Sustainable Approach to Eradicate the Inhibitory Effect of Free-Cyanide on Simultaneous Nitrification and Aerobic Denitrification during Wastewater Treatment
Abstract
1. Introduction
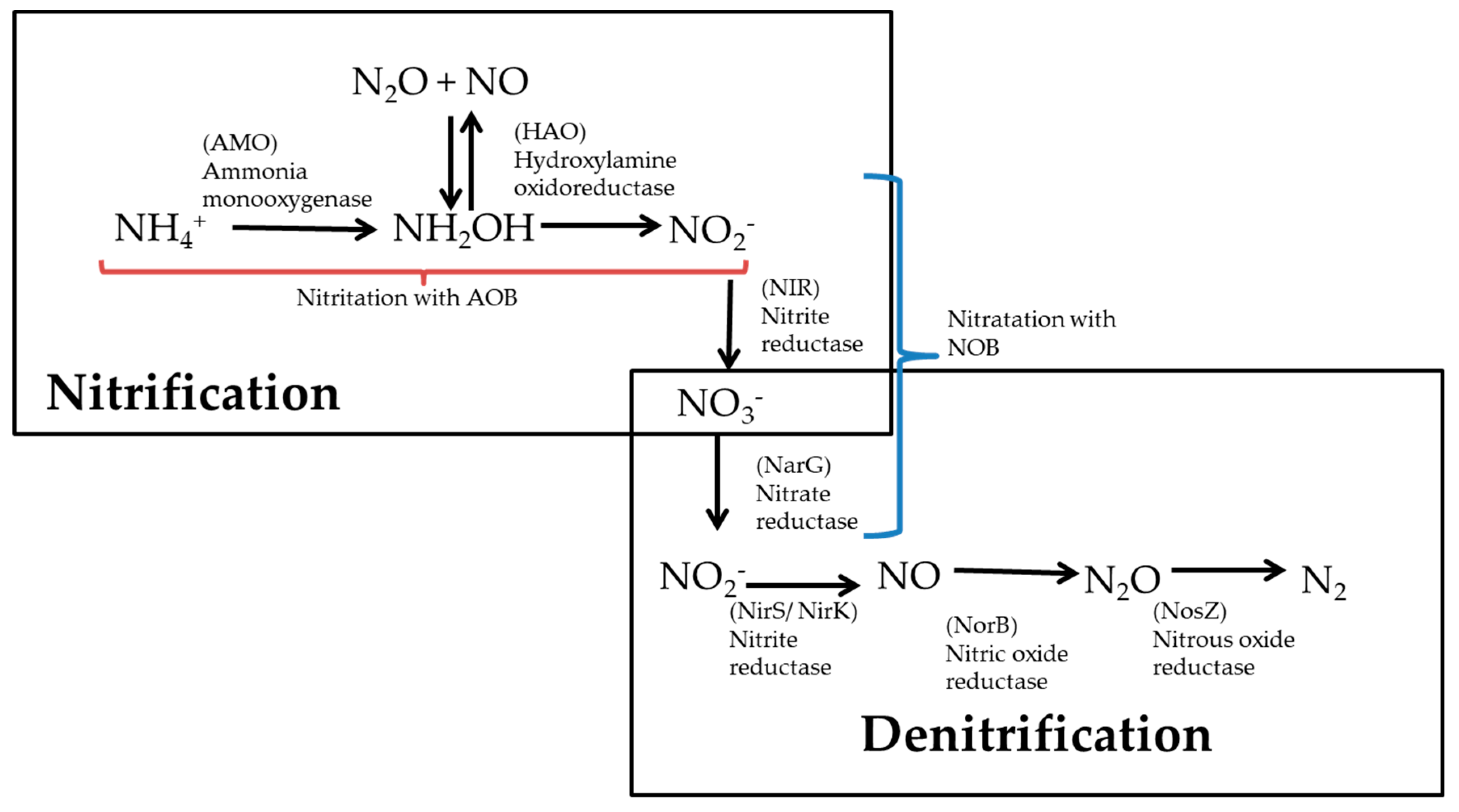
2. Multi-Stage Nitrification and Subsequent Denitrification: An Obsolete Technology
3. Recent Advances in Nitrification and Denitrification Processes: Future Perspectives
Overall Remarks on Simultaneous Nitrification and Aerobic Denitrification (SNaD): Advances and Limitations
4. Challenges in Simultaneous Nitrification and Aerobic Denitrification (SNaD) Processes
4.1. Prevention of Biomass Washout During the Start-Up of SNaD
4.2. Inhibition Mechanism of Simultaneous Nitrification and Aerobic Denitrification by Pollutants
4.3. FCN Wastewater in Municipal Wastewater Sewage Systems (MWSSs) and Its Impact on Nitrification and Denitrification: A Culture of Illegal Wastewater Dumping
5. Current Solutions to the Challenges in Simultaneous Nitrification and Aerobic Denitrification (SNaD)
5.1. Physical Process Used as Remedial Strategy to Decrease the Inhibitory Effect of FCN on SNaD
5.2. Biological Systems Responsible for Lowering FCN Concentration Prior to SNaD
5.3. Overall Remarks on Remedial Strategies in Place to Mitigate FC in SNaD
6. A Proposed Sustainable Solution: Environmental Benignity at the Core of SNaD Development
Application of FCN Resistant Microorganisms in Simultaneous Nitrification and Aerobic Denitrification (SNaD) Under Cyanogenic Conditions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, M.; Okabe, S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere 2015, 141, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Fang, H.; Su, B.; Chen, J.; Lin, J. Characterization of halophilic heterotrophic nitrification–aerobic denitrification bacterium and its application on the treatment of saline wastewater. Bioresour. Technol. 2015, 179, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Norton-Brandão, D.; Scherrenberg, S.; van Lier, J. Reclamation of used urban waters for irrigation purposes—A review of treatment technologies. J. Environ. Manag. 2013, 122, 85–98. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Park, D.; Lee, D.S.; Park, J.M. Inhibitory effects of toxic compounds on nitrification process for cokes wastewater treatment. J. Hazard. Mater. 2008, 152, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ryu, H.; Kim, M.; Kim, J.; Lee, S. Enhancing struvite precipitation potential for ammonia nitrogen removal in municipal landfill leachate. J. Hazard. Mater. 2007, 146, 81–85. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Cho, K.; Park, J. Long term assessment of factors affecting nitrifying bacteria communities and N-removal in a full-scale biological process treating high strength hazardous wastewater. Bioresour. Technol. 2013, 134, 180–189. [Google Scholar] [CrossRef]
- Han, Y.; Jin, X.; Wang, Y.; Liu, Y.; Chen, X. Inhibitory effect of cyanide on nitrification process and its eliminating method in a suspended activated sludge process. Environ. Sci. Pollut. Res. 2014, 21, 2706–2713. [Google Scholar] [CrossRef]
- Shoda, M.; Ishikawa, Y. Heterotrophic nitrification and aerobic denitrification of a wastewater from a chemical company by Alcaligenes faecalis no. 4. Int. J. Water Wastewater Treat. 2015, 1, 1–5. [Google Scholar]
- Chen, P.; Li, J.; Li, Q.; Wang, Y.; Li, S.; Ren, T.; Wang, L. Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcus sp. CPZ24. Bioresour. Technol. 2012, 116, 266–270. [Google Scholar] [CrossRef]
- Banning, N.C.; Maccarone, L.D.; Fisk, L.M.; Murphy, D.V. Ammonia-oxidising bacteria not archaea dominate nitrification activity in semi-arid agricultural soil. Sci. Rep. 2015, 5, 11146. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Levy-Booth, D.; Prescott, C.; Grayston, S. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Shoda, M.; Ishikawa, Y. Heterotrophic nitrification and aerobic denitrification of high-strength ammonium in anaerobically digested sludge by Alcaligenes faecalis strain No. 4. J. Biosci. Bioeng. 2014, 117, 737–741. [Google Scholar] [CrossRef]
- Toyoda, S.; Yoshida, N.; Koba, K. Isotopocule analysis of biologically produced nitrous oxide in various environments. Mass Spectrom. Rev. 2015, 36, 135–160. [Google Scholar] [CrossRef]
- Clough, T.J.; Lanigan, G.J.; de Klein, C.A.; Samad, M.S.; Morales, S.E.; Rex, D.; Bakken, L.R.; Johns, C.; Condron, L.M.; Grant, J.; et al. Influence of soil moisture on codenitrification fluxes from a urea-affected pasture soil. Sci. Rep. 2017, 7, 2185. [Google Scholar] [CrossRef]
- He, T.; Li, Z.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Ai, G.; Miao, L.; Zheng, H.; Liu, Z. The characteristics of a novel heterotrophic nitrification–aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, G.M.; Miao, L.L.; Liu, Z.P. Marinobacter strain NNA5, a newly isolated and highly efficient aerobic denitrifier with zero N2O emission. Bioresour. Technol. 2016, 206, 9–15. [Google Scholar] [CrossRef]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.; Zhou, J.; Zhang, H. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioproc. Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Z.; Chen, M.; Dong, X.; Zhou, J. Evaluation of simultaneous nitrification and denitrification under controlled conditions by an aerobic denitrifier culture. Bioresour. Technol. 2015, 175, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, L.; Yang, S.; Zhang, K.; Huang, T.; Wen, G. Heterotrophic nitrification and aerobic denitrification at low nutrient conditions by a newly isolated bacterium, Acinetobacter sp. SYF26. Microbiology 2015, 161, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, A.; Zhang, X.; Ma, F. Regulation of dissolved oxygen from accumulated nitrite during the heterotrophic nitrification and aerobic denitrification of Pseudomonas stutzeri T13. Appl. Microbiol. Biotechnol. 2014, 99, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Khardenavis, A.; Kapley, A.; Purohit, H. Simultaneous nitrification and denitrification by diverse Diaphorobacter sp. Appl. Microbiol. Biotechnol. 2007, 77, 403–409. [Google Scholar] [CrossRef]
- Zheng, M.; He, D.; Ma, T.; Chen, Q.; Liu, S.; Ahmad, M.; Gui, M.; Ni, J. Reducing NO and N2O emission during aerobic denitrification by newly isolated Pseudomonas stutzeri PCN-1. Bioresour. Technol. 2014, 162, 80–88. [Google Scholar] [CrossRef]
- Pal, R.R.; Khardenavis, A.A.; Purohit, H.J. Identification and monitoring of nitrification and denitrification genes in Klebsiella pneumoniae EGD-HP19-C for its ability to perform heterotrophic nitrification and aerobic denitrification. Funct. Integr. Genom. 2015, 15, 63–76. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Yang, F.; Xue, Y.; Wang, T. The development of simultaneous partial nitrification, ANAMMOX and denitrification (SNAD) process in a single reactor for nitrogen removal. Bioresour. Technol. 2009, 100, 1548–1554. [Google Scholar] [CrossRef]
- Ntwampe, S.K.; Santos, B.A. Potential of agro-waste extracts as supplements for the continuous bioremediation of free cyanide contaminated wastewater. J. Environ. Chem. Eng. 2013, 7, 493–497. [Google Scholar]
- Santos, B.A.Q.; Ntwampe, S.K.O.; Doughari, J.H.; Muchatibaya, G. Application of Citrus sinensis solid waste as a pseudo-catalyst for free cyanide conversion under alkaline conditions. BioResources 2013, 8, 3461–3467. [Google Scholar] [CrossRef]
- Mekuto, L.; Jackson, V.A.; Ntwampe, S.K. Biodegradation of free cyanide using Bacillus sp. consortium dominated by Bacillus safensis, Lichenformis and Tequilensis strains: A bioprocess supported solely with whey. J. Bioremediat. Biodegrad. 2013, 2–7. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Srivastava, R.K. Sequencing batch reactor technology for biological wastewater treatment: A review. Asia-Pac. J. Chem. Eng. 2011, 6, 3–13. [Google Scholar] [CrossRef]
- Aybar, M.; Pizarro, G.; Boltz, J.P.; Downing, L.; Nerenberg, R. Energy-efficient wastewater treatment via the air-based, hybrid membrane biofilm reactor (hybrid MfBR). Water Sci. Technol. 2014, 69, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Han, Y.; Ma, W.; Han, H.; Zhu, H.; Xu, C.; Li, K.; Wang, D. Enhanced nitrogen removal from coal gasification wastewater by simultaneous nitrification and denitrification (SND) in an oxygen-limited aeration sequencing batch biofilm reactor. Bioresour. Technol. 2017, 244, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Mahvi, A.H. Sequencing batch reactor: A promising technology in wastewater treatment. J. Environ. Health Sci. 2008, 5, 79–90. [Google Scholar]
- He, Q.; Zhang, W.; Zhang, S.; Wang, H. Enhanced nitrogen removal in an aerobic granular sequencing batch reactor performing simultaneous nitrification, endogenous denitrification and phosphorus removal with low superficial gas velocity. Chem. Eng. J. 2017, 326, 1223–1231. [Google Scholar] [CrossRef]
- Koch, G.; Egli, K.; Van der Meer, J.R.; Siegrist, H. Mathematical modeling of autotrophic denitrification in a nitrifying biofilm of a rotating biological contactor. Water Sci. Technol. 2000, 41, 191–198. [Google Scholar] [CrossRef]
- Sin, G.; Kaelin, D.; Kampschreur, M.J.; Takacs, I.; Wett, B.; Gernaey, K.V.; Rieger, L.; Siegrist, H.; van Loosdrecht, M. Modelling nitrite in wastewater treatment systems: A discussion of different modelling concepts. Water Sci. Technol. 2008, 58, 1155–1171. [Google Scholar] [CrossRef]
- Seifi, M.; Fazaelipoor, M.H. Modeling simultaneous nitrification and denitrification (SND) in a fluidized bed biofilm reactor. Appl. Math. Model. 2012, 36, 5603–5613. [Google Scholar] [CrossRef]
- Kanyenda, G.; Ntwampe, S.K.O.; Mpongwana, N.; Godongwana, B. Mathematical Exposition of Simultaneous Nitrification and Aerobic Denitrification. In Proceedings of the 10th lnt’I Conference on Advances in Science, Engineering, Technology & Healthcare (ASETH-18), Cape Town, South Africa, 19–20 November 2018; pp. 242–245, ISBN 978-81-938365-2-1. Available online: https://doi.org/10.17758/EARES4.EAP1118258 (accessed on 28 October 2019).
- Edwards, J.S.; Covert, M.; Palsson, B. Metabolic modelling of microbes: The flux-balance approach. Environ. Microbiol. 2002, 4, 133–140. [Google Scholar] [CrossRef]
- Cui, J.; Wang, X.; Yuan, Y.; Guo, X.; Gu, X.; Jian, L. Combined ozone oxidation and biological aerated filter processes for treatment of cyanide containing electroplating wastewater. Chem. Eng. 2014, 241, 184–189. [Google Scholar] [CrossRef]
- Papirio, S.; Zou, G.; Ylinen, A.; Di Capua, F.; Pirozzi, F.; Puhakka, J.A. Effect of arsenic on nitrification of simulated mining water. Bioresour. Technol. 2014, 164, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Lochmatter, S.; Holliger, C. Optimization of operation conditions for the startup of aerobic granular sludge reactors biologically removing carbon, nitrogen, and phosphorous. Water Res. 2014, 59, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Hermansson, M.; Modin, O.; Persson, F.; Wilén, B.M. Effects of wash-out dynamics on nitrifying bacteria in aerobic granular sludge during start-up at gradually decreased settling time. Water 2016, 8, 172. [Google Scholar] [CrossRef]
- Li, G.; Puyol, D.; Carvajal-Arroyo, J.M.; Sierra-Alvarez, R.; Field, J.A. Inhibition of anaerobic ammonium oxidation by heavy metals. J. Chem. Technol. Biotechnol. 2015, 90, 830–837. [Google Scholar] [CrossRef]
- Aslan, S.; Sozudogru, O. Individual and combined effects of nickel and copper on nitrification organisms. Ecol. Eng. 2017, 99, 126–133. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Pan, X. Simultaneous biological removal of nitrogen–sulfur–carbon: Recent advances and challenges. Biotechnol. Adv. 2013, 31, 409–420. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Han, Y.; Zhang, X. Start-up and bacterial communities of single-stage nitrogen removal using anammox and partial nitritation (SNAP) for treatment of high strength ammonia wastewater. Bioresour. Technol. 2014, 169, 652–657. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Moreno-Vivián, C.; Roldán, M.D. Biodegradation of cyanide wastes from mining and jewellery industries. Curr. Opin. Biotechnol. 2016, 38, 9–13. [Google Scholar] [CrossRef]
- Huang, X.; Urata, K.; Wei, Q.; Yamashita, Y.; Hama, T.; Kawagoshi, Y. Fast start-up of partial nitritation as pre-treatment for anammox in membrane bioreactor. Biochem. Eng. J. 2016, 105, 371–378. [Google Scholar] [CrossRef]
- Daverey, A.; Chen, Y.C.; Dutta, K.; Huang, Y.T.; Lin, J.G. Start-up of simultaneous partial nitrification, anammox and denitrification (SNAD) process in sequencing batch biofilm reactor using novel biomass carriers. Bioresour. Technol. 2015, 190, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Chen, Y.C.; Sung, S.; Lin, J.G. Effect of zinc on anammox activity and performance of simultaneous partial nitrification, anammox and denitrification (SNAD) process. Bioresour. Technol. 2014, 165, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Gunatilake, S.K. Methods of removing heavy metals from industrial wastewater. Methods 2015, 1, 14. [Google Scholar]
- Kim, Y.; Cho, H.; Lee, D.; Park, D.; Park, J. Comparative study of free cyanide inhibition on nitrification and denitrification in batch and continuous flow systems. Desalination 2011, 279, 439–444. [Google Scholar] [CrossRef]
- Akinpelu, E.; Ntwampe, S.; Mpongwana, N.; Nchu, F.; Ojumu, T. Biodegradation kinetics of free cyanide in Fusarium oxysporum-Beta vulgaris waste-metal (As, Cu, Fe, Pb, Zn) Cultures under Alkaline Conditions. BioResources 2016, 11, 2470–2482. [Google Scholar] [CrossRef]
- Wu, D.; Senbayram, M.; Well, R.; Brüggemann, N.; Pfeiffer, B.; Loick, N.; Stempfhuber, B.; Dittert, K.; Bol, R. Nitrification inhibitors mitigate N2O emissions more effectively under straw-induced conditions favoring denitrification. Soil Biol. Biochem. 2017, 104, 197–207. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- De Sanctis, D.; Ascenzi, P.; Bocedi, A.; Dewilde, S.; Burmester, T.; Hankeln, T.; Moens, L.; Bolognesi, M. Cyanide binding and heme cavity conformational transitions in Drosophila melanogaster hexacoordinate hemoglobin. Biochemistry 2006, 45, 10054–10061. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Malamis, S.; Omirkhan, A.; Nauruzbayeva, J.; Makhtayeva, Z.; Seidakhmetov, T.; Kudarova, A. Investigating the inhibitory effect of cyanide, phenol and 4-nitrophenol on the activated sludge process employed for the treatment of petroleum wastewater. J. Environ. Manag. 2017, 203, 825–830. [Google Scholar] [CrossRef]
- Safa, Z.J.; Aminzadeh, S.; Zamani, M.; Motallebi, M. Significant increase in cyanide degradation by Bacillus sp. M01 PTCC 1908 with response surface methodology optimization. AMB Express 2017, 7, 200. [Google Scholar] [CrossRef]
- Tiong, B.E.L.I.N.D.A.; Bahari, Z.M.; Lee, N.S.I.S.; Jaafar, J.; Ibrahim, Z.; Shahir, S. Cyanide degradation by Pseudomonas pseudoalcaligenes strain W2 isolated from mining effluent. Sains Malays. 2015, 44, 233–238. [Google Scholar] [CrossRef]
- Itoba-Tombo, E.F. Spatial and temporal distribution of pollutants from different land use/land-cover types of the bottelary river catchment. In New Horizon in wastewater Management Emerging Monitoring and Remediation Strategies; Fosso-Kankeu, E., Ed.; Nova Science Publishers: New York, NY, USA, 2019; Volume 3, pp. 65–86. [Google Scholar]
- Luque-Almagro, V.M.; Blasco, R.; Martínez-Luque, M.; Moreno-Vivián, C.; Castillo, F.; Roldán, M.D. Bacterial cyanide degradation is under review: Pseudomonas pseudoalcaligenes CECT5344, a case of an alkaliphilic cyanotroph. Biochem. Soc. Trans. 2011, 39, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Balomajumder, C.; Agarwal, V. Enzymatic mechanism and biochemistry for cyanide degradation: A review. J. Hazard. Mater. 2010, 176, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.; Kut, Ö.; Prenosil, J.; Bourne, J. Kinetics of enzymatic degradation of cyanide. Biotechnol. Bioeng. 1992, 39, 629–634. [Google Scholar] [CrossRef]
- Han, Y.; Jin, X.; Wang, F.; Liu, Y.; Chen, X. Successful startup of a full-scale acrylonitrile wastewater biological treatment plant (ACN-WWTP) by eliminating the inhibitory effects of toxic compounds on nitrification. Water Sci. Technol. 2013, 69, 553–559. [Google Scholar] [CrossRef]
- Khamar, Z.; Makhdoumi-Kakhki, A.; Mahmudy Gharaie, M. Remediation of cyanide from the gold mine tailing pond by a novel bacterial co-culture. Int. Biodeterior. Biodegrad. 2015, 99, 123–128. [Google Scholar] [CrossRef]
- Rinágelová, A.; Kaplan, O.; Veselá, A.B.; Chmátal, M.; Křenková, A.; Plíhal, O.; Pasquarelli, F.; Cantarella, M.; Martínková, L. Cyanide hydratase from Aspergillus niger K10: Overproduction in Escherichia coli, purification, characterization and use in continuous cyanide degradation. Process. Biochem. 2014, 49, 445–450. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Cabello, P.; Sáez, L.P.; Olaya-Abril, A.; Moreno-Vivián, C.; Roldán, M.D. Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1067–1074. [Google Scholar] [CrossRef]
- Murugesan, T.; Durairaj, N.; Ramasamy, M.; Jayaraman, K.; Palaniswamy, M.; Jayaraman, A. Analeptic agent from microbes upon cyanide degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1557–1565. [Google Scholar] [CrossRef]
- Dwivedi, N.; Balomajumder, C.; Mondal, P. Comparative evaluation of cyanide removal by adsorption, biodegradation, and simultaneous adsorption and biodegradation (SAB) process using Bacillus cereus and almond shell. J. Environ. Biol. 2016, 37, 551. [Google Scholar]
- Ramavandi, B. Adsorption potential of NH4Br-soaked activated carbon for cyanide removal from wastewater. Indian J. Chem. Technol. 2016, 22, 183–193. [Google Scholar]
- Singh, N.; Balomajumder, C. Simultaneous removal of phenol and cyanide from aqueous solution by adsorption onto surface modified activated carbon prepared from coconut shell. J. Water Process. Eng. 2016, 9, 233–245. [Google Scholar] [CrossRef]
- Özel, Y.K.; Gedikli, S.; Aytar, P.; Ünal, A.; Yamaç, M.; Çabuk, A.; Kolankaya, N. New fungal biomasses for cyanide biodegradation. J. Biosci. Bioeng. 2010, 110, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Akhter, Y.; Chatterjee, S. A review on remediation of cyanide containing industrial wastes using biological systems with special reference to enzymatic degradation. World J. Microbiol. Biotechnol. 2019, 35, 70. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.M.; Liu, J.K.; Lou, H.R.; Lin, C.S.; Chen, S.C. Biotransformation of cyanide to methane and ammonia by Klebsiella oxytoca. Chemosphere 2003, 50, 1055–1061. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.K.O.; Jackson, V.A. Biodegradation of free cyanide and subsequent utilisation of biodegradation by-products by Bacillus consortia: Optimisation using response surface methodology. Environ. Sci. Pollut. Res. Int. 2015, 22, 10434–10443. [Google Scholar] [CrossRef]
- Chen, C.; Kao, C.; Chen, S. Application of Klebsiella oxytoca immobilized cells on the treatment of cyanide wastewater. Chemosphere 2008, 71, 133–139. [Google Scholar] [CrossRef]
- Lin, J.; Meng, Y.; Shi, Y.; Lin, X. Complete Genome Sequences of Colwellia sp. Arc7-635, a Denitrifying Bacterium Isolated from Arctic Seawater. Curr. Microbiol. 2019, 76, 1–5. [Google Scholar] [CrossRef]
- Watts, M.P.; Moreau, J.W. New insights into the genetic and metabolic diversity of thiocyanate-degrading microbial consortia. Appl. Microbiol. Biotechnol. 2016, 100, 1101–1108. [Google Scholar] [CrossRef]
- Ryu, B.G.; Kim, W.; Nam, K.; Kim, S.; Lee, B.; Park, M.S.; Yang, J.W. A comprehensive study on algal–bacterial communities shift during thiocyanate degradation in a microalga-mediated process. Bioresour. Technol. 2015, 191, 496–504. [Google Scholar] [CrossRef]
- Salazar-Benites, G.; Dyer, C.; Bott, C.; Kennedy, A.; Williamson, A.; DeVries, A. Some Serious BNR Intensification: Combining cost-effective cyanide treatment, chemically-enhanced primary treatment, and advanced aeration controls to achieve nitrogen removal in a high-rate activated sludge plant. Proc. Water Environ. Fed. 2016, 2016, 4651–4680. [Google Scholar] [CrossRef]
- Mekuto, L.; Kim, Y.M.; Ntwampe, S.K.; Mewa-Ngongang, M.; Mudumbi, N.; Baptist, J.; Dlangamandla, N.; Itoba-Tombo, E.F.; Akinpelu, E.A. Heterotrophic nitrification-aerobic denitrification potential of cyanide and thiocyanate degrading microbial communities under cyanogenic conditions. Environ. Eng. Sci. 2018, 24, 254–262. [Google Scholar] [CrossRef]
- Mpongwana, N.; Ntwampe, S.K.; Mekuto, L.; Akinpelu, E.A.; Dyantyi, S.; Mpentshu, Y. Isolation of high-salinity-tolerant bacterial strains, Enterobacter sp., Serratia sp., Yersinia sp., for nitrification and aerobic denitrification under cyanogenic conditions. Water Sci. Technol. 2016, 73, 2168–2175. [Google Scholar] [CrossRef] [PubMed]


| Category of Affected Process | Gene or Locus | Encoded Gene Product and Their Functions |
|---|---|---|
| Regulation | anr | Fumarate and nitrate reductase (FNR)-like global redox regulator for the expression of denitrification genes. |
| Dnr, fnrD | FNR-like regulator that affects the expression of nirS and norCB. | |
| Fixk2 | FNR-like regulator that affects anaerobic growth on nitrate. | |
| fnrP | FNR-like regulator that affects the expression of narGH. | |
| narL | Nitrate responsive transcription factor of Pseudomonas of a narXL two- component system. | |
| nirI | A membrane protein with similarity to NosR affects nirS expression. | |
| nirR | Pseudomonas locus that affects the synthesis of nirS and LysR regulator. | |
| nirY (orf 286) | FNR-like regulator that affects expression nirS and norCB in Paracoccus and Rhodobacter sp. | |
| nnrS | Activate transcription of nirK and nor genes in Rhodobacter sphaeroides. | |
| nosR | Membrane-bound regulator required for transcription of nosZ. | |
| rpoN | Sigma factors affect denitrification in Ralstonia eutropha | |
| Nitrate respiration | narD | Plasmid bone locus for eutropha respiratory nitrate reduction. |
| narG | α-subunit of nitrate reductase respiration that binds to molybdopterin guanine dinucleotide (MGD). | |
| narH | Β-subunit of nitrate reductse respiration that binds to Fe-S cluster. | |
| narI | Cytochrome b subunit of respiratory nitrate reductase. | |
| narJ | Protein required for nitrate reductase assembles. | |
| Periplasmic nitrate reduction | napA | The large subunit of periplasmic of nitrate reductase that binds to bis- molybdopterin guanine dinucleotide (MGD) and Fe-S cluster. |
| napB | Small subunit of periplasmic of nitrate reductase, a diheme cytochrome c. | |
| napD | Cytoplasmic protein with presumed maturation function, homologous to Escherichia Coli napD (YojF). | |
| napE | Putative monotopic membrane protein; there are no known homologs. | |
| Nitrite respiration | nirB | Cytochrome c552. |
| nirC | Monoheme cytochrome c with a putative function in NirS maturation. | |
| nirK, nirU | Cu-containing nitrite reductase. | |
| nirN orf507 | It affects anaerobic growth and in-vivo nitrite reduction, similar to NirS. | |
| nirQ | Gene product that affects catalytic functions of NirS and NorCB. | |
| nirS (denA) | Cytochrome cd, nitrate reductase. | |
| Heme D1 Biosynthesis | nirD | Gene product affects heme D. Biosynthesis or processing. |
| nirE | S-Adenosyl-l-Methionine uropophyrinogen III methyltransferase. | |
| nirF | Needed for heme D biosynthesis and processing; similar to NirS. | |
| nirG | Gene product affects heme D. Biosynthesis or processing. | |
| nirH | Gene product affects heme D. Biosynthesis or processing. | |
| nirJ, orf393 | Needed for heme D biosynthesis and processing; similar to PqqE, NifB, and MoaA. | |
| nirL | Gene product affects heme D. Biosynthesis or processing. | |
| NO respiration | norB | Cytochrome b subunit of NO reductase. |
| norC | Cytochrome c subunit of NO reductase. | |
| norD, orf6 | Affect availability under denitrifying conditions. | |
| norE, orf2, orf175 | Membrane protein: homologous with COX III. | |
| norF | Affect NO and nitrite reductase. | |
| norQ | Affect NirS and NorCB function; homolog of NirQ. | |
| N2O respiration | Fhp | R. eutropha flavohemoglobin affects N2O and NO reduction. |
| nosA, oprC | Channel-forming outer membrane protein; Cu-processing for NosZ. | |
| nosD | Periplasmic plastic involved in Cu insertion into NosZ. | |
| nosF | ATP or GDP binding protein involved in Cu insertion into NosZ. | |
| nosL | Part of nos gene cluster; putative outer membrane lipoprotein. | |
| nosX | Affect nitrous oxide reduction in Sinorhizobium meliloti. | |
| nosY | Inner membrane protein involved in Cu processing for NosZ. | |
| nosZ | Nitrous oxide reductase. | |
| Electron transfer | azu | Azurin. |
| cycA | Cytochrome C2 (C550). | |
| napC | Tetraheme cytochrome c; homologous to NirT. | |
| nirM (denB) | Cytochrome C551. | |
| nirT | Putative membrane-anchored tetraheme c-type cytochrome. | |
| paz | Pseudoazurin. | |
| Functionally unassigned | Orf396 | A putative 12 span membrane protein of Pseudomonas stutzeri homologous to NnrS. |
| nirX | A Paracoccus putative cytoplasmic protein; homologous to NosX. | |
| orf7, orf63 | Pseudomonas gene downstream of dnr and fnrD. | |
| orf247 | Putative member of the short-chain alcohol dehydrogenase family. |
| Microorganism | Description of Process Examined | Reference |
|---|---|---|
| Bacillus sp | Free cyanide (FCN) biodegradation subsequent nitrification and aerobic denitrification | [78] |
| CN− degrading consortium | Heterotrophic nitrification—aerobic denitrification potential of cyanide and thiocyanate degrading microbial communities under cyanogenic conditions | [84] |
| Enterobacter sp., Yersinia sp. And Serratia sp | Nitrification and aerobic denitrification under cyanogenic conditions | [85] |
| Pseudomonas fluorescens | Elimination of cyanide inhibition through cultivation of cyanide degrading bacteria | [8] |
| Thiobacillus and Micractinium | Simultaneously remove SCN (thiocyanate) and total nitrogen | [82] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpongwana, N.; Ntwampe, S.K.O.; Omodanisi, E.I.; Chidi, B.S.; Razanamahandry, L.C. Sustainable Approach to Eradicate the Inhibitory Effect of Free-Cyanide on Simultaneous Nitrification and Aerobic Denitrification during Wastewater Treatment. Sustainability 2019, 11, 6180. https://doi.org/10.3390/su11216180
Mpongwana N, Ntwampe SKO, Omodanisi EI, Chidi BS, Razanamahandry LC. Sustainable Approach to Eradicate the Inhibitory Effect of Free-Cyanide on Simultaneous Nitrification and Aerobic Denitrification during Wastewater Treatment. Sustainability. 2019; 11(21):6180. https://doi.org/10.3390/su11216180
Chicago/Turabian StyleMpongwana, Ncumisa, Seteno K. O. Ntwampe, Elizabeth I. Omodanisi, Boredi S. Chidi, and Lovasoa C. Razanamahandry. 2019. "Sustainable Approach to Eradicate the Inhibitory Effect of Free-Cyanide on Simultaneous Nitrification and Aerobic Denitrification during Wastewater Treatment" Sustainability 11, no. 21: 6180. https://doi.org/10.3390/su11216180
APA StyleMpongwana, N., Ntwampe, S. K. O., Omodanisi, E. I., Chidi, B. S., & Razanamahandry, L. C. (2019). Sustainable Approach to Eradicate the Inhibitory Effect of Free-Cyanide on Simultaneous Nitrification and Aerobic Denitrification during Wastewater Treatment. Sustainability, 11(21), 6180. https://doi.org/10.3390/su11216180







