Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Pot Experiment
2.3. Measurement of Phytometric Features of the Plants
2.4. Methods of Drying
2.5. Modelling of Drying Kinetics
- A—coefficient corresponding to the initial value of MR,
- k—drying constant,
- n—exponent.
- M—current moisture content,
- M0—initial moisture content.
2.6. Plant Extract
2.7. Total Polyphenolic Content
2.8. Antioxidant Activities (ABTS Method)
2.9. Colour Change
2.10. Head Space–Solid Phase Microextraction HS-SPME
2.11. Chromatographic Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Phytometric Features of Plants
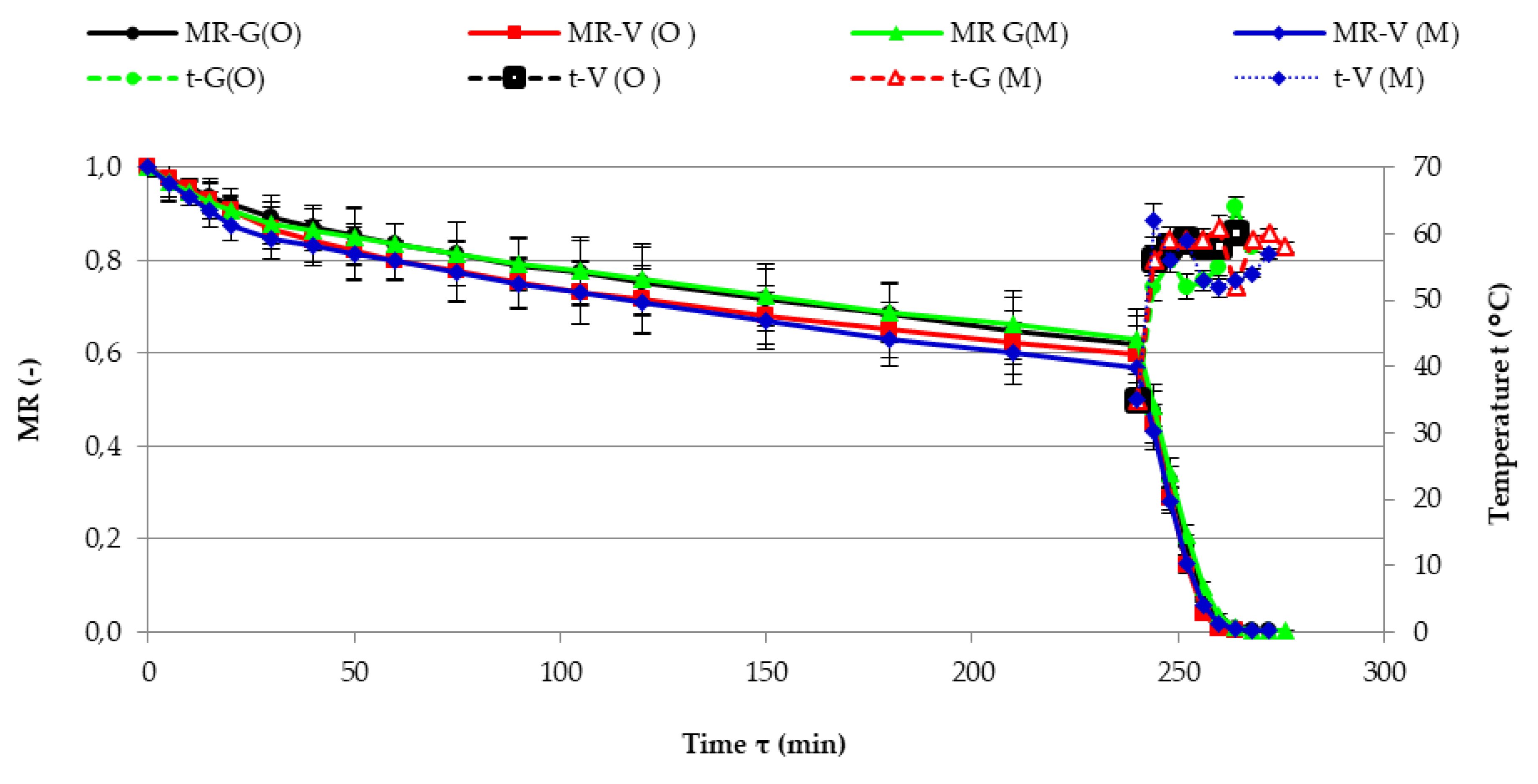
3.2. The Kinetics of Drying
3.3. Colour
3.4. Bioactive Compounds Content
3.5. Chemical Composition of Volatile Fraction HS-SPME
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Borowski, B.; Dzida, K. Yield and chemical composition of basil herb depending on cultivar and foliar feeding with nitrogen. Acta Scientarium Pol. Hortorum Cultus 2011, 10, 207–219. [Google Scholar]
- Paton, A.; Harley, R.M.; Harley, M.M. Ocimum: An Overview of Relationships and Classification. In Medicinal and Aromatic Plants—Industrial Profiles; Holm, Y., Hiltunen, R., Eds.; Harwood Academic: Amsterdam, The Netherlands, 1999; pp. 1–38. [Google Scholar]
- Makri, O.; Kintzios, S. Ocimum sp. (Basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2007, 13, 123–150. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Orlic, S.; Politeo, O.; Strikic, F.; Kolak, I.; Milos, M.; Satovic, Z. Composition and antibacterial activities of essentials oils of seven Ocimum taxa. Food Chem. 2010, 119, 196–201. [Google Scholar] [CrossRef]
- Moghaddam, A.M.D.; Shayegh, J.; Mikaili, P.; Sharaf, J.D. Antimicrobial activity of essentials oil extract of Ocimum basilicum L. leaves on a variety of pathogenic bacteria. J. Med. Plants Res. 2011, 5, 3453–3456. [Google Scholar]
- De Vincenzi, M.; Silano, M.; Maialetti, F.; Scazzocchio, B. Constituents of aromatic plants: II. Estragole. Fitoterapia 2000, 71, 725–729. [Google Scholar] [CrossRef]
- Kaledin, V.I.; Pakharukova, M.Y.; Pivovarova, E.N.; Kropachev, K.Y.; Baginskaya, N.V.; Vasilieva, E.D.; Ilnitskaya, S.I.; Nikitenko, E.V.; Kobzev, V.F.; Merkulova, T.I. Correlation between hepatocarcinogenic effect of estragole and its influence on glucocorticoid induction of liver-specific enzymes and activities of FOXA and HNF4 transcription factors in mouse and rat liver. Biochemistry 2009, 74, 377–384. [Google Scholar] [CrossRef]
- Rakic, Z.; Johnson, C.B. Influence of environmental factors (including UV-B radiation) on the composition of the essential oil of Ocimum basilicum—Sweet basil. J. Herbs Spices Med. Plants 2002, 9, 157–162. [Google Scholar] [CrossRef]
- Biesiada, A.; Kuś, A. The effect of nitrogen fertilization and irrigation on yielding and nutritional status of sweet basil (Ocimum basilicum L.). ACTA Sci. Pol. Hortic. Hortorum Cultus 2010, 9, 3–12. [Google Scholar]
- Mordalski, R.; Buchwald, W.; Kucharski, W.A.; Zalińska, H.; Forycka, A. Impact of mineral fertilization on yield of common basil (Ocimum basilicum L.) herb. Post Fitoter 2018, 19, 143–148. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Sweet basil (Ocimum basilicum L.) flowering affected by foliar nitrogen application. Acta Agrobot. 2011, 64, 57–64. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Kwee, E.M.; Niemeyer, E.D. Potassium rate alters the antioxidant capacity and phenolic concentration of basil (Ocimum basilicum L.) leaves. Food Chem. 2010, 123, 1235–1241. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Rożek, E.; Borowski, B. Response of different basil cultivars to nitrogen and potassium fertilization: Total and mineral nitrogen content in herb. ACTA Sci. Pol. Hortic. Hortorum Cultus 2011, 10, 217–232. [Google Scholar]
- Ramezani, S.; Rezaei, M.R.; Sotoudehnia, P. Improved growth, field and essentials oil content of basil grown under different levels of phosphorus sprays in the field. J. Appl. Biol. Sci. 2009, 3, 96–101. [Google Scholar]
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of nutritional properties of Stinging nettle (Urtica dioica) flour with wheat and barley flours. Food Sci. Nutr. 2016, 4, 119–124. [Google Scholar] [CrossRef]
- Kochan, E.A.; Balcerczak, E.; Szymczyk, P.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Szymańska, G. Abscicis acid gerulates the 3-hydroxy-3-methylglutaryl CoA reductase gene promoter and ginsenoside production in Panax quinquefolium hairy root culures. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Stępień, A.E.; Gorzelany, J.; Matłok, N.; Lech, K.; Figiel, A. The effect of drying methods on the energy consumption, bioactive potential and colour of dried leaves of Pink Rock Rose (Cistus creticus). J. Food Sci. Technol. 2019, 56, 2386–2394. [Google Scholar] [CrossRef]
- Alibas, I. Determination of drying parameters, ascorbic acid contents and color characteristics of nettle leaves during microwave-, air- and combined microwave-air-drying. J. Food Process Eng. 2010, 33, 213–233. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Aouadhi, C.; Kaddour, R.; Gruber, M.; Zargouni, H.; Zaouali, W.; Ben Hamida, N.; Ben Nasri, M.; Ouerghi, Z.; Hosni, K. Comparison of antioxidant and antimicrobial activities of two cultivated Cistus species from Tunisia. J. Biosci. 2016, 32, 226–237. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sifola, M.I.; Barbieri, G. Growth, yield and essentials oil content of three cultivars of basil grown under different levels of nitrogen in the field. Sci. Hortic. 2006, 108, 408–413. [Google Scholar] [CrossRef]
- Rao, E.V.S.P.; Puttana, K.; Rao, R.S.G.; Ramesh, S. Nitrogen and potassium nutrition of French basil (Ocimum basilicum Linn). J. Spices Aromat. Crop. 2007, 16, 99–105. [Google Scholar]
- Sarab, D.; Badi, H.N.; Nasi, M.; Makkizadeh, M.; Midi, H. Changes in essentials oil content and yield of basil in response to different levels of nitrogen and plant density. J. Med. Plants 2008, 7, 60–70. [Google Scholar]
- Zheljazkov, V.D.; Cantrell, C.L.; Ebelhar, M.W.; Rowe, D.; Coker, C.E. Productivity, oil content, and oil composition of sweet basil as a function of nitrogen and sulphur fertilization. Hortic. Sci. 2008, 43, 1415–1422. [Google Scholar]
- Daneshian, A.; Gurbuz, B.; Cosge, B.; Ipek, A. Chemical components of essential oils from basil (Ocimum basilicum L.) grown at different nitrogen levels. Int. J. Nat. Eng. Sci. 2009, 3, 8–12. [Google Scholar]
- Krokida, M.K.; Maroulis, Z.B.; Saravacos, G.D. The effect of the method of drying on the color of dehydrated products. Int. J. Food Sci. Technol. 2001, 36, 53–59. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M.; Okyay, M.H. Evaluation of drying methods with respect to drying parameters, some nutritional and colour characteristics of peppermint (Mentha x piperita L.). Energy Convers. Manag. 2010, 51, 2769–2775. [Google Scholar] [CrossRef]
- Sarimeseli, A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energy Convers. Manag. 2011, 52, 1449–1453. [Google Scholar] [CrossRef]
- Jaroszewska, A. Assimilatory pigment contentof stone fruit trees leaves in relation to irrigation and fertilization, infrastructure and ecology of rural areas. Kom. Tech. Infrastruktury Wsi 2011, 6, 155–164. [Google Scholar]
- Śledź, M.; Witrowa-Rajchert, D. Influence of microwave-convective drying on chlorophyll content and colour of herbs. Acta Agrophys. 2012, 19, 865–876. [Google Scholar]
- Therdthai, N.; Zhou, W. Characterization of microwave vacuum drying and hot air drying of mint leaves (Mentha cordifolia Opiz ex Fresen). J. Food Eng. 2009, 91, 482–489. [Google Scholar] [CrossRef]
- Szymanowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Eckelmann, S. Biodiversitat der Gattung Ocimum L., Insbesondere der Kultursippen. Die Doktorarbeit, University of Kassel: Kassel, Germany, 2002; Volume 19, p. 236. Available online: http://www.genres.de/infos/igrreihe.htm (accessed on 21 November 2019).

| Fertilization Method | Substratum Composition | Substratum | Nitrogen Fertilization | |||||
|---|---|---|---|---|---|---|---|---|
| pH in KCl | (mg 100 g Substratum−1) | N Total (%) | Dose (g N Plant−1) | Type of Fertilizer | ||||
| P2O5 | K2O | Mg | ||||||
| mineral | neutral peat (100%) | 6.4 | 40.4 | 244.2 | 62.0 | 0.74 | 0.15 | Ammonium nitrate |
| organic | neutral peat (70%), extract of common nettle (10%), horse manure (20%) | 5.3 | 237.0 | 1282.0 | 269.0 | 1.18 | 0.15 | Bioilsa N 12.5 |
| Variety | Fertilization Method | Plant Height (cm) | Plant Weight (g) | Leaf Weight (g) | ||
|---|---|---|---|---|---|---|
| B1 ** | B2 ** | B3 ** | ||||
| Genovese | organic | 20.41 ± 1.32 a | 5.47 ± 0.34 a | 0.38 ± 0.06 a | 0.52 ± 0.06 a | 0.38 ± 0.07 a |
| mineral | 15.93 ± 1.24 b | 3.76 ± 0.78 b | 0.26 ± 0.07 a | 0.44 ± 0.14 a | 0.19 ± 0.03 b | |
| Violetto | organic | 21.24 ± 1.57 c | 5.21 ± 0.57 c | 0.19 ± 0.04 b | 0.20 ± 0.03 c | 0.26 ± 0.04 c |
| mineral | 17.16 ± 1.20 d | 2.85 ± 0.53 d | 0.13 ± 0.02 b | 0.17 ± 0.03 d | 0.18 ± 0.04 d | |
| Variety | Fertilization Method | Drying Period | Constants | Statistics | Drying Time (min) | Final Mcwb (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| A | k | n | R2 | RMSE ‡ | |||||
| Genovese | organic | CPD | 1 | 0.01000 | 0.7036 | 0.9992 | 0.003543 | 272 | 2.15 |
| VMFD | 0.6119 | 0.02408 | 1.5908 | 0.9981 | 0.011675 | ||||
| mineral | CPD | 1 | 0.01203 | 0.6591 | 0.9977 | 0.005573 | 276 | 1.94 | |
| VMFD | 0.6239 | 0.02460 | 1.5534 | 0.9982 | 0.010332 | ||||
| Violetto | organic | CPD | 1 | 0.01845 | 0.6139 | 0.9966 | 0.007820 | 264 | 2.15 |
| VMFD | 0.5915 | 0.02454 | 1.6536 | 0.9971 | 0.015425 | ||||
| mineral | CPD | 1 | 0.01773 | 0.6266 | 0.9956 | 0.008989 | 272 | 2.15 | |
| VMFD | 0.5641 | 0.02691 | 1.5836 | 0.9989 | 0.008025 | ||||
| Variety | Fertilization Method | L* | a* | b* |
|---|---|---|---|---|
| Genovese | organic | 51.02 ± 1.40 a | −0.02 ± 0.03 c | 11.63 ± 0.63 f |
| mineral | 50.25 ± 1.50 a | −0.06 ± 0.04 c | 13.63 ± 1.74 f | |
| Violetto | organic | 45.02 ± 0.74 b | −1.99 ± 0.15 d | 3.60 ± 0.46 g |
| mineral | 44.12 ± 0.24 b | −2.50 ± 0.13 e ** | 6.33 ± 0.32 h** |
| Variety | Fertilization Method | ABTS (Trolox mg 100 ml−1) | Total Phenolic Content (Gallic Acid Equivalent mg ml−1) |
|---|---|---|---|
| Genovese | organic | 665.43 ± 0.19 b | 77.57 ± 1.13 d |
| mineral | 231.48 ± 1.87 c* | 14.68 ± 1.13 e* | |
| Violetto | organic | 579.57 ± 3.04 g | 50.37 ± 1.57 i |
| mineral | 303.88 ± 5.11 h* | 20.21 ± 1.46 j* |
| No. | RT (min) | Peak Share in the Chromatogram (%) | Ordinary Substance Name | Systematic Substance Name | No. CAS | |||
|---|---|---|---|---|---|---|---|---|
| V(O) | V(M) | G(O) | G(M) | |||||
| 1 | 9.684 | 8.475 | 4.47 | 32.31 | 7.866 | eucalyptol | 4,7,7-trimethyl-8-oxabicyclo[2.2.2]octane | 470–82–6 |
| 2 | 10.98 | 12.19 | 18.94 | 12.60 | 22.57 | linalool | (±)-3,7-Dimethyl-1,6-octadien-3-ol, (±)-3,7-Dimethyl-3-hydroxy-1,6-octadiene | 78–70–6 |
| 3 | 11.26 | 0.839 | 0.587 | – | – | fenchol | 1,3,3-trimethylbicyclo[2.2.1]heptan-2-ol | 1632–73–1 |
| 4 | 12.56 | 1.016 | 1.216 | 0.932 | 1.611 | (-)-α -terpineol | 2-[(1S)-4-methyl-1-cyclohex-3-enyl]propan-2-ol | 10482–56–1 |
| 5 | 13.04 | 3.006 | 3.618 | – | – | fenchyl acetate | 1,3,3-trimethyl-2-norbornanyl acetate | 13851–11–1 |
| 6 | 13.37 | trace | 0.340 | – | 0.501 | carvacryl methyl ether | 4-isopropyl-2-methoxy-1-methylbenzene | 6379–73–3 |
| 7 | 14.04 | trace | 0.458 | 1.350 | 2.481 | (−)-bornyl acetate | endo-(1S)-1,7,7-Trimethylbicyclo[2.2.1]hept-2-yl acetate | 5655–61–-8 |
| 8 | 14.92 | 1.134 | 0.330 | trace | trace | α -terpinyl acetate | 2-(4-methyl-3-cyclohexen-1-yl)-2-propanyl acetate | 80–26–2 |
| 9 | 14.96 | 1.134 | 0.310 | trace | trace | (-)-α -cubebene | 4,10-dimethyl-7-propan-2-yltricyclo[4.4.0.01,5]dec-3-ene | 17699–14–8 |
| 10 | 15.02 | 5.068 | 3.949 | 9.588 | 15.02 | eugenol | 2-Methoxy-4-(2-propenyl)phenol, 4-Allyl-2-methoxyphenol, 4-Allylguaiacol | 97–53–0 |
| 11 | 15.36 | trace | 0.485 | trace | 0.506 | α -copaene | 1,3-dimethyl-8-(1-methyl ethyl) tricyclo(4.4.0.0.02,7-)dec-3-ene stereoisomer | 3856–25–5 |
| 12 | 15.50 | 1.629 | 1.346 | 1.316 | 0.936 | (−)-β-elemene | β-Elemene, (1S,2S,4R)-(−)-2,4-Diisopropenyl-1-methyl-1-vinylcyclohexane, (1S,2S,4R)-1-Ethenyl-1-methyl-2,4-bis(1-methylethenyl)cyclohexane | 515–13–9 |
| 13 | 15.63 | 35.25 | 39.17 | 11.27 | 19.39 | methyl eugenol | 4-Allyl-1,2-dimethoxybenzene, Eugenol methyl ether, Eugenyl methyl ether | 93–15-–2 |
| 14 | 15.73 | trace | 0.344 | trace | trace | 3,5-diisopropylphenol | 3,5-diisopropylphenol;Einecs 248-086-0;Phenol, 3,5-bis(1-methylethyl)-;3,5-bis(1-methylethyl)phenol | 26886–05–5 |
| 15 | 15.98 | 3.273 | 2.761 | trace | trace | β-caryophyllene | (−)-trans-Caryophyllene, trans-(1R,9S)-8-Methylene-4,11,11-trimethylbicyclo[7.2.0]undec-4-ene | 87–44–5 |
| 16 | 16.11 | 2.045 | 1.446 | 20.34 | 19.34 | α -bergamotene | (1R*,5R*,6R*)-2,6-dimethyl-6-(4-methylpent-3-en-1-yl)bicyclo[3.1.1]hept-2-ene | 17699–05–7 |
| 17 | 16.11 | 1.100 | 1.373 | 0.802 | 0.497 | α -guaiene | 1,4-dimethyl-7-prop-1-en-2-yl-1,2,3,4,5,6,7,8-octahydroazulene | 3691–12–1 |
| 18 | 16.31 | 2.450 | 2.099 | 0.790 | 0.945 | nerolidol | 3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol, 3-Hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene | 7212–44–-4 |
| 19 | 16.42 | 1.216 | 0.964 | 1.716 | 1.325 | α-Humulene | α-Caryophyllene, trans,trans,trans-2,6,6,9-Tetramethyl-1,4,8-cycloundecatriene | 6753–98–6 |
| 20 | 17.15 | 2.33 | 1.607 | 2.709 | 2.363 | ƴ-muurolene | (1R,4aR,8aS)-7-methyl-4-methylidene-1-propan-2-yl-2,3,4a,5,6,8a-hexahydro-1H-naphthalene | 30021–74–0 |
| TOTAL | 82.17 | 85.81 | 95.75 | 95.37 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matłok, N.; Gorzelany, J.; Stępień, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. https://doi.org/10.3390/su11236590
Matłok N, Gorzelany J, Stępień AE, Figiel A, Balawejder M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability. 2019; 11(23):6590. https://doi.org/10.3390/su11236590
Chicago/Turabian StyleMatłok, Natalia, Józef Gorzelany, Agnieszka Ewa Stępień, Adam Figiel, and Maciej Balawejder. 2019. "Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.)" Sustainability 11, no. 23: 6590. https://doi.org/10.3390/su11236590
APA StyleMatłok, N., Gorzelany, J., Stępień, A. E., Figiel, A., & Balawejder, M. (2019). Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability, 11(23), 6590. https://doi.org/10.3390/su11236590








