery (%) | |||
|---|---|---|---|---|---|
| Untreated | Optimal pH 1 | Untreated 2 | Optimal pH | At pH 8.5 | |
| 1 | 8.0 | 9.9 | 0 | 59 | 57 |
| 2 | 7.8 | 10.1 | 0 | 49 | 37 |
| 3 | 7.8 | 10.1 | 0 | 60 | 32 |
| 4 | 8.1 | 9.5 | 0 | 49 | 27 |
| Location | NaOH (g m−3) | Air Sparging (g m−3) | ||
|---|---|---|---|---|
| Predicted | Actual 1 | Predicted | Actual 1 | |
| 1 | 342 | 347 ± 7.1 | 355 | 353 ± 4.0 |
| 2 | 73.4 | 71.4 ± 0.3 | 74.1 | 74.4 ± 1.1 |
| 3 | 70.1 | 71.1 ± 0.4 | 92.9 | 93.8 ± 19.8 |
| 4 | 28.8 | 27.1 ± 0.2 | 30.9 | 30.7 ± 0.3 |
| Location | P Load Reduction | Struvite | P Value 2 | N+P Value 2 | |
|---|---|---|---|---|---|
| Filtrate-Scale | WWTP-Scale | ||||
| ------------------%------------------ | kg P yr−1 | USD | USD | ||
| 1 | 59 | 24 | 1514 | $2665 | $3346 |
| 1 + Mg 1 | 97 | 43 | 1961 | $3451 | $4332 |
| 2 | 43 | 10 | 223 | $392 | $492 |
| 3 | 41 | 10 | 111 | $195 | $245 |
| 4 | 61 | 3 | 17 | $30 | $36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallas, J.F.; Mackowiak, C.L.; Wilkie, A.C.; Harris, W.G. Struvite Phosphorus Recovery from Aerobically Digested Municipal Wastewater. Sustainability 2019, 11, 376. https://doi.org/10.3390/su11020376
Hallas JF, Mackowiak CL, Wilkie AC, Harris WG. Struvite Phosphorus Recovery from Aerobically Digested Municipal Wastewater. Sustainability. 2019; 11(2):376. https://doi.org/10.3390/su11020376
Chicago/Turabian StyleHallas, John F., Cheryl L. Mackowiak, Ann C. Wilkie, and Willie G. Harris. 2019. "Struvite Phosphorus Recovery from Aerobically Digested Municipal Wastewater" Sustainability 11, no. 2: 376. https://doi.org/10.3390/su11020376





