Waste to Carbon: Estimating the Energy Demand for Production of Carbonized Refuse-Derived Fuel
Abstract
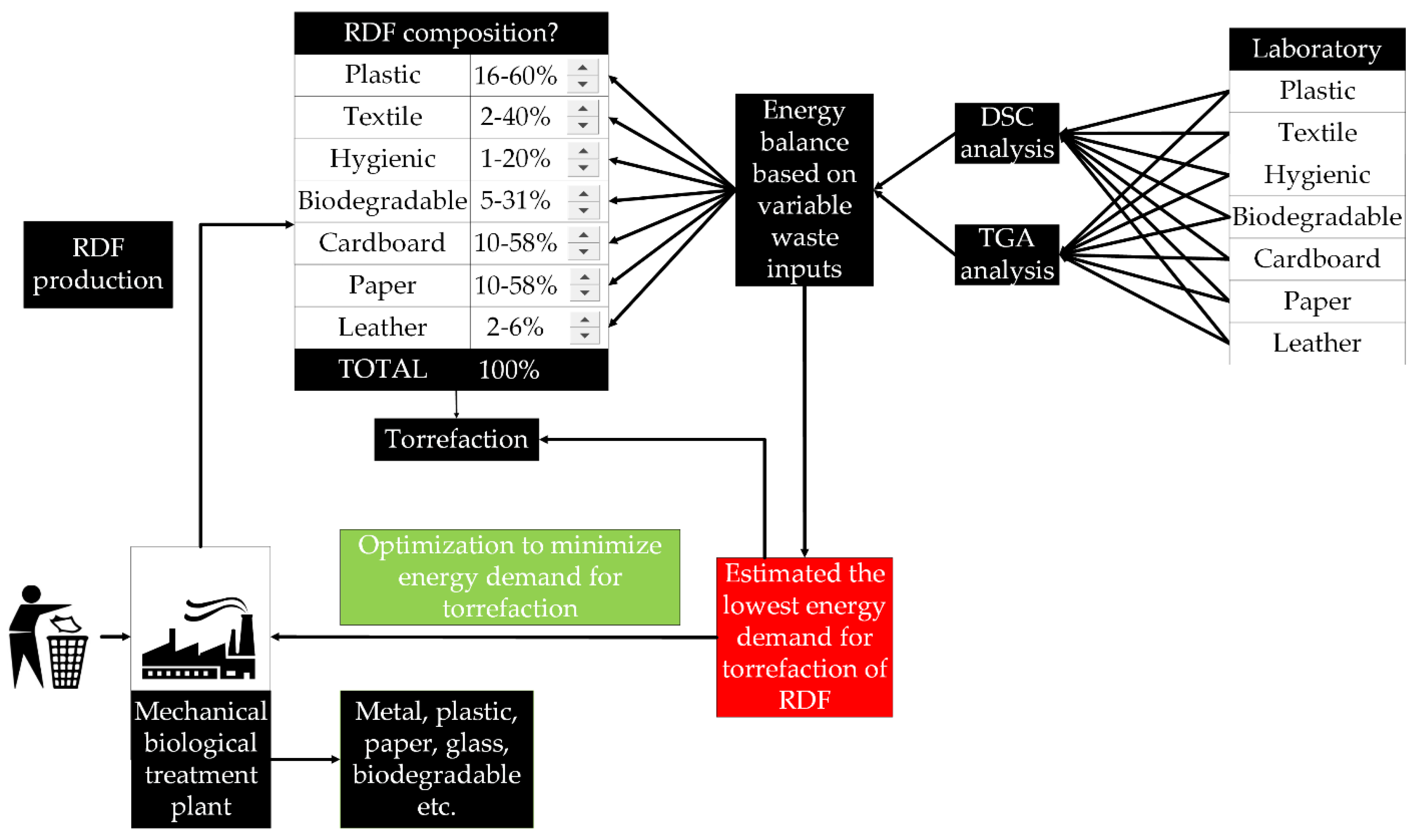
:1. Introduction
- recovery of recyclable materials (e.g., plastics, metals, glass, paper),
- biological stabilization of biodegradable waste,
- separation of inert waste, which is destined for the landfill,
- production of fuel (RDF) from high-calorific waste (which cannot be recycled).
2. Materials and Methods
2.1. Feedstock Preparation
2.2. Thermogravimetric Analysis (TGA)
2.3. Differential Scanning Calorimetry (DSC)
2.4. Modeling Energy Demand of Torrefaction of RDF
2.5. Calculation of the Heating Cost of the Sample
2.6. Statistical Analysis
3. Results
3.1. RDF Weight Reduction during Torrefaction—TGA
3.2. Specific Heat during Torrefaction—Evidence of Endo- and Exothermic Reactions—DSC Analyses
3.3. Mathematical Modeling Results
4. Discussion
4.1. TGA
4.2. DSC
4.3. Mathematical Modeling
- (1)
- adding a more extensive database of TGA and DSC for other materials present in RDF,
- (2)
- adding the effects related to the thermal conductivity and scale-up, and
- (3)
- considering possible interactions during the decomposition of various RDF mixed wastes.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Transformation No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| Waste Component | Start Point, °C | End Point, °C | Start Point, °C | End Point, °C | Start Point, °C | End Point, °C |
| Chicken meat | 171en | 205en | 245en | 278en | 281en | 291en |
| Diaper | 124en | 131en | 156en | 172en | 220en | > 300en |
| Gauze | 230ex | 260ex | 260en | 300en | — | — |
| Egg package | 165en | 235en | 235en | 290en | — | — |
| Paper | 165en | 235en | 235en | 290en | — | — |
| Cotton | 200ex | 230ex | 240ex | 300ex | — | — |
| Genuine leather | 120en | 145en | 150ex | 200ex | 220ex | 260ex |
| PP | 150en | 200en | — | — | — | — |
References
- Rada, E.C.; Ragazzi, M. RDF/SRF evolution in the MSW sector: Coexistence of BMT and selective collection. Int. J. Sustain. Dev. Plan. 2015. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, W.T. Semi-dry carbonation process using fly ash from solid refused fuel power plant. Sustainability 2019, 11, 908. [Google Scholar] [CrossRef]
- Velis, C.A.; Longhurst, P.J.; Drew, G.H.; Smith, R.; Pollard, S.J.T. Production and quality assurance of solid recovered fuels using mechanical-biological treatment (MBT) of waste: A comprehensive assessment. Crit. Rev. Environ. Sci. Technol. 2010. [Google Scholar] [CrossRef]
- Jędrczak, A.; Suchowska-Kisielewicz, M. A comparison of waste stability indices for mechanical–biological waste treatment and composting plants. Int. J. Environ. Res. Public Health 2018, 15, 2585. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Azapagic, A. Assessing the environmental sustainability of energy recovery from municipal solid waste in the UK. Waste Manag. 2016. [Google Scholar] [CrossRef]
- Białowiec, A.; Micuda, M.; Szumny, A.; Łyczko, J.; Koziel, J.A. Quantification of VOC emissions from carbonized refuse-derived fuel using solid-phase microextraction and gas chromatography-mass spectrometry. Molecules 2018, 11, 3233. [Google Scholar] [CrossRef]
- Białowiec, A.; Micuda, M.; Szumny, A.; Łyczko, J.; Koziel, J.A. Waste to carbon: Influence of structural modification on voc emission kinetics from stored carbonized refuse-derived fuel. Sustainability 2019, 11, 935. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Godina, R.; de Oliveira Matias, J.C.; Nunel, L.J.R. Future perspectives of biomass torrefaction: Review of the current state-of-the-art and research development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef]
- Białowiec, A.; Pulka, J.; Stępień, P.; Manczarski, P.; Gołaszewski, J. The RDF/SRF torrefaction: An effect of temperature on characterization of the product – Carbonized Refuse Derived Fuel. Waste Manag. 2017. [Google Scholar] [CrossRef]
- Stępień, P.; Pulka, J.; Serowik, M.; Białowiec, A. Thermogravimetric and calorimetric characteristics of alternative fuel in terms of its use in low-temperature pyrolysis. Waste Biomass Valor. 2018. [Google Scholar] [CrossRef]
- Nowak, A.; Cichy, B. A wide spectrum of thermal analysis capabilities in research and industry. Chemik 2014, 10, 11–12. [Google Scholar]
- Sever Akdağ, A.; Atimtay, A.; Sanin, F.D. Comparison of fuel value and combustion characteristics of two different RDF samples. Waste Manag. 2016. [Google Scholar] [CrossRef] [PubMed]
- Barba, D.; Capocelli, M.; Cornacchia, G.; Matera, D.A. Theoretical and experimental procedure for scaling-up RDF gasifiers: The Gibbs Gradient Method. Fuel 2016. [Google Scholar] [CrossRef]
- Stępień, P.; Serowik, M.; Koziel, J.A.; Białowiec, A. Waste to carbon energy demand model and data based on the TGA and DSC analysis of individual MSW components. Data 2019, 4, 53. [Google Scholar] [CrossRef]
- Lasdon, L.S.; Fox, R.L.; Ratner, M.W. Nonlinear optimization using the generalized reduced gradient method. Recherche Opérationnelle 1974, 8, 73–103. [Google Scholar] [CrossRef] [Green Version]
- Wagland, S.T.; Kilgallon, P.; Coveney, R.; Garg, A.; Smith, R.; Longhurst, P.J.; Pollard, S.J.T.; Simms, N. Comparison of coal/solid recovered fuel (SRF) with coal/refuse derived fuel (RDF) in a fluidised bed reactor. Waste Manag. 2011. [Google Scholar] [CrossRef]
- Soltani, A.; Sadiq, R.; Hewage, K.; Reza, B.; Ruparathna, R. Environmental and economic aspects of production and utilization of RDF as alternative fuel in cement plants: A case study of Metro Vancouver Waste Management. Resour. Conserv. Recycl. 2013. [Google Scholar] [CrossRef]
- Stępień, P.; Białowiec, A. Kinetic parameters of torrefaction process of alternative fuel produced from municipal solid waste and characteristic of carbonized refuse derived fuel. Detritus 2018. [Google Scholar] [CrossRef]
- Kara, M. Environmental and economic advantages associated with the use of RDF in cement kilns. Resour. Conserv. Recycl. 2012. [Google Scholar] [CrossRef]
- Hogland, W.; Marques, M. Physical, biological and chemical processes during storage and spontaneous combustion of waste fuel. Resour. Conserv. Recycl. 2003. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Fate and behavior of inorganic constituents of RDF in a two stage fluid bed-plasma gasification plant. Fuel 2015. [Google Scholar] [CrossRef]
- Agon, N.; Hrabovský, M.; Chumak, O.; Hlína, M.; Kopecký, V.; Mašláni, A.; Bosmans, A.; Helsen, L.; Skoblja, S.; Van Oost, G.; et al. Plasma gasification of refuse derived fuel in a single-stage system using different gasifying agents. Waste Manag. 2015. [Google Scholar] [CrossRef]
- Krüger, B.; Mrotzek, A.; Wirtz, S. Separation of harmful impurities from refuse derived fuels (RDF) by a fluidized bed. Waste Manag. 2014. [Google Scholar] [CrossRef] [PubMed]
- OBI. Węgiel kamienny Orzech. Available online: https://www.obi.pl/paliwa/wegiel-kamienny-orzech-26-28-mj-25-kg/p/6319800 (accessed on 29 May 2019).
- OBI. Pellet Magma Premium. Available online: https://www.obi.pl/paliwa/pellet-magma-premium-17-4-mj-15-kg/p/5510698 (accessed on 29 May 2019).
- Żontała, K.; Łopacka, J.; Lipińska, A.; Sakowska, A. Application of differential scanning calorimetry in food analysis. Postępy Tech. Przetwórstwa Spożywczego 2015, 1, 113–117. [Google Scholar]
- Alongi, J.; Ciobanu, M.; Tata, J.; Carosio, F.; Malucelli, G. Thermal stability and flame retardancy of polyester, cotton, and relative blend textile fabrics subjected to sol-gel treatments. J. Appl. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Wang, P.; Howard, B.H. Impact of thermal pretreatment temperatures on woody biomass chemical composition, physical properties and microstructure. Energies 2018, 11, 25. [Google Scholar] [CrossRef]
- Rawoteea, S.A.; Mudhoo, A.; Kumar, S. Co-composting of vegetable wastes and carton: Effect of carton composition and parameter variations. Bioresour. Technol. 2017. [Google Scholar] [CrossRef]
- Shahedifar, V.; Rezadoust, A.M. Thermal and mechanical behavior of cotton/vinyl ester composites: Effects of some flame retardants and fiber treatment. J. Reinf. Plast. Compos. 2013. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Czégény, Z.; Badea, E.; Carsote, C.; Şendrea, C.; Barta-Rajnai, E.; Bozi, J.; Miu, L.; Jakab, E. Thermal characterization of new, artificially aged and historical leather and parchment. J. Anal. Appl. Pyrol. 2015. [Google Scholar] [CrossRef]
- DEMILEC (USA) LLC. Karta charakterystyki preparatu Izocyjanian A 500. Available online: https://www.ekopian.pl/PDF/techniczne_skladnikA.pdf (accessed on 23 March 2019).
- Jaworski, T. Problems of mathematical modeling of processes of thermal treatment of solid waste. Piece Przemysłowe & Kotły 2015, 1, 8–14. [Google Scholar]
- Cordella, M.; Bauer, I.; Lehmann, A.; Schulz, M.; Wolf, O. Evolution of disposable baby diapers in Europe: Life cycle assessment of environmental impacts and identification of key areas of improvement. J. Clean. Prod. 2015. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007. [Google Scholar] [CrossRef]
- Alvarenga, L.M.; Xavier, T.P.; Barrozo, M.A.S.; Bacelos, M.S.; Lira, T.S. Determination of activation energy of pyrolysis of carton packaging wastes and its pure components using thermogravimetry. Waste Manag. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kijeńska, D.; Błajet, B. Threats in the production of freon free polyurethane foams. Bezpieczeństwo Pracy Nauka i Praktyka 1999, 10, 11–12. [Google Scholar]
- Ntihuga, J.N.; Seen, T.; Gschwind, P.; Kohlus, R. Estimating energy- and eco-balances for continuous bio-ethanol production using a blenke cascade system. Energies 2013, 6, 2065. [Google Scholar] [CrossRef]









| Sample Name | RDF Composition, % | |||||
|---|---|---|---|---|---|---|
| Plastics | Paper | Diapers | Textiles | Kitchen and Garden Waste | ||
| RDF 1—UK [16] | 23.16 | 61.05 | — | 15.79 | — | |
| RDF 2—Poland [10] | 36.17 | 13.45 | 9.86 | 2.67 | 37.74 | |
| RDF 3—Canada [17] | 37.01 | 50.39 | — | 10.08 | 2.52 | |
| RDF 4—Poland [18] | 36.24 | 13.52 | 9.93 | 2.63 | 37.68 | |
| RDF 5—Turkey [19] | 16.90 | 17.10 | — | 66.00 | — | |
| RDF 6—Sweden [20] | 43.00 | 48.00 | — | 9.00 | ||
| RDF 7—UK [21] | 50.55 | 27.18 | — | 12.12 | 10.14 | |
| RDF 8—Turkey [12] | 20.00 | 20.00 | — | 60.00 | — | |
| RDF 9—Belgium [22] | 53.41 | 14.77 | — | 11.36 | 20.45 | |
| RDF 10—Germany [23] | 37.11 | 10.31 | 5.15 | 20.62 | 26.80 | |
| Optimized RDF | Min. | 16.90 47.00 | 10.00 | 5.00 | 2.20 | 2.46 |
| Max. | 58.00 | 8.30 | 66.00 | 31.57 | ||
| RDF Material Type | Source of Data | The Total Energy Demand of Torrefaction, J∙g−1 | % Difference between Experiment and Model |
|---|---|---|---|
| Genuine leather | Exp. | 497.21 | 4.34% |
| Model | 476.55 | ||
| (Exp.—Model) | (−20.66) | ||
| Egg package | Exp. | 1317.18 | 0.39% |
| Model | 1312.03 | ||
| (Exp.—Model) | (−5.15) | ||
| PP | Exp. | 657.68 | 0.35% |
| Model | 655.39 | ||
| (Exp.—Model) | (−2.29) | ||
| Diaper | Exp. | 1278.76 | 0.21% |
| Model | 1276.11 | ||
| (Exp.—Model) | (−2.26) | ||
| Paper receipt | Exp. | 1062.19 | 0.03% |
| Model | 1061.29 | ||
| (Exp.—Model) | (−0.90) | ||
| Chicken meat | Exp. | 215.95 | 0.03% |
| Model | 215.90 | ||
| (Exp.—Model) | (−0.05) | ||
| Cotton | Exp. | 423.86 | 0.00% |
| Model | 423.86 | ||
| (Exp.—Model) | (0.00) | ||
| Gauze | Exp. | 257.45 | 0.00% |
| Model | 257.45 | ||
| (Exp.—Model) | (0.00) |
| Name Of Sample | RDF Composition, % | The Total Energy Demand for Torrefaction, J∙g−1 | The Cost of Heat Production | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken Meat | Diaper | Gauze | Egg Package | Paper Receipt | Cotton | Genuine Leather | PP | Wood Pellets, €∙Mg of RDF | Hard Coal,€∙Mg of RDF’s | ||
| RDF 1 | 0.00 | 0.00 | 5.26 | 30.53 | 30.53 | 5.26 | 5.26 | 23.16 | 937.27 | 14.3 | 7.93 |
| RDF 2 | 37.74 | 9.86 | 0.89 | 6.78 | 6.78 | 0.89 | 0.89 | 36.17 | 615.57 | 9.41 | 5.21 |
| RDF 3 | 2.52 | 0.00 | 3.36 | 25.19 | 25.19 | 3.36 | 3.36 | 37.02 | 884.75 | 13.53 | 7.49 |
| RDF 4 | 37.68 | 9.93 | 0.88 | 6.76 | 6.76 | 0.88 | 0.88 | 36.23 | 616.21 | 9.42 | 5.21 |
| RDF 5 | 0.00 | 0.00 | 22.00 | 8.55 | 8.55 | 22.00 | 22.00 | 16.90 | 568.43 | 8.69 | 4.81 |
| RDF 6 | 9.00 | 0.00 | 0.00 | 24.00 | 24.00 | 0.00 | 0.00 | 43.00 | 870.84 | 13.32 | 7.37 |
| RDF 7 | 10.14 | 0.00 | 4.04 | 13.59 | 13.59 | 4.04 | 4.04 | 50.56 | 722.51 | 11.05 | 6.11 |
| RDF 8 | 0.00 | 0.00 | 20.00 | 10.00 | 10.00 | 20.00 | 20.00 | 20.00 | 600.00 | 9.18 | 5.08 |
| RDF 9 | 20.45 | 0.00 | 3.79 | 7.39 | 7.39 | 3.79 | 3.79 | 53.40 | 701.13 | 10.72 | 5.93 |
| RDF 10 | 26.80 | 5.15 | 6.87 | 5.15 | 5.15 | 6.87 | 6.87 | 37.14 | 568.57 | 8.69 | 4.81 |
| Optimized RDF | 14.28 | 5.00 | 39.42 | 10.00 | 10.00 | 2.20 | 2.20 | 16.90 | 564.05 | 8.63 | 4.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, P.; Serowik, M.; Koziel, J.A.; Białowiec, A. Waste to Carbon: Estimating the Energy Demand for Production of Carbonized Refuse-Derived Fuel. Sustainability 2019, 11, 5685. https://doi.org/10.3390/su11205685
Stępień P, Serowik M, Koziel JA, Białowiec A. Waste to Carbon: Estimating the Energy Demand for Production of Carbonized Refuse-Derived Fuel. Sustainability. 2019; 11(20):5685. https://doi.org/10.3390/su11205685
Chicago/Turabian StyleStępień, Paweł, Małgorzata Serowik, Jacek A. Koziel, and Andrzej Białowiec. 2019. "Waste to Carbon: Estimating the Energy Demand for Production of Carbonized Refuse-Derived Fuel" Sustainability 11, no. 20: 5685. https://doi.org/10.3390/su11205685
APA StyleStępień, P., Serowik, M., Koziel, J. A., & Białowiec, A. (2019). Waste to Carbon: Estimating the Energy Demand for Production of Carbonized Refuse-Derived Fuel. Sustainability, 11(20), 5685. https://doi.org/10.3390/su11205685







