1. Introduction
Replacement control is a method of invasive species control which aims to naturally eliminate invasive plants by deliberately manipulating vegetation composition [
1]. It is based on the principles of interspecific competition and community succession. The method was introduced by Chinese researchers in the 1980s to control invasive plants [
2], and has gained attention for its economic and ecological benefits. Besides competitive native plants, sufficiently competitive non-invasive and even less-invasive exotic competitors have been explored for replacement control. An increasing number of examples have proven that the introduction of appropriate exotic species which are less invasive is a promising strategy for eradicating invasive plants and restoring native communities. Zhou et al. [
3] used exotic
Sonneratia apetala and
S. caseolaris to control invasive
Spartina alterniflora through shading and allelopathy, promoting the restoration of native mangroves, and
Setaria sphacelata and
Lolium perenne competitively suppress the growth of invasive
Ageratina adenophora [
4,
5].
Kuebbing and Nunez [
6] concluded that competitive interactions prevailed between nonnative plants and these interactions were found in 39.8% of all the non-native plant interaction studies. Interspecific competition commonly results in the suppression of growth and/or decline in populations of the species that are competitively subordinate [
7]; it can even result in exclusion of some species from particular habitats [
8]. Interspecific competition influences the adaptation and colonization of invaders to nearby communities, and therefore, can play a significant role in influencing plant distributions at relatively small spatial scales [
8]. An exotic species, which is not capable of regenerating in particular habitats or is competitively subordinate to native species, will achieve natural eradication of itself and promote the restoration of native communities after excluding the targeted invasive plant [
3]. Thus, controlling invasive plants with other plants has broad promise for invasive weed management.
Traits that allow plants to compete for limiting resources (e.g., light, water, and soil nutrients) drive interspecific competition [
8]. Resource competition is comprised of the direct capture and use of common resources, and the indirect impacts of one plant on resource availability to its neighbor. For example, taller plants can achieve greater photosynthetic rates by capturing more sunlight; they also shade their neighbors, suppressing photosynthesis of these adjacent plants [
9,
10]. Thus, competitive abilities in plants include the suppressive effects on its neighbors and tolerance of these competitive effects from neighbors [
11]. Resource competition models predict a positive correlation between competitive (suppressive) effects and tolerances (responses); however, other theoretical models predict the opposite—negative correlation between these interactions [
8]. In other words, a species with a strong ability to suppress its neighbors will not necessarily have a strong tolerance for competition. Therefore, a competitive response, as well as competitive effects, should be considered to evaluate the potential of a species to be used for replacement control.
Chromolaena odorata (L.) King and Robinson is one of the most destructive invasive plants in South China. In the area where it has invaded,
C. odorata can inhibit soil biota [
12], decrease the richness of almost all native plants and form mono-dominant stands, suppress regeneration in native communities [
13], and alter the fire regime in savannas [
14]. It is tolerant of severe stresses, such as mowing and fire [
14], and is considered as a serious threat to local biodiversity, agricultural production, and ecological security [
15]. So far, biological control methods using plant pathogens and herbivore have not controlled
C. odorata effectively [
16]. It is susceptible to shading, and prefers warm, wet, and fertile habitats [
17,
18]; replacement control by introducing a taller and faster growing plant would provide shade, reducing the microenvironmental temperature and soil fertility; thus, controlling
C. odorata through replacement control has been considered. Hybrid giant Napier (
Pennisetum hydridum) is a tall, multipurpose crop with high photosynthetic efficiency [
19,
20]. It is a strong competitor, has little invasion risk, does not produce allelochemicals, can be easily removed [
21], and is effective in replacement control of
Eupatorium adenophorum in South China [
22]. Thus, we proposed that hybrid giant Napier should be able to control
C. odorata. To evaluate potential impacts of
P. hydridum on
C. odorata, we conducted a series of replacement experiments in a glasshouse and assessed the competitive outcomes. For better predictions regarding the ecological mechanisms behind the observed outcomes, we measured the plant traits, including biomass, morphological and physiological traits, and soil traits, including nutrient concentrations. We hypothesized that
P. hydridum constrains
C. odorata through light and nutrient competition. Our research would provide data for field practices on the control of the harmful, invasive weed.
2. Materials and Methods
2.1. Materials and Site
Seeds of
C. odorata were collected in March 2015 at the suburb (20°02′ N, 110°11′ E) of Haikou City in Hainan, China, and were stored at −18 ℃. Fully developed and average-sized seeds were selected and sown in seedbeds in a greenhouse after immersion in 40 ℃ water overnight for incubation.
Pennisetum hydridum is a perennial, tussock-forming plant, and formed as a hybrid of elephant grass and African
Pennisetum (
P. purpureum × P. typhoideum). It is recorded and conserved by International Center for Tropical Agriculture (CIAT), coded as CIAT6263 [
23]. Stem cuttings of
P. hydridum were collected from the Teaching and Research Farm (23°13′ N, 113°38′ E) of South China Agricultural University in Zengcheng, Guangzhou, China, and were placed in seedbeds to produce new individuals. Ten weeks after sowing and cutting, uniform, ~20 cm tall seedlings of each species were carefully transplanted in different proportions into plastic pots (upper diameter of 23 cm, basal diameter of 16 cm, and height of 21 cm) and filled with 5 kg of topsoil from local, barren agricultural land near the experimental station. Soil total nitrogen (TN) contetnt was 0.86 g kg
−1, total phosphorus (TP) contetnt was 0.66 g kg
−1, total potassium (TK) contetnt was 25.14 g kg
−1, available N (AN) contetnt was 384.18 mg kg
−1, available P (AP) contetnt was 37.73 mg kg
−1, available K (AK) contetnt was 152.34 mg kg
−1, catalase activity (CAT) was 2.37 mL g
−1, acid phosphatase activity (ACP) was 14.42 mg kg
−1, and urease activity was 477.76 mg kg
−1.
Five replicates (pots) of each of five treatments with the following initial plantings were set up: (1) monoculture of P. hydridum (4P), (2) monoculture of C. odorata (4C), (3) one P. hydridum and three C. odorata (1P3C), (4) two P. hydridum and two C. odorata (2P2C), and (5) three P. hydridum and one C. odorata (3P1C). All treatments had the same initial plant density: four plants per pot. Seedlings were watered daily and were not provided with fertilizers. Soil was collected after plant morphological and photosynthetic traits were measured on 3–25 December 2015.
2.2. Measurements
One individual from each species in each replicate was randomly selected and five sun-exposed leaves were sampled from each individual for leaf gas-exchange measurements. Light-saturated photosynthetic rate (A) and stomatal conductance (gs) were measured between 09:00 and 11:00 on a sunny day using a portable photosynthesis system (Li-6400; Li-Cor, Lincoln, NE, USA), with a red/blue LED light source (6400-02B). During measurements, the photosynthetic photon flux density (PPFD) was 1400 μmol m−2 s−1 (above the light level required for photosynthetic saturation in both of the species); leaf chamber CO2 concentration and relative humidity were maintained at 380 μmol mol−1 and 50%–70%, respectively. Prior to gas exchange measurements, leaves were exposed to the above conditions for 5 min to allow for the stabilization of photosynthetic parameters.
Total biomass, shoot biomass, root biomass, total leaf area (LA), total number of branches (BN), and mean branch length (BL) of every individual were measured immediately after harvest and we used averages of these measurements from individuals within each pot for each replicate of each species. Leaves were scanned and LA was determined using Adobe Photoshop CS2 9.0.2 (Adobe Systems, San Jose, CA, USA), and BL was measured with a ruler. Biomasses of the individuals were measured after oven drying at 70 °C for 72 h. Specific leaf area (SLA) was calculated by dividing leaf area by dry mass.
All the soils were carefully separated from roots and collected from each pot. After transportation to the laboratory, they were air-dried, passed through a 5 mm sieve after removal of fine roots, and thoroughly homogenized before nutrient concentrations were determined. TN was determined using the Kjeldahl method [
24]. TP content was determined by Mo-Sb spectrophotometry after a boiling NaOH digestion [
25]. TK was measured by NaOH flame photometry. Soil available N content was determined using alkali-diffusion, available P content using hydrochloric acid-sulfuric acid extraction, and available K content using ammonium acetate extraction flame photometry [
26].
2.3. Data Analysis
Total relative yield (RYT), competitive balance index (CB), and relative yield (RY) of each species were calculated as follows [
27]:
where RY
P and RY
C are the relative yields of
P. hydridum and
C. odorata in the mixtures, respectively. Y
PC is the yield of
P. hydridum in the presence of
C. odorata; Y
CP is the yield of
C. odorata in the presence of
P. hydridum. Y
P and Y
C are the yields of
P. hydridum and
C. odorata grown in monocultures, respectively.
p and
q were the proportions of
P. hydridum and
C. odorata in mixtures, respectively (
p +
q = 1). Since CB
P = −CB
C, we used the competitive balance index of
C. odorata (CB
C) to indicate the competitive balance between
P. hydridum and
C. odorata. RYT < 1 in a mixture indicates that interspecific competition exceeds intraspecific competition, and RYT > 1 indicates the opposite. RYT = 1, indicates that intra- and interspecific competition is balanced. CB
C > 0 indicates that
C. odorata is more competitive in the two-species condition, and CB
C < 0 indicates that it is less competitive.
The RYT and RY values in a given treatment were compared with the expected values calculated based on the initial proportions, which were 1 (treatment 4P), 0.75 (3P1C), 0.5 (2P2C), and 0.25 (1P3C) for P. hydridum. An analogous method was used for C. odorata. The RY of a given species and the RYT were shown as a function of the species proportion. The expected values (Xexpected) of soil nutrient contents when species grew equally well in mixtures and monocultures were calculated based on the values in monocultures (Xi) and the species’ proportions: Xexpected = XP × p + XC × q.
Significant differences among treatments were evaluated by LSD test (
P values were corrected with a multiple-test Holm’s procedure). The significant differences between the observed and expected values were evaluated by one sample
t-tests. All the analyses were performed in R version 3.5.3 [
28].
3. Results
Most morphological and physiological trait values of
P. hydridum were higher than those of
C. odorata in the monocultures (
Table 1).
P. hydridum had larger total, shoot, and root biomass than
C. odorata.
P. hydridum also had higher
A and SLA than
C. odorata. However, the BL, BN, and
gs of
P. hydridum were lower than those of
C. odorata. Values of root/shoot and LA of two plants were not significantly different between the species.
P. hydridum’s competitive advantage was clear from differences in its trait distributions relative to those of
C. odorata. RY values were significantly higher than expected in the 3P1C and 1P3C treatments for
P. hydridum, and were lower than expected in all mixtures for
C. odorata (
Figure 1a). CB
C was significantly lower than 0 in 3P1C and 1P3C, but was near 0 in 2P2C (
Figure 1b). Moreover, RYT was significantly lower than expected when
P. hydridum and
C. odorata grew together (
Figure 1a).
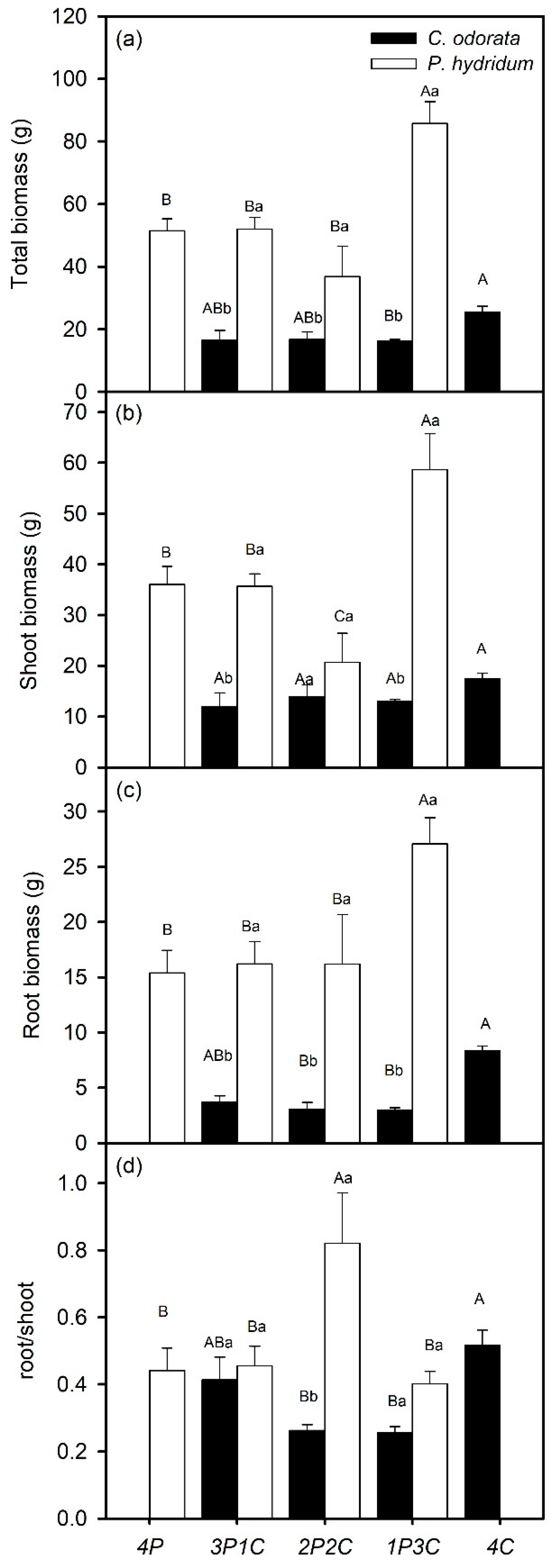
Biomasses in the different treatments showed strong responses of
C. odorata to competitive stress from
P. hydridum. Total and root biomasses in all the treatments were reduced compared to those in the monoculture for
C. odorata, but remained constant between the treatments (
Figure 2a,c). There was no difference between the treatments in shoot biomass for
C. odorata (
Figure 2b). For
C. odorata, root/shoot ratio was significantly lower in 1P3C and 2P2C than that in monoculture (
Figure 2d). The presence of
P. hydridum reduced total and root biomasses of
C. odorata by 34.89%–36.81% and 45.84%–66.48%, respectively. In contrast, responses of
P. hydridum to
C. odorata were weak. Total and shoot biomasses were lower in 2P2C, but significantly higher in 1P3C than those in the monoculture for
P. hydridum, and root biomass was higher in 1P3C than that in the other treatments (
Figure 2a–c). Root/shoot ratio of
P. hydridum in 2P2C was significantly higher than that in the other treatments (
Figure 2d). Total, shoot, and root biomasses of
P. hydridum were higher than those of
C. odorata in all the mixture treatments, except for shoot biomass in 2P2C (
Figure 2a–c). Root/shoot ratios of
P. hydridum in 3P1C and 1P3C were not different from those of
C. odorata, but were significantly higher in 2P2C (
Figure 2d).
Morphological traits in the treatments also showed weak tolerance of
C. odorata to competitive effects from
P. hydridum. The LA and BN values of
C. odorata decreased with its abundance across the treatments (
Figure 3a,c). The BL of
C. odorata was slightly lower in all mixture treatments than that in the monoculture (
Figure 3b), but
P. hydridum showed strong tolerance to competitive effects from
C. odorata. The LA of
P. hydridum slightly decreased with its abundance across 4P, 3P1C, and 2P2C, but increased in 1P3C. There were no differences in the BL and BN values of
P. hydridum among all treatments (
Figure 3). The LAs of
P. hydridum were higher than those of
C. odorata in 3P1C and 1P3C (
Figure 3a), but the BN of
P. hydridum was lower than that of
C. odorata in 2P2C and 1P3C (
Figure 3c). There was no significant difference in BL between species in the mixture treatments (
Figure 3b).
The two species showed differential physiological responses to growth in the presence of heterospecifics.
A values were lower in 2P2C and 3P1C than those in the monoculture for
C. odorata, and was lowest in 3P1C (
Figure 4a). The
gs of
C. odorata was significantly lower in 3P1C than in its monoculture (
Figure 4b).
A and
gs of
P. hydridum were significantly lower in mixtures than those in the monoculture, and were lowest in 1P3C (
Figure 4a,b).
A of
P. hydridum was higher than that of
C. odorata in all treatments, but
gs of
P. hydridum was significantly lower than that of
C. odorata in 2P2C and 1P3C (
Figure 4a,b). SLA was significantly higher in 1P3C than that in the monoculture for
C. odorata, and there were no differences between the treatments for
P. hydridum (
Figure 4c).
The two plant species had multiple impacts on soil nutrients when they were grown together. There were no differences in the contents of soil TN, TP, TK, or AN among the treatments (
Figure 5a-d). Soil AP and AK contents were significantly lower in 4P than in 4C. AP content was highest in 4C and lowest in 3P1C, and AK content was highest in 4C and lowest in 2P2C than that in the other treatments (
Figure 5e,f). Moreover, soil nutrient contents decreased in mixture treatments compared with the expected values based on the monocultures. AP contents in 3P1C, 2P2C, and 1P3C, and AK contents in 3P1C and 2P2C, were significantly lower than expected. However, AN content in 1P3C was higher than expected (
Figure 5).
4. Discussion
We found that
P. hydridum was larger and had both higher photosynthetic capacity and stronger nutrient uptake than
C. odorata in monocultures (
Table 1). As a result, it was the more competitive species in heterospecific plantings. RYT indicated intense competition between the two species, especially in 2P2C. Differences between the observed and expected values of RY indicated stronger interspecific than intraspecific competition for
C. odorata, and the opposite, stronger intraspecific than interspecific competition for
P. hydridum (
Figure 1a). CB
C indicated that
C. odorata was weaker when competing with
P. hydridum in all treatments, and interspecific competition was nearly balanced in 2P2C (
Figure 1b).
C. odorata was much more suppressed by
P. hydridum than that
P. hydridum was suppressed by
C. odorata. In other words,
P. hydridum had superior competitive capacity and stronger tolerance than
C. odorata when they were intercropped.
P. hydridum inhibited growth of
C. odorata aboveground through shading and interfering with its leaf and branch formations. Even though Ismail et al. [
29] found biomass and leaf area of
C. odorata was reduced when shade alone increased, our results only found leaf area declined as the abundance of the neighbor species increased. Our results are in line with previous studies that suggested
C. odorata is a facultatively shade tolerant weed with low biomass accumulation under low light conditions [
18,
30]. The relatively large size of
P. hydridum reduced light availability of
C. odorata and depressed its biomass accumulation. However,
C. odorata tolerated shade and prevented further reduction in biomass when the plant proportion of
P. hydridum increased. These results suggest that a high population of
P. hydridum is not necessary for controlling
C. odorata. On the other hand,
P. hydridum also inhibited the growth of
C. odorata by competing belowground with stronger roots and stronger uptake of phosphorus and potassium. Moreover, the outcome of nutrient competition between the two species was a more oligotrophic soil condition that would decrease the competitive advantage of
C. odorata over native plants [
31]. Quan et al. [
17] found that the root biomass of
C. odorata was not influenced by nutrient concentration, and root/shoot ratios significantly increased with nutrient decreases. However, root biomass and root/shoot ratios of
C. odorata decreased with soil nutrient reduction resulting from the presence of
P. hydridum in this study. Our results indicated the reduction of root biomass and root fraction of
C. odorata was due to not only soil nutrient decreases but also root interactions. Aerts et al. [
32] suggested that investment in below-ground biomass competition was positively correlated with species competitiveness. Greater relative investment in root biomass by
P. hydridum likely contributes to enhancement of its competitive dominance compared with
C. odorata.
C. odorata also accumulates soil pathogens that inhibit native plants [
12], and produces allelochemicals that elicit strong allelopathic effects on coexisting species [
13]. However, the absence of competition-driven decreases in the morphological traits (such as biomass, leaf area, and branch length) of
P. hydridum indicated that
P. hydridum is not vulnerable to the allelopathic effects of
C. odorata.
Additionally, we found the two species had different strategies for competing with each other.
P. hydridum adopted biomass reallocation, shifting biomass to the roots in 2P2C; indeed, proportional root biomass was closely related to plant competitive ability [
32,
33]. In contrast,
C. odorata noticeably reduced root allocation when growing with heterospecific neighboring plants in mixtures [
34,
35]. Shade due to the presence of neighbors can trigger the stem-elongation of plants, but
C. odorata adopted shade tolerance instead, to maximize its performance when facing challenge that it was unlikely to outgrow the taller
P. hydridum [
9].
C. odorata increased SLA to promote light-capture efficiency under low shade stress (in 1P3C) and decreased photosynthetic capacity when the shade stress increased (in 2P2C and 3P1C).
The couples of soil nutrients and RYT indicate that soil P and K availabilities played an important role in the competition between
P. hydridum and
C. odorata. Soil phosphorus and potassium availabilities were positively correlated with plant biomass [
36]. Many studies have found that alien plants reduced soil P and K availabilities more than native species did [
37,
38]. In addition, the high availability of soil K facilitates the success of invasive plants [
39]. The reduction of AK uptaken by
P. hydridum could play an important role in inhibiting invasive weeds.
Besides its inhibition on
C. odorata’s growth, low light and soil AK conditions produced by
P. hydridum will also suppress the germination and regeneration of
C. odorata in the field [
29,
40]. Additionally, removing
P. hydridum can provide favorable conditions for the restoration of native communities [
21]. Moreover, the results from mixed-species treatments indicated intensive intraspecific competition within
P. hydridum; the existence of
C. odorata released
P. hydridum from its intraspecific competition, and led to increased biomass and RY. Therefore, the potential intraspecific influences of
P. hydridum in a high population density should be considered for the field-based implementation of controlling
C. odorata.
In conclusion, P. hydridum exhibited strong competitive effects and tolerance when competing with C. odorata, indicating that it is a promising candidate for replacement control of C. odorata. P. hydridum inhibited the growth of C. odorata by reducing the availability of light and by both interfering with root formation and reducing availabilities of soil nutrients belowground. Considering its strong intraspecific competition, a relatively low population density of P. hydridum is suggested for the maintenance of high production and economic efficiency.