Effect of Biochar and Irrigation on the Interrelationships among Soybean Growth, Root Nodulation, Plant P Uptake, and Soil Nutrients in a Sandy Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site and Design
2.2. Measurements of Plant Dry Mass and Nutrient Concentrations
2.3. Measurements of the Plant Growth Parameters and Yield Components
2.4. Nodule Sampling and Leghemoglobin Content Analysis
2.5. Soil Analyses
2.6. Acid and Alkaline Phosphomonoesterase Activity Assays
2.7. Statistical Analysis
3. Results
3.1. Plant Growth Parameters and Soybean Yield Components
3.2. Nodulation, Lb Content, and Phosphatase Activity
3.3. Soil Chemical Properties
3.4. Principal Component Analysis
3.5. Redundancy Analysis
4. Discussion
4.1. Plant Growth Response to Irrigation and Biochar Addition
4.2. Biochar Influence on Soil C
4.3. Root Nodulation Response to Soil N, K, Mg, and S Based on Biochar Addition
4.4. The Relationships Among Biochar, Phosphatase Activity, and Root P
4.5. Liming Effect on Soil CEC, Root Nodulation, and AKP Activity
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lanza, G.; Rebensburg, P.; Kern, J.; Lentzsch, P.; Wirth, S. Impact of Chars and Readily Available Carbon on Soil Microbial Respiration and Microbial Community Composition in a Dynamic Incubation Experiment. Soil Tillage Res. 2016, 164, 18–24. [Google Scholar] [CrossRef]
- Smith, P.; Gregory, P.J. Climate Change and Sustainable Food Production. Proc. Nutr. Soc. 2013, 72, 21–28. [Google Scholar] [CrossRef] [PubMed]
- McBeath, A.V.; Smernik, R.J. Variation in the Degree of Aromatic Condensation of Chars. Org. Geochem. 2009, 40, 1161–1168. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An Investigation into the Reactions of Biochar in Soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Boonchan, S.; Britz, M.L.; Stanley, G.A. Degradation and Mineralization of High Molecular Weight Polycyclic Armoatic Hydrocarbons by Defined Fungal Bacterial Cocultures. Appl. Environ. Microbiol. 2000, 66, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Hagemann, N.; Harter, J.; Behrens, S. Elucidating the Impacts of Biochar Applications on Nitrogen Cycling Microbial Communities; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Gong, H.; Meng, D.; Li, X.; Zhu, F. Soil Degradation and Food Security Coupled with Global Climate Change in Northeastern China. Chin. Geogr. Sci. 2013, 23, 562–573. [Google Scholar] [CrossRef]
- Massah, J.; Azadegan, B. Effect of Chemical Fertilizers on Soil Compaction and Degradation. Agric. Mech. Asia Afr. Lat. Am. 2016, 47, 44–50. [Google Scholar]
- Salvagiotti, F.; Cassman, K.G.; Weiss, A.; Dobermann, A. Nitrogen Uptake, Fixation and Response to N Fertilizer in Soybeans. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Sahrawat, K.L.; Upadhyaya, H.D.; Mengoni, A.; Galardini, M.; Bazzicalupo, M.; Biondi, E.G.; Hungria, M.; Kaschuk, G.; Blair, M.W.; et al. Advances in Host Plant and Rhizobium Genomics to Enhance Symbiotic Nitrogen Fixation InGrain Legumes; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 129. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from Water Hyacinth (Eichornia Crassipes) and Its Impact on Soil Biological Activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Głodowska, M.; Schwinghamer, T.; Husk, B.; Smith, D. Biochar Based Inoculants Improve Soybean Growth and Nodulation. Agric. Sci. 2017, 8, 1048–1064. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Wirth, S.J.; Alam, P.; Alyemeni, M.N.; Ahmad, P. Interactive Effects of Nutrients and Bradyrhizobium Japonicum on the Growth and Root Architecture of Soybean (Glycine Max L.). Front. Microbiol. 2018, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.W. N2 Fixation and Growth of Legumes as Affected by Sulphur Fertilization. Biol. Fertil. Soils 1996, 23, 449–453. [Google Scholar] [CrossRef]
- Scherer, H.W.; Pacyna, S.; Spoth, K.R.; Schulz, M. Low Levels of Ferredoxin, ATP and Leghemoglobin Contribute to Limited N2 Fixation of Peas (Pisum Sativum L.) and Alfalfa (Medicago Sativa L.) under S Deficiency Conditions. Biol. Fertil. Soils 2008, 44, 909–916. [Google Scholar] [CrossRef]
- Scheifele, M.; Hobi, A.; Buegger, F.; Gattinger, A.; Schulin, R.; Boller, T.; Mäder, P. Impact of Pyrochar and Hydrochar on Soybean (Glycine Max L.) Root Nodulation and Biological Nitrogen Fixation. J. Plant Nutr. Soil Sci. 2017, 180, 199–211. [Google Scholar] [CrossRef]
- Anderson, A.J.; Spencero, D. Molybdenum in nitrogen metabolism of legumes and non-legumes. Aust. J. Sci. Res. 1950, 3, 414–430. [Google Scholar] [CrossRef]
- El Msehli, S.; Lambert, A.; Baldacci-Cresp, F.; Hopkins, J.; Boncompagni, E.; Smiti, S.A.; Hérouart, D.; Frendo, P. Crucial Role of (Homo)Glutathione in Nitrogen Fixation in Medicago Truncatula Nodules. New Phytol. 2011, 192, 496–506. [Google Scholar] [CrossRef]
- Kalloniati, C.; Krompas, P.; Karalias, G.; Udvardi, M.K.; Rennenberg, H.; Herschbach, C.; Flemetakis, E. Nitrogen-Fixing Nodules Are an Important Source of Reduced Sulfur, Which Triggers Global Changes in Sulfur Metabolism in Lotus japonicus. Plant Cell 2015, 27, 2384–2400. [Google Scholar] [CrossRef]
- Davidian, J.C.; Kopriva, S. Regulation of Sulfate Uptake and Assimilation—The Same or Not the Same? Mol. Plant 2010, 3, 314–325. [Google Scholar] [CrossRef]
- Sorrenti, G.; Muzzi, E.; Toselli, M. Root growth dynamic and plant performance of nectarine trees amended with biochar and compost. Sci. Hortic. 2019, 257, 108710. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Fu, S.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Effect of Biochar in Cadmium Availability and Soil Biological Activity in an Anthrosol Following Acid Rain Deposition and Aging. Water Air Soil Pollut. 2015, 226, 164. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Cely, P.; Plaza, C.; Méndez, A. Influence of pig manure and its biochar on soil CO2 emissions and soil enzymes. Ecol. Eng. 2016, 5, 19–24. [Google Scholar] [CrossRef]
- Das, S.K.; Varma, A. Soil Enzymology. Sci. Prog. 2011, 64, 275–285. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Phosphorus Cycle Enzymes. In Methods of Soil Enzymology; Dick, R.P., Ed.; SSSA Book Series, No. 9; Soil Science Society of America: Madison, WI, USA, 2011; pp. 161–183. [Google Scholar] [CrossRef]
- Benavente, I.; Gasco, G.; Plaza, C.; Paz-Ferreiro, J.; Mendez, A. Choice of pyrolysis parameters for urban wastes affects soil enzymes and plant germination in a Mediterranean soil. Sci. Total Environ. 2018, 634, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Martínez, V.; Tabatabai, M.A. Enzyme Activities in a Limed Agricultural Soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Li, S.; Liang, C.; Shangguan, Z. Effects of Apple Branch Biochar on Soil C Mineralization and Nutrient Cycling under Two Levels of N. Sci. Total Environ. 2017, 607–608, 109–119. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Foster, E.J.; Fogle, E.J.; Cotrufo, M.F. Sorption to biochar impacts β-glucosidase and phosphatase enzyme activities. Agriculture 2018, 8, 158. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Bound, S.A.; Doyle, R.; Bowman, J.P. Effects of Biochar and Compost Amendments on Soil Physico-Chemical Properties and the Total Community within a Temperate Agricultural Soil. Appl. Soil Ecol. 2016, 98, 243–253. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A. Stabilizing Effect of Biochar on Soil Extracellular Enzymes after a Denaturing Stress. Chemosphere 2016, 142, 114–119. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Oram, N.J.; van de Voorde, T.F.J.; Ouwehand, G.-J.; Bezemer, T.M.; Mommer, L.; Jeffery, S.; Van Groenigen, J.W. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef]
- Deng, B.; Tammeorg, P.; Luukkanen, O.; Helenius, J.; Starr, M. Effects of Acacia seyal and biochar on soil properties and sorghum yield in agroforestry systems in South Sudan. Agrofor. Syst. 2017, 91, 137. [Google Scholar] [CrossRef]
- Lehmann, J.; Kuzyakov, Y.; Pan, G.; Ok, Y.S. Biochars and the plant-soil interface. Plant Soil 2015, 395, 1–5. [Google Scholar] [CrossRef]
- Ulrich, A.; Klimke, G.; Wirth, S. Diversity and Activity of Cellulose-Decomposing Bacteria, Isolated from a Sandy and a Loamy Soil after Long-Term Manure Application. Microb. Ecol. 2008, 55, 512. [Google Scholar] [CrossRef]
- Wilson, D.O.; Reisenauer, H.M. Determination of Leghemoglobin in Legume Nodules. Anal. Biochem. 1963, 6, 27–30. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon and Organic Matter. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; ASA-SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- ISO 13536:1995. Soil Quality—Determination of the Potential Cation Exchange Capacity and Exchangeable Cations Using Barium Chloride Solution Buffered at pH = 8.1; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Principal Component Analysis and Redundancy Analysis. Anal. Ecol. Data Stat. Biol. Heal. 2007, 193–224. [Google Scholar] [CrossRef]
- Bonser, S.P.; Aarssen, L.W. Allometry and Plasticity of Meristem Allocation throughout Development in Arabidopsis Thaliana. J. Ecol. 2001, 89, 72–79. [Google Scholar] [CrossRef]
- Bonser, S.P.; Aarssen, L.W. A Llometry and Development in Herbaceous Plants: Functional Responses of Meristem Allocation To Light and Nutrient Availability 1. Am. J. Bot. 2003, 90, 404–412. [Google Scholar] [CrossRef]
- Caton, B.P.; Foin, T.C.; Hill, J.E. Phenotypic Plasticity of Ammannia Spp. in Competition with Rice. Weed Res. 1997, 37, 33–38. [Google Scholar] [CrossRef]
- Gascó, G.; Cely, P.; Paz-Ferreiro, J.; Plaza, C.; Méndez, A. Relation between biochar properties and effects on seed germination and plant development. Biol. Agric. Hortic. 2016, 32, 237–247. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, D.; Huang, Y.; Japhet, W.; Sun, D. Plasticity and Allometry of Meristem Allocation in Response to Density in Three Annual Plants with Different Architectures. Botany 2008, 86, 1291–1298. [Google Scholar] [CrossRef]
- Roitsch, T. Source-Sink Regulation. Curr. Opin. Plant Biol. 1999, 2, 198–206. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, X.; Fu, S.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Biochar alters the resistance and resilience to drought in a tropical soil. Environ. Res. Lett. 2014, 9, 064013. [Google Scholar] [CrossRef]
- Bellaloui, N.; Mengistu, A.; Kassem, M.A. Effects of Genetics and Environment on Fatty Acid Stability in Soybean Seed. Food Nutr. Sci. 2013, 4, 165–175. [Google Scholar] [CrossRef]
- Rajabbeigi, E.; Eichholz, I.; Beesk, N.; Ulrichs, C.; Kroh, L.W.; Rohn, S.; Huyskens-Keil, S. Interaction of Drought Stress and UV-B Radiation-Impact on Biomass Production and Flavonoid Metabolism in Lettuce (Lactuca sativa L.). J. Appl. Bot. Food Qual. 2013, 86, 190–197. [Google Scholar] [CrossRef]
- Güereña, D.T.; Lehmann, J.; Thies, J.E.; Enders, A.; Karanja, N.; Neufeldt, H. Partitioning the Contributions of Biochar Properties to Enhanced Biological Nitrogen Fixation in Common Bean (Phaseolus vulgaris). Biol. Fertil. Soils 2015, 51, 479–491. [Google Scholar] [CrossRef]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar Characteristics and Application Rates Affecting Corn Growth and Properties of Soils Contrasting in Texture and Mineralogy. Geoderma 2015, 237, 105–116. [Google Scholar] [CrossRef]
- Novak, J.; Busscher, W.; Laird, D.; Ahmedna, M.; Watts, D.W.; Niandou, M. Impact of Biochar Amendment on Fertility of a Southeastern Coastal Plain Soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Shinogi, Y.; Yoshida, H.; Koizumi, T.; Yamaoka, M.; Saito, T. Basic Characteristics of Low-Temperature Carbon Products from Waste Sludge. Adv. Environ. Res. 2003, 7, 661–665. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of Soil-Applied Black Carbon: Downward Migration, Leaching and Soil Respiration. Glob. Chang. Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Appleby, C.A. Leghaemoglobin and Rhizobium respiration. Annu. Rev. Plant Physiol. 1984, 35, 443–478. [Google Scholar] [CrossRef]
- Ohyama, T.; Yashima, H.; Tanabata, S.; Ishikawa, S.; Sato, T.; Nishiwaki, T.; Ohtake, N.; Sueyoshi, K.; Ishii, S.; Fujimaki, S. Effect of Nitrate on Nodulation and Nitrogen Fixation of Soybean. Soybean Physiol. Biochem. 2011, 333–364. [Google Scholar] [CrossRef]
- Singh, S.; Varma, A. Rhizobium Biology and Biotechnology 15Structure, Function, and Estimation of Leghemoglobin. Soil Biol. 2017, 50, 309–330. [Google Scholar] [CrossRef]
- Clúa, J.; Roda, C.; Zanetti, M.E.; Blanco, F.A. Compatibility between Legumes and Rhizobia for the Establishment of a Successful Nitrogen-Fixing Symbiosis. Genes 2018, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.I. The effects of water stress on nitrogen-fixing root nodules. New Phytol. 1971, 70, 9–17. [Google Scholar] [CrossRef]
- Graham, P.H. Stress Tolerance in Rhizobium and Bradyrhizobium, and Nodulation under Adverse Soil Conditions. Can. J. Microbiol. 1992, 38, 475–484. [Google Scholar] [CrossRef]
- Serraj, R.; Vadez, V.; Denison, R.F.; Sinclair, T.R. Involvement of Ureides in Nitrogen Fixation Inhibition in Soybean1. Plant Physiol. 1999, 119, 289–296. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Hua, M.; Wirth, S.; Bellingrath-kimura, D. Potential Effects of Biochar-Based Microbial Inoculants in Agriculture. Environ. Sustain. 2018, 1, 19–24. [Google Scholar] [CrossRef]
- Peng, W.T.; Zhang, L.D.; Zhou, Z.; Fu, C.; Chen, Z.C.; Liao, H. Magnesium Promotes Root Nodulation through Facilitation of Carbohydrate Allocation in Soybean. Physiol. Plant. 2018, 163, 372–385. [Google Scholar] [CrossRef]
- Sangakkara, U.R.; Hartwig, U.A.; Nösberger, J. Soil Moisture and Potassium Affect the Performance of Symbiotic Nitrogen Fixation in Faba Bean and Common Bean. Plant Soil 1996, 184, 123–130. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Ogaya, R. Experimental Drought Reduced Acid and Alkaline Phosphatase Activity and Increased Organic Extractable P in Soil in a Quercus Ilex Mediterranean Forest. Eur. J. Soil Biol. 2008, 44, 509–520. [Google Scholar] [CrossRef]
- Yu, O.Y.; Raichle, B.; Sink, S. Impact of Biochar on the Water Holding Capacity of Loamy Sand Soil. Int. J. Energy Environ. Eng. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Colvan, S.R.; Syers, J.K.; O’Donnell, A.G.O. Effect of Long-Term Fertiliser Use on Acid and Alkaline Phosphomonoesterase and Phosphodiesterase Activities in Managed Grassland. Biol. Fertil. Soils 2001, 34, 258–263. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Phosphorus in Action. Soil Biol. 2011, 26, 215–243. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil-Concepts and Mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Blackwell, M.S.A.; Brookes, P.C.; de la Fuente-Martinez, N.; Gordon, H.; Murray, P.J.; Snars, K.E.; Williams, J.K.; Bol, R.; Haygarth, P.M. Phosphorus Solubilization and Potential Transfer to Surface Waters from the Soil Microbial Biomass Following Drying–Rewetting and Freezing–Thawing, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 106. [Google Scholar] [CrossRef]
- Antoun, H. Beneficial Microorganisms for the Sustainable Use of Phosphates in Agriculture. Procedia Eng. 2012, 46, 62–67. [Google Scholar] [CrossRef]
- Vassilev, N.; Martos, E.; Mendes, G.; Martos, V.; Vassileva, M. Biochar of Animal Origin: A Sustainable Solution to the Global Problem of High-Grade Rock Phosphate Scarcity? J. Sci. Food Agric. 2013, 93, 1799–1804. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical Cycling of Nitrogen and Phosphorus in Biochar-Amended Soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Rose, T.; Herridge, D.; Kimber, S.; Rust, J.; Cowie, A.; Morris, S. Enhanced Biological N2 Fixation and Yield of Faba Bean (Vicia faba L.) in an Acid Soil Following Biochar Addition: Dissection of Causal Mechanisms. Plant Soil 2015, 395, 7–20. [Google Scholar] [CrossRef]
- Marschner, H.; Marschner, H. 7–Nitrogen Fixation. Miner. Nutr. High. Plants 1995, 2, 201–228. [Google Scholar] [CrossRef]
- Quilliam, R.S.; DeLuca, T.H.; Jones, D.L. Biochar Application Reduces Nodulation but Increases Nitrogenase Activity in Clover. Plant Soil 2013, 366, 83–92. [Google Scholar] [CrossRef]
- Indrasumunar, A.; Menzies, N.W.; Dart, P.J. Calcium Affects the Competitiveness of Acid-Sensitive and Acid-Tolerant Strains of Bradyrhizobium Japonicum in Nodulating and Fixing Nitrogen with Two Soybean Cultivars in Acid Soil. Soil Biol. Biochem. 2012, 46, 115–122. [Google Scholar] [CrossRef]
- Haynes, R.J. Effects of Liming on Phosphate Availability in Acid Soils—A Critical Review. Plant Soil 1982, 68, 289–308. [Google Scholar] [CrossRef]

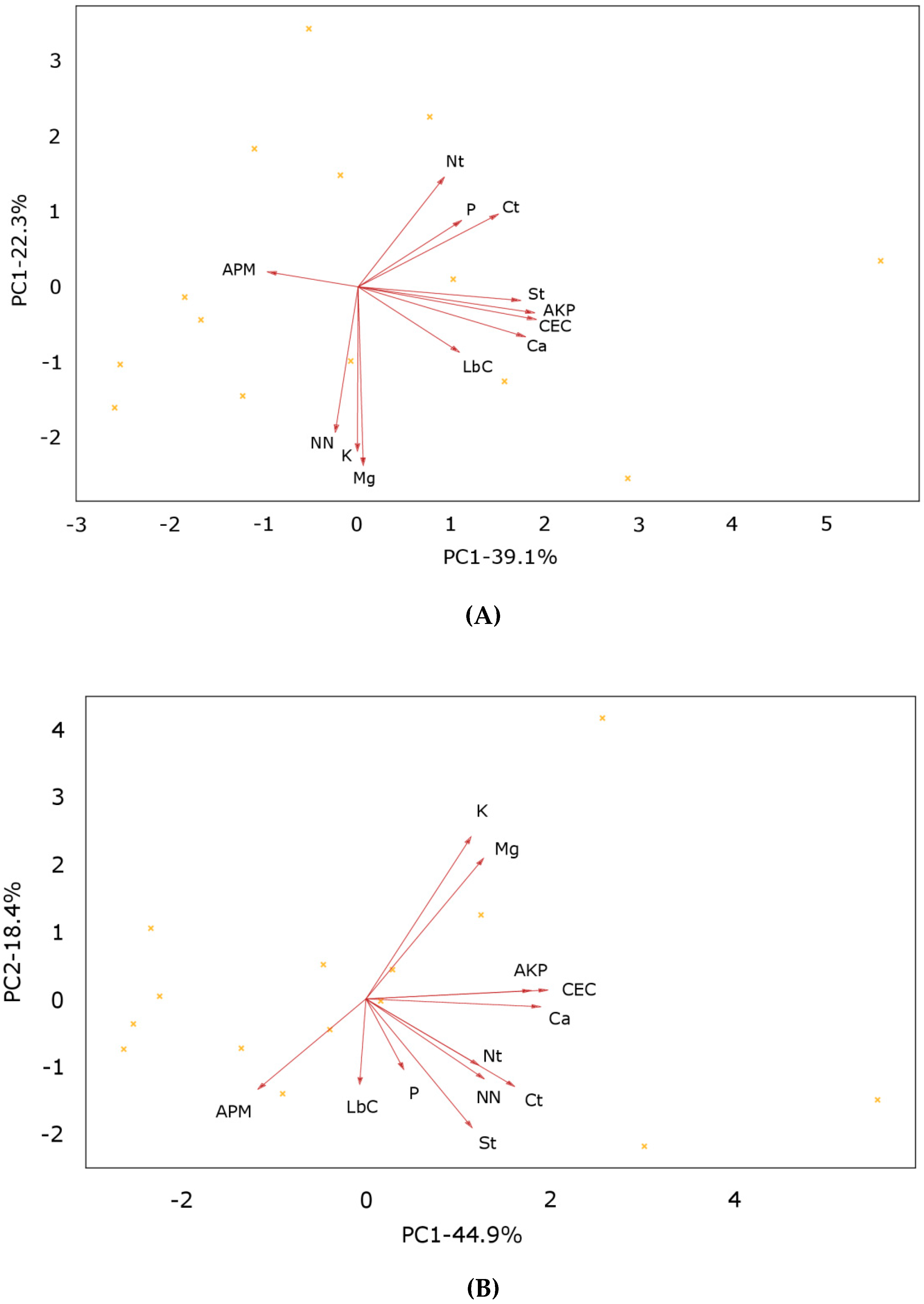
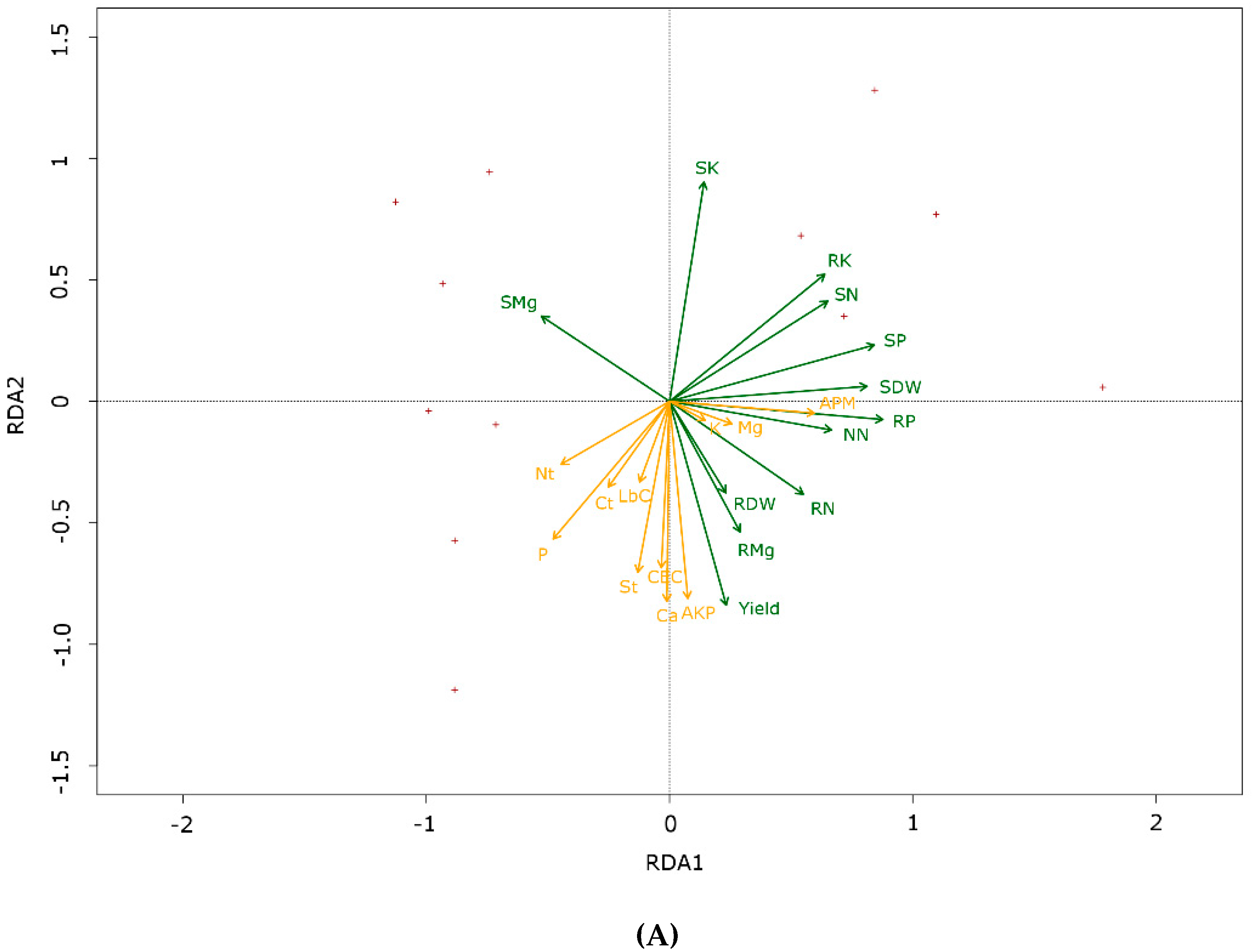

| Plant Measures | Shoot Dry Weight (g m−2) | Root Dry Weight (g m−2) | Grain Yield (t ha−1) | Shoot Dry Matter Content (%) | Root Dry Matter Content (%) |
|---|---|---|---|---|---|
| Irrigation + biochar | 694 ± 17 a | 70 ± 2 a | 2.85 ± 0.24 a | 26.96 ± 0.37 b | 67.08 ± 1.23 b |
| Irrigation − biochar | 697 ± 17 a | 71 ± 3 a | 2.72 ± 0.12 a | 26.52 ± 0.27 b | 68.60 ± 2.35 b |
| Rainfed + biochar | 616 ± 14 b | 66 ± 3 a | 2.59 ± 0.09 a | 30.49 ± 0.32 a | 74.83 ± 1.63 a |
| Rainfed − biochar | 625 ± 10 b | 68 ± 3 a | 2.55 ± 0.10 a | 30.39 ± 0.41 a | 75.29 ± 1.41 a |
| Plant Nutrient Concentration | Shoot | ||||
| N (g kg−1) | P (g kg−1) | K (g kg−1) | Mg (g kg−1) | ||
| Irrigation + biochar | 23.69 ± 0.69 a | 3.51 ± 0.08 a | 20.92 ± 0.32 a | 3.38 ± 0.09 a | |
| Irrigation − biochar | 23.21 ± 0.85 a | 3.53 ± 0.04 a | 21.42 ± 0.58 a | 3.61 ± 0.05 a | |
| Rainfed + biochar | 21.68 ± 0.25 b | 3.06 ± 0.05 b | 20.80 ± 0.20 a | 3.69 ± 0.09 a | |
| Rainfed − biochar | 21.49 ± 0.35 b | 3.06 ± 0.12 b | 20.45 ± 0.20 a | 3.66 ± 0.10 a | |
| Plant Nutrient Concentration | Root | ||||
| N (g kg−1) | P (g kg−1) | K (g kg−1) | Mg (g kg−1) | ||
| Irrigation + biochar | 7.55 ± 0.41 a | 1.67 ± 0.12 a | 12.23 ± 0.48 a | 2.49 ± 0.14 a | |
| Irrigation − biochar | 7.84 ± 0.36 a | 1.50 ± 0.09 a | 12.20 ± 0.36 a | 2.47 ± 0.11 a | |
| Rainfed + biochar | 6.61 ± 0.09 b | 1.04 ± 0.03 b | 10.40 ± 0.44 a | 2.25 ± 0.09 a | |
| Rainfed − biochar | 6.42 ± 0.12 b | 1.00 ± 0.04 b | 10.28 ± 0.35 a | 2.31 ± 0.06 a | |
| Nodule Number | Lb Content | AKP Activity | APM Activity | Soil Water Content | |
|---|---|---|---|---|---|
| Unit | plant−1 | mg gFW−1 | µmol pNP g−1 h−1 | µmol pNP g−1 h−1 | % |
| Irrigation + biochar | 35.46 ± 4.32 a | 70 ± 2 a | 3.19 ± 0.89 a | 7.47 ± 0.42 a | 6.9 ± 0.8 a |
| Irrigation − biochar | 25.88 ± 3.61 b | 71 ± 3 a | 2.67 ± 0.72 a | 7.15 ± 0.52 ab | 6.3 ± 0.7 a |
| Rainfed + biochar | 17.38 ± 3.97 c | 66 ± 3 a | 2.70 ± 0.36 a | 6.13 ± 0.40 b | 6.5 ± 0.5 a |
| Rainfed − biochar | 12.24 ± 1.28 c | 68 ± 3 a | 2.82 ± 0.46 a | 5.97 ± 0.22 b | 5.8 ± 0.5 a |
| Soil Properties | C | N | S | P | K | Ca | Mg | CEC | pH (H2O) |
|---|---|---|---|---|---|---|---|---|---|
| Unit | g kg−1 | g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | cmol+ kg−1 | |
| Irrigation + biochar | 11.00 ± 0.44 ab | 1.01 ± 0.05 a | 0.23 ± 0.01 a | 379 ± 4 a | 1066 ± 41 a | 1441 ± 168 a | 924 ± 22 a | 4.88 ± 0.43 a | 6.51 ± 0.17 a |
| Irrigation − biochar | 10.30 ± 0.43 ab | 1.06 ± 0.05 a | 0.24 ± 0.01 a | 379 ± 8 a | 1040 ± 45 a | 1352 ± 162 a | 945 ± 34 a | 4.58 ± 0.44 a | 6.32 ± 0.18 a |
| Rainfed + biochar | 11.48 ± 0.41 a | 1.10 ± 0.04 a | 0.24 ± 0.00 a | 396 ± 8 a | 1017 ± 55 a | 1404 ± 90 a | 864 ± 43 a | 4.93 ± 0.29 a | 6.52 ± 0.07 a |
| Rainfed − biochar | 10.12 ± 0.23 b | 1.06 ± 0.04 a | 0.23 ± 0.01 a | 390 ± 8 a | 1035 ± 70 a | 1325 ± 86 a | 890 ± 52 a | 4.59 ± 0.28 a | 6.41 ± 0.17 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Egamberdieva, D.; Wirth, S.; Li, Q.; Omari, R.A.; Hou, M.; Bellingrath-Kimura, S.D. Effect of Biochar and Irrigation on the Interrelationships among Soybean Growth, Root Nodulation, Plant P Uptake, and Soil Nutrients in a Sandy Field. Sustainability 2019, 11, 6542. https://doi.org/10.3390/su11236542
Ma H, Egamberdieva D, Wirth S, Li Q, Omari RA, Hou M, Bellingrath-Kimura SD. Effect of Biochar and Irrigation on the Interrelationships among Soybean Growth, Root Nodulation, Plant P Uptake, and Soil Nutrients in a Sandy Field. Sustainability. 2019; 11(23):6542. https://doi.org/10.3390/su11236542
Chicago/Turabian StyleMa, Hua, Dilfuza Egamberdieva, Stephan Wirth, Qirui Li, Richard Ansong Omari, Mudan Hou, and Sonoko D. Bellingrath-Kimura. 2019. "Effect of Biochar and Irrigation on the Interrelationships among Soybean Growth, Root Nodulation, Plant P Uptake, and Soil Nutrients in a Sandy Field" Sustainability 11, no. 23: 6542. https://doi.org/10.3390/su11236542
APA StyleMa, H., Egamberdieva, D., Wirth, S., Li, Q., Omari, R. A., Hou, M., & Bellingrath-Kimura, S. D. (2019). Effect of Biochar and Irrigation on the Interrelationships among Soybean Growth, Root Nodulation, Plant P Uptake, and Soil Nutrients in a Sandy Field. Sustainability, 11(23), 6542. https://doi.org/10.3390/su11236542






