Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
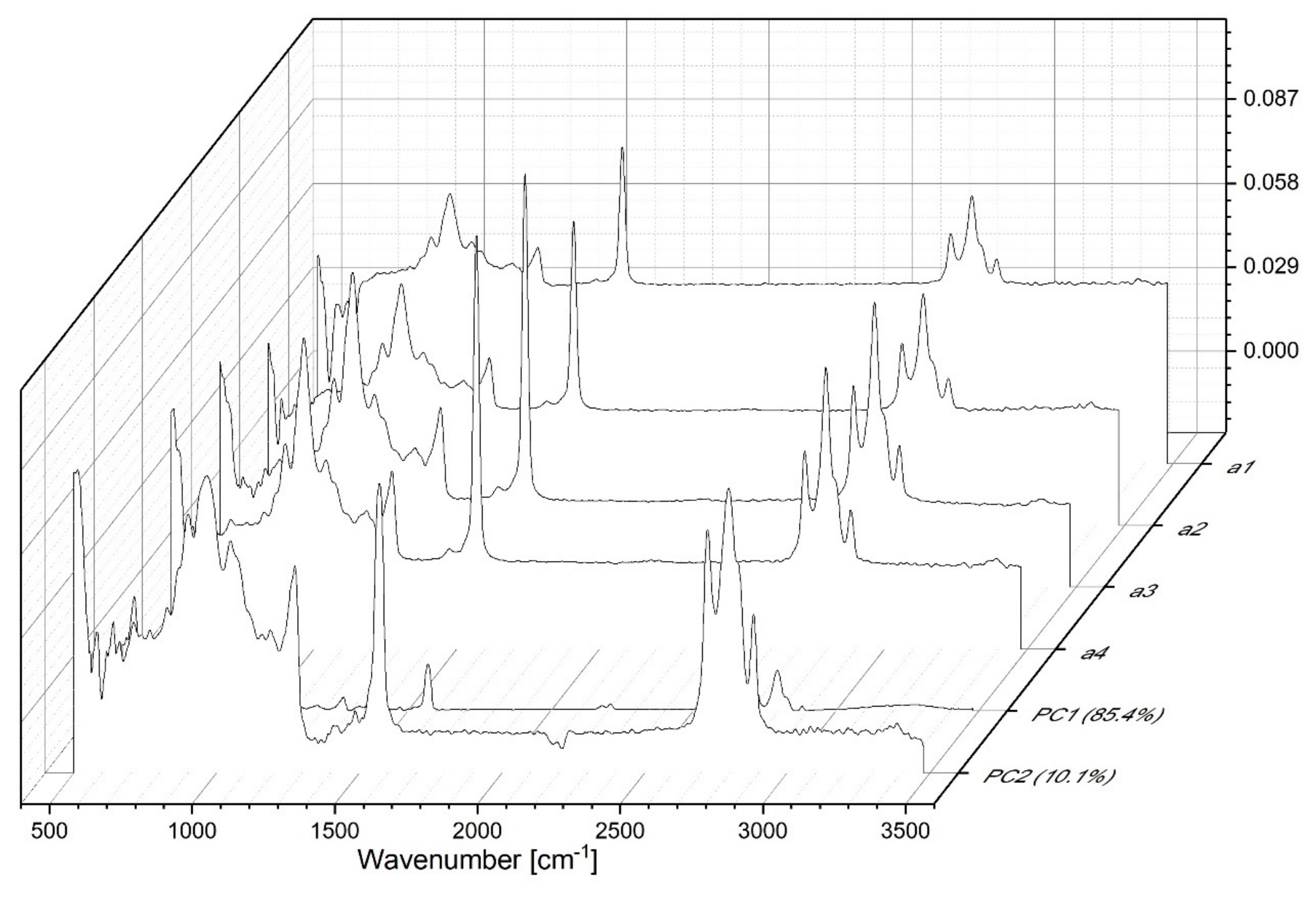
3.1. FTIR Spectroscopic Analysis of Moldavian Dragonhead Oil Samples
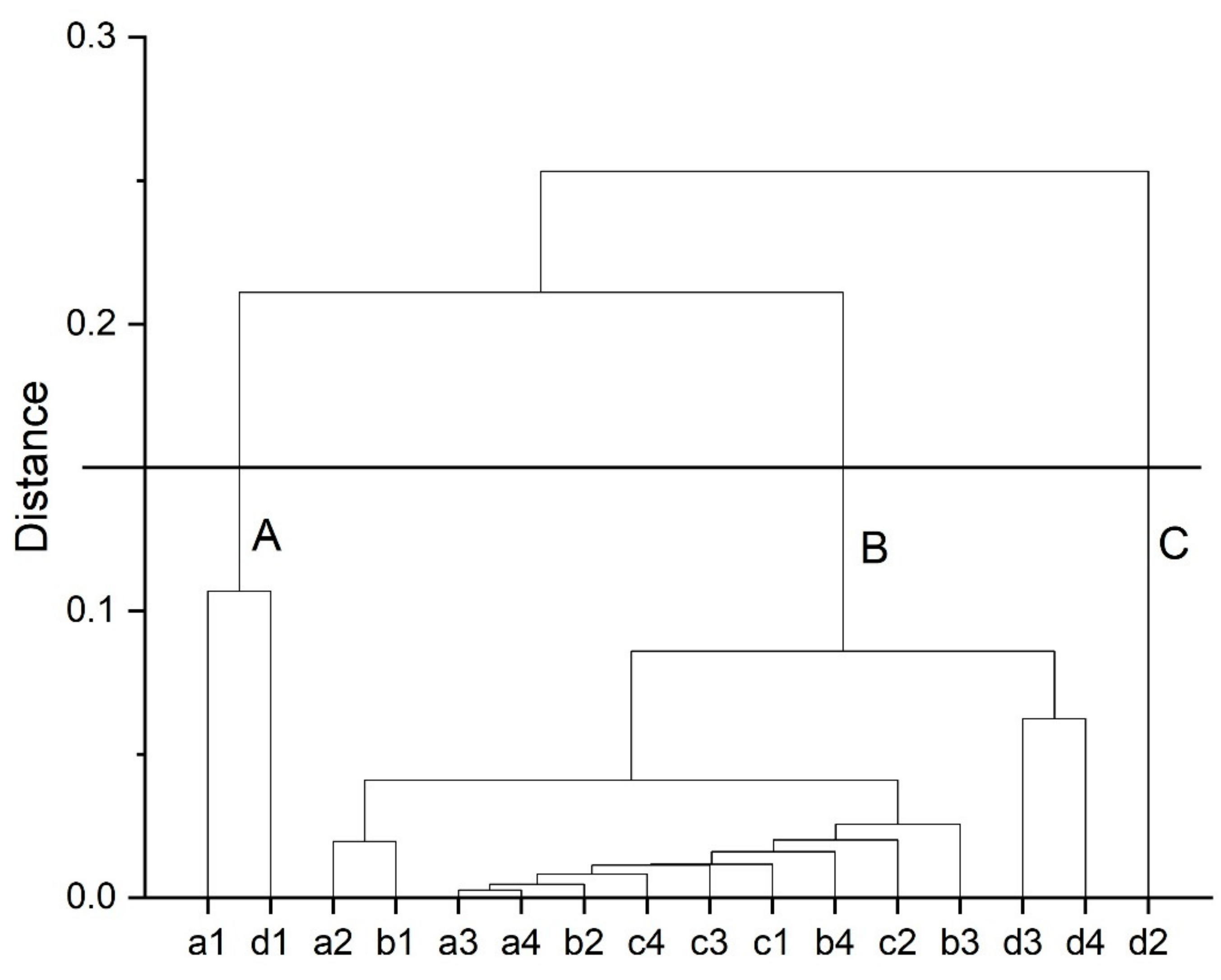
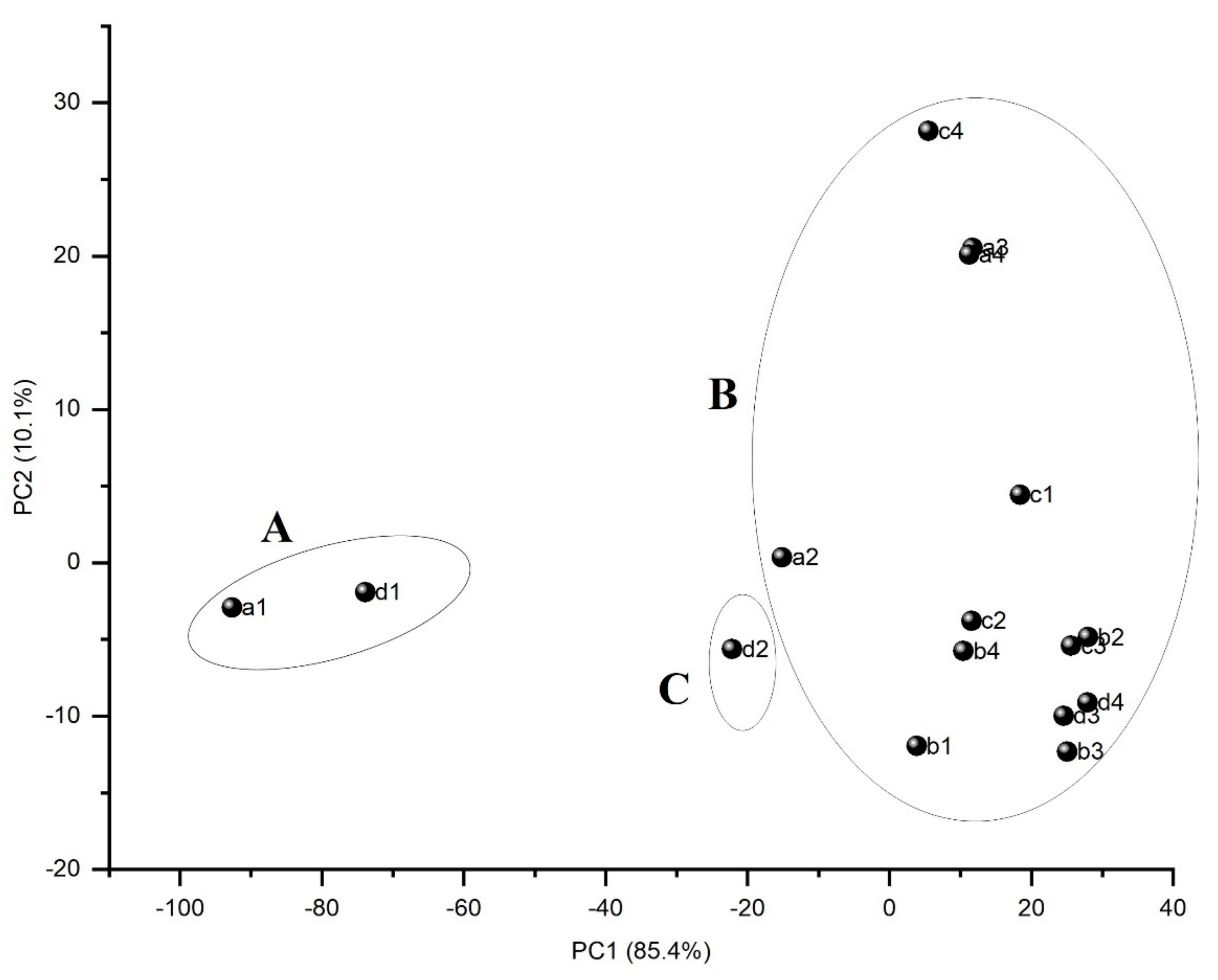
3.2. Chemometrics Studies
4. Conclusions
- Principal component analysis (PCA) was used to identify the main sources of variance in the Fourier-transforms infrared (FTIR) spectra of oil samples obtained from Moldavian dragonhead seeds and stored under different conditions. PCA combined with HCA allowed the samples to be explored in terms of their similarities, relative to the storage method with respect to their FTIR spectra. Due to its inherent simplicity, quick and non-invasive character, this method may prove useful in monitoring the physicochemical changes in oils or e.g., the oxidative state in oils relative to the time and conditions under which they are stored.
- The analyzed oil samples were characterized by a very good fatty acids profile, which confirmed their value as food products with significant health benefits. Spectral analysis revealed significant changes with respect to bands associated in the literature to various fat fractions contained in the oil. The noticeable changes occurring after 8 weeks in storage in infrared spectra located within the ranges of 1720–1500 cm−1 and ~1426 cm−1, 1369 and 1237 cm−1, constituted markers which are evidence of the advancement of the ageing processes in the analyzed samples. Changes related to aging of the sample were related to the intensification of bands reflecting the vibrations of C-C, C=C, and C=O groups; as such, they constitute perfect marker bands which can be easily correlated with the given oil’s shelf life and the oxidative processes that affect it. However, only a detailed chemometric analysis allowed us to complement and fully follow differences between the respective samples which reflected the particular storage conditions.
- The advent of FTIR with multivariate analysis has revolutionized many research fields. FTIR offers unique advantages, as it reflects the overall vibrations of the components and their interactions within the samples as spectra, in addition to being non-invasive and label-free, unlike conventional methods of this kind.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oniszczuk, A.; Olech, M.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Wójtowicz, A. Extraction methods, LC-ESI-MS/MS analysis of phenolic compounds and antiradical properties of functional food enriched with elderberry flowers or fruits. Arab. J. Chem. 2016, in press. [Google Scholar] [CrossRef]
- Wójtowicz, A.; Oniszczuk, A.; Oniszczuk, T.; Kocira, S.; Wojtunik, K.; Mitrus, M.; Kocira, A.; Widelski, J.; Skalicka-Wożniak, K. Application of Moldavian dragonhead (Dracocephalum moldavica L.) leaves addition as a functional component of nutritionally valuable corn snacks. J. Food Sci. Technol. 2017, 54, 3218–3229. [Google Scholar] [CrossRef]
- Wolski, T.; Kwiatkowski, S. Biology of growth and development of Moldavian dragonhead (Dracocephalum moldavica L.)–aromatic and medicinal plant. Post. Fitoter. 2006, 1, 2–10. [Google Scholar]
- Kocira, S.; Sujak, A.; Kocira, A.; Wójtowicz, A.; Oniszczuk, A. Effect of Fylloton application on photosynthetic activity of Moldavian dragonhead (Dracocephalum moldavica L.). Agric. Agric. Sci. Procedia 2015, 7, 108–112. [Google Scholar] [CrossRef]
- Dmitruk, M.; Weryszko-Chmielewska, E.; Sulborska, A. Flowering and nectar secretion in two forms of the Moldavian dragonhead (Dracocephalum moldavica L.)—A plant with extraordinary apicultural potential. J. Apic. Sci. 2018, 62, 97–110. [Google Scholar] [CrossRef]
- Ehsani, A.; Alizadeh, O.; Hashemi, M.; Afshari, A.; Aminzare, M. Phytochemical, antioxidant and antibacterial properties of Melissa officinalis and Dracocephalum moldavica essential oils. Vet. Res. Forum 2017, 8, 223–229. [Google Scholar] [PubMed]
- Domokos, J.; Peredi, J.; Halasz-Zelnik, K. Characterization of seed oils of dragonhead (Dracocephalum moldavica L.) and catnip (Nepeta cataria var. citriodora Balb.). Ind. Crop. Prod. 1994, 3, 91–94. [Google Scholar] [CrossRef]
- Dziki, D.; Miś, A.; Gładyszewska, B.; Laskowski, J.; Kwiatkowski, S.; Gawlik-Dziki, U. Physicochemical and grinding characteristics of dragonhead seeds. Int. Agrophys. 2013, 27, 403–408. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Relationships between the composition of edible oils and lard and the ratio of the absorbance of specific bands of their Fourier transform infrared spectra. Role of some bands of the fingerprint region. J. Agric. Food Chem. 1998, 46, 1788–1793. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.C. Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Res. Int. 2010, 43, 886–892. [Google Scholar] [CrossRef]
- Nawrocka, A.; Lamorska, J. Determination of food quality by using spectroscopic methods. In Advances in Agrophysical Research; Intech Open: Rijeka, Croatia, 2013; pp. 347–368. [Google Scholar] [CrossRef]
- Yasushi, E. Analytical methods to evaluate the quality of edible fats and oils: The JOCS standard methods for analysis of fats, oils and related materials (2013) and advanced methods. J. Oleo Sci. 2018, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.P.; Março, P.H.; Valderrama, P. Thermal edible oil evaluation by UV–Vis spectroscopy and chemometrics. Food Chem. 2014, 163, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Leder, P.J.S.; Porcu, O.M. The importance of UV-Vis spectroscopy: Application in food products characterization. Scholar. J. Food Nutr. 2018, 1. [Google Scholar] [CrossRef]
- Kruzlcova, D.; Mocak, J.; Katsoyannos, E.; Lankmayr, E. Classification and characterization of olive oils byUV-Vis absorption spectrometry and sensorial analysis. J. Food Nutr. Res. 2008, 47, 181–188. [Google Scholar]
- Alexa, E.; Dragomirescu, A.; Pop, G.; Jianu, C.; Drago, D. The use of FT-IR spectroscopy in the identification of vegetable oils adulteration. J. Food Agric. Environ. 2009, 7, 20–24. [Google Scholar] [CrossRef]
- Nigri, S.; Oumeddour, R. Fourier transform infrared and fluorescence spectroscopy for analysis of vegetable oils. MATEC Web Conf. 2013, 5, 04028. [Google Scholar] [CrossRef]
- Kluczyk, D.; Matwijczuk, A.; Górecki, A.; Karpińska, M.M.; Szymanek, M.; Niewiadomy, A.; Gagoś, M. Molecular organization of dipalmitoylphosphatidylcholine bilayers containing bioactive compounds 4-(5-heptyl-1, 3, 4-thiadiazol-2-yl) benzene-1, 3-diol and 4-(5-methyl-1, 3, 4-thiadiazol-2-yl) benzene-1, 3-diols. J. Phys. Chem. B 2016, 120, 12047–12063. [Google Scholar] [CrossRef]
- Rohman, A. Infrared spectroscopy for quantitative analysis and oil parameters of olive oil and virgin coconut oil: A review. Int. J. Food Prop. 2017, 20, 1447–1456. [Google Scholar] [CrossRef]
- Siddiqui, N.; Ahmad, A. Infrared spectroscopic studies on edible and medicinal oils. Int. J. Sci. Environ. Tech. 2013, 2, 1297–1306. [Google Scholar]
- Karim, M.M.; Karim, S.F.E.; Rana, A.A.; Masum, S.M.; Mondol, A.; Israt, S.S. ATR-FTIR spectroscopy and chemometric techniques for the identification of edible vegetable oils. Bangladesh J. Sci. Ind. Res. 2015, 50, 233–240. [Google Scholar] [CrossRef]
- Al-Alawi, A.; Van De Voort, F.R.; Sedman, J.; Ghetler, A. Automated, FTIR analysis of free fatty acids or moisture in edible oils. J. Assoc. Lab. Autom. 2006, 11, 23–29. [Google Scholar] [CrossRef]
- Hayati, I.N.; Che Man, Y.B.; Tan, C.P.; Aini, I.N. Monitoring peroxide value in oxidized emulsions by Fourier transform infrared spectroscopy. Eur. J. Lipid Sci.Technol. 2005, 107, 886–895. [Google Scholar] [CrossRef]
- Hendl, O.; Howell, J.A.; Lowery, J.; Jones, W. A rapid andsimple method for the determination of iodine values usingderivative Fourier transform infrared measurements. Anal. Chim. Acta 2001, 427, 75–81. [Google Scholar] [CrossRef]
- Dubois, J.; Van De Voort, F.R.; Sedman, J.; Ismail, A.A.; Ramaswamy, H.R. Quantitative Fourier transform infrared analysis for anisidine value and aldehydes in thermally stressed oils. J. Am. Oil Chem. Soc. 1996, 73, 787–794. [Google Scholar] [CrossRef]
- Sherazi, S.T.H.; Talpur, M.Y.; Mahesar, S.A.; Kandhro, A.A.; Arain, S. Main fatty acid classes in vegetable oils bySB-ATR-Fourier transform infrared (FTIR) spectroscopy. Talanta 2009, 80, 600–606. [Google Scholar] [CrossRef]
- Maggio, R.M.; Kaufman, T.S.; Del Carlo, M.; Cerretani, L.; Bendini, A.; Cichelli, A.; Compagnone, D. Monitoring of fatty acid composition in virgin oliveoil by Fourier transformed infrared spectroscopy coupled with partial least squares. Food Chem. 2009, 114, 1549–1554. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Herrero, C.; Barranco, A.; López-Márquez, D.M.; Berrueta, L.A.; Gallo, B.; Vicente, F. Polyphenolic compositions of Basque natural ciders: A chemometric study. Food Chem. 2006, 97, 438–446. [Google Scholar] [CrossRef]
- Woodcock, T.; Downey, G.; Kelly, J.D.; O’Donnell, C. Geographical classification of honey samples by near-infrared spectroscopy: A feasibility study. J. Agric. Food Chem. 2007, 55, 9128–9134. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y.; Durmaz, G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009, 112, 721–726. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrophotometric Identification of Organic Compounds, 7th ed.; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Yang, H.; Irudayaraj, J. Comparison of near-infrared, Fourier transform-infrared, and Fourier transform-Raman methods for determining olive pomace oil adulteration in extra virgin olive oil. J. Amer. Oil Chem. Soc. 2001, 78, 889. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, N.; Duponchel, L.; Huvenne, J.P.; Sombret, B.; Legrand, P. Classification of edible fats and oils by principal component analysis of Fourier transform infrared spectra. Food Chem. 1996, 57, 245–251. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Elzey, B.; Pollard, D.; Sayo, O.F. Determination of adulterated neem and flaxseed oil compositions by FTIR spectroscopy and multivariate regression analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B. Analysis of cod-liver oil adulteration using Fourier transform infrared (FTIR) spectroscopy. J. Am. Oil Chem. Soc. 2009, 86, 1149. [Google Scholar] [CrossRef]
- Hirri, A.; Bassbasi, M.; Platikanov, S.; Tauler, R.; Oussama, A. FTIR spectroscopy and PLS-DA classification and prediction of four commercial grade virgin olive oils from Morocco. Food Anal. Methods 2016, 9, 974–981. [Google Scholar] [CrossRef]
- Gurdeniz, G.; Ozen, B. Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data. Food Chem. 2009, 116, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.W.; Kemsley, E.K.; Wilson, R.H. Potential of Fourier transform infrared spectroscopy for the authentication of vegetable oils. J. Agric. Food Chem. 1994, 42, 1154–1159. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Di Virgilio, F.; Belloni, P.; Bonoli-Carbognin, M.; Lercker, G. Preliminary evaluation of the application of the FTIR spectroscopy to control the geographic origin and quality of virgin olive oils. J. Food Qual. 2007, 30, 424–437. [Google Scholar] [CrossRef]
- De Luca, M.; Restuccia, D.; Clodoveo, M.L.; Puoci, F.; Ragno, G. Chemometric analysis for discrimination of extra virgin olive oils from whole and stoned olive pastes. Food Chem. 2016, 202, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Terouzi, W.; Ioele, G.; Kzaiber, F.; Oussama, A.; Oliverio, F.; Tauler, R.; Ragno, G. Derivative FTIR spectroscopy for cluster analysis and classification of morocco olive oils. Food Chem. 2011, 124, 1113–1118. [Google Scholar] [CrossRef]
- Cebia, N.; Yilmaz, M.T.; Sagdic, O.; Yuce, H.; Yelboga, E. Prediction of peroxide value in omega-3 rich microalgae oil by ATR-FTIR spectroscopy combined with chemometrics. Food Chem. 2017, 225, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Mahboubifar, M.; Hemmateenejad, B.; Javidnia, K.; Yousefinejad, S. Evaluation of long-heating kinetic process of edible oils using ATR–FTIR and chemometrics tools. J. Food Sci. Technol. 2017, 54, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
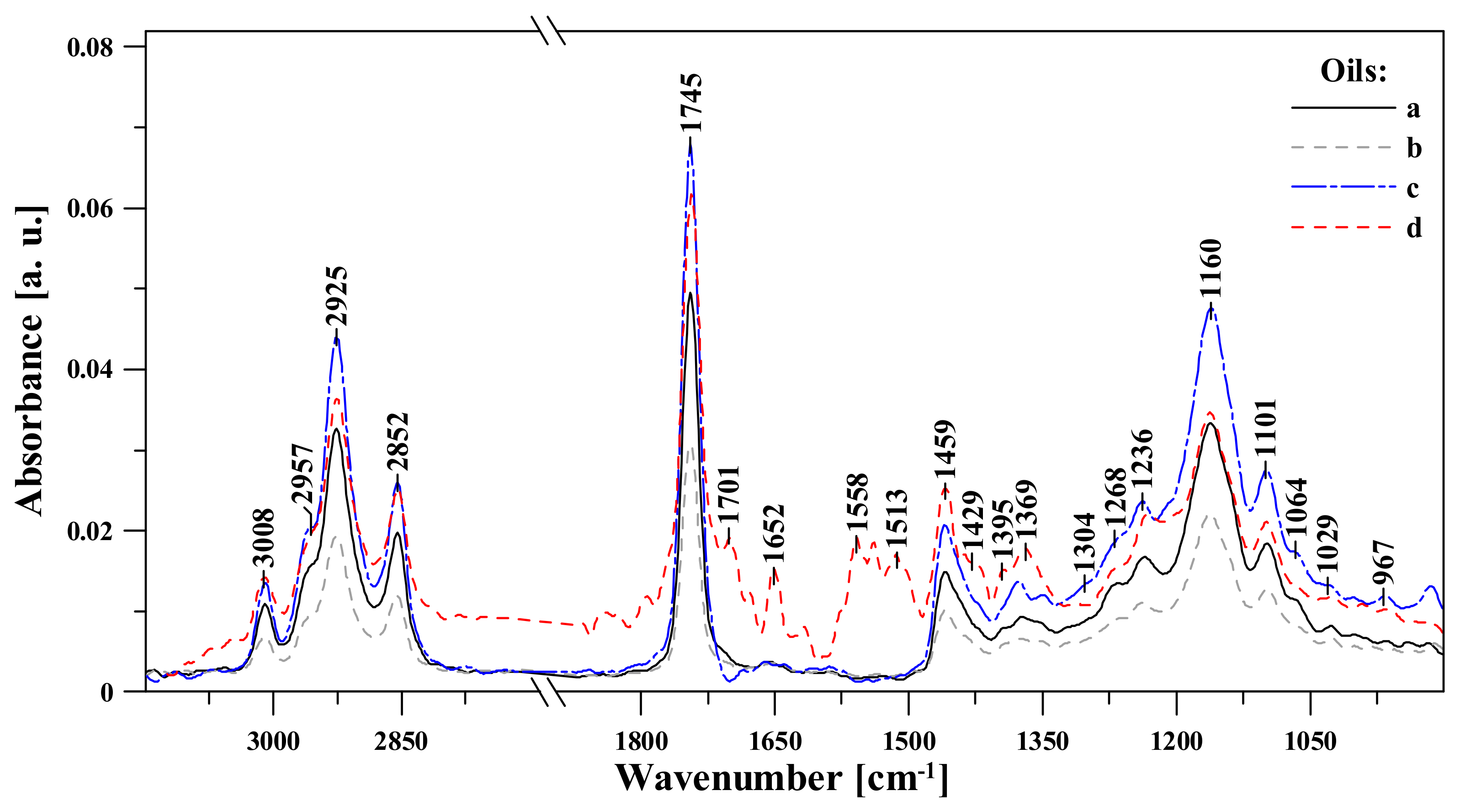
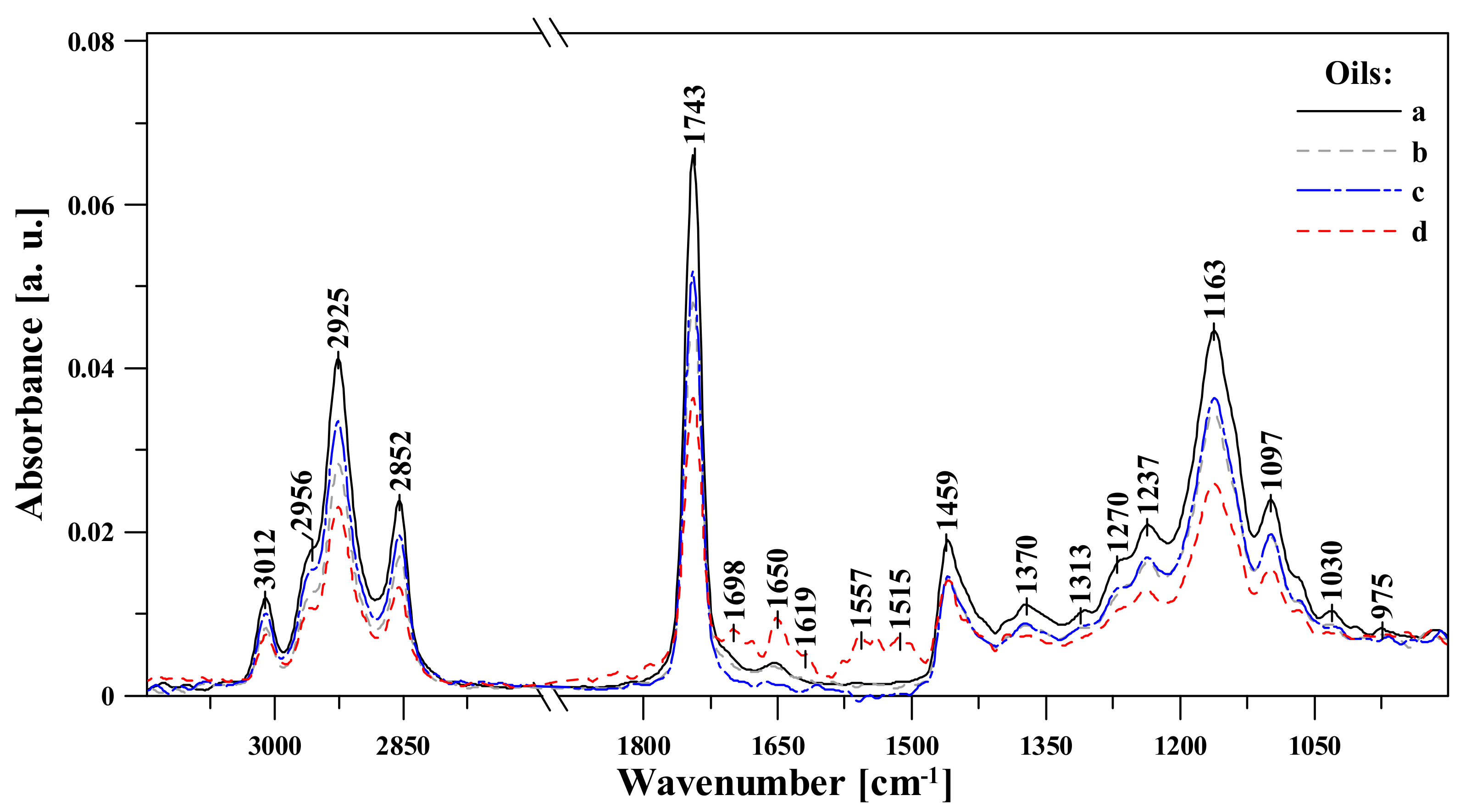
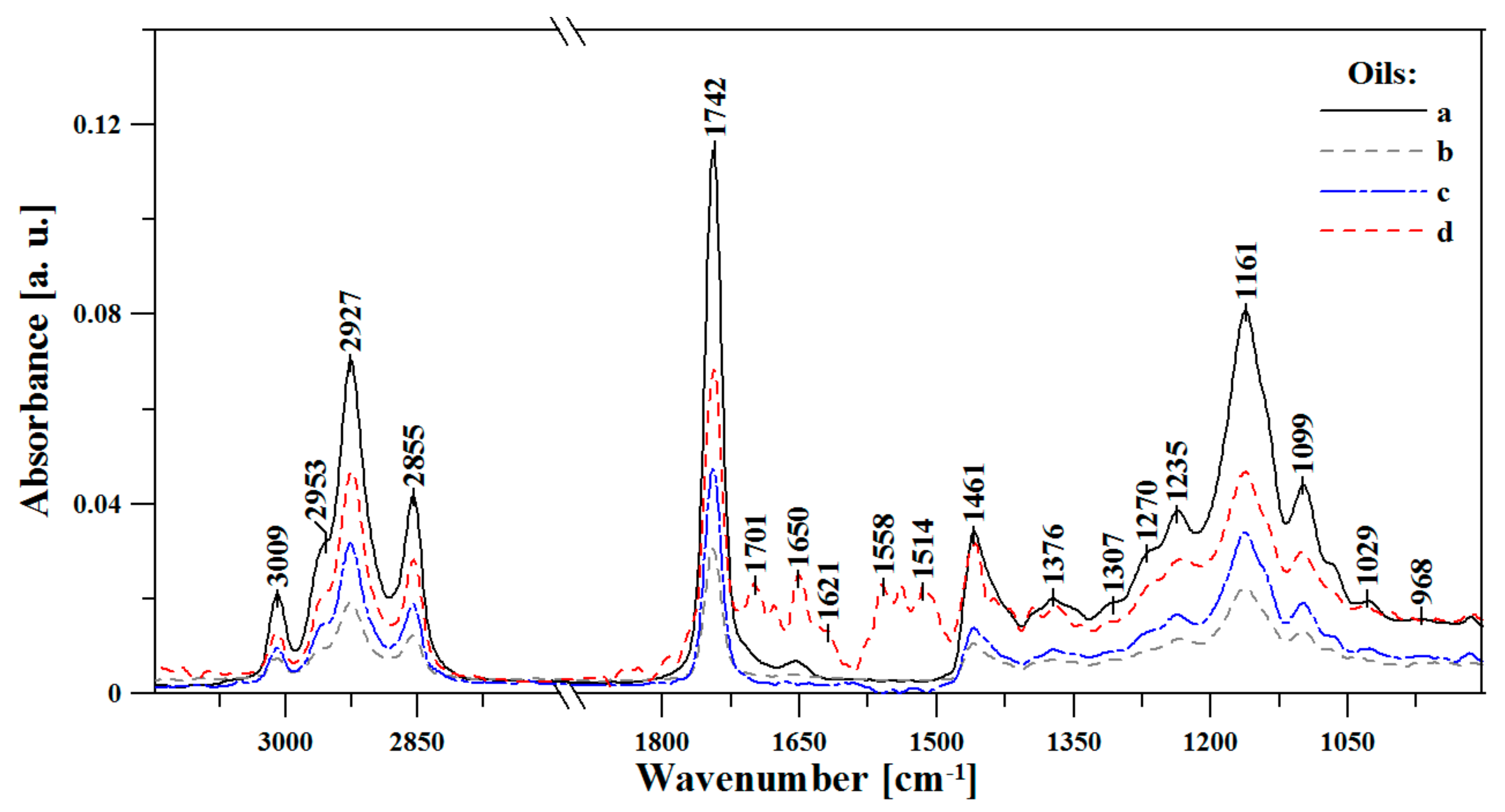
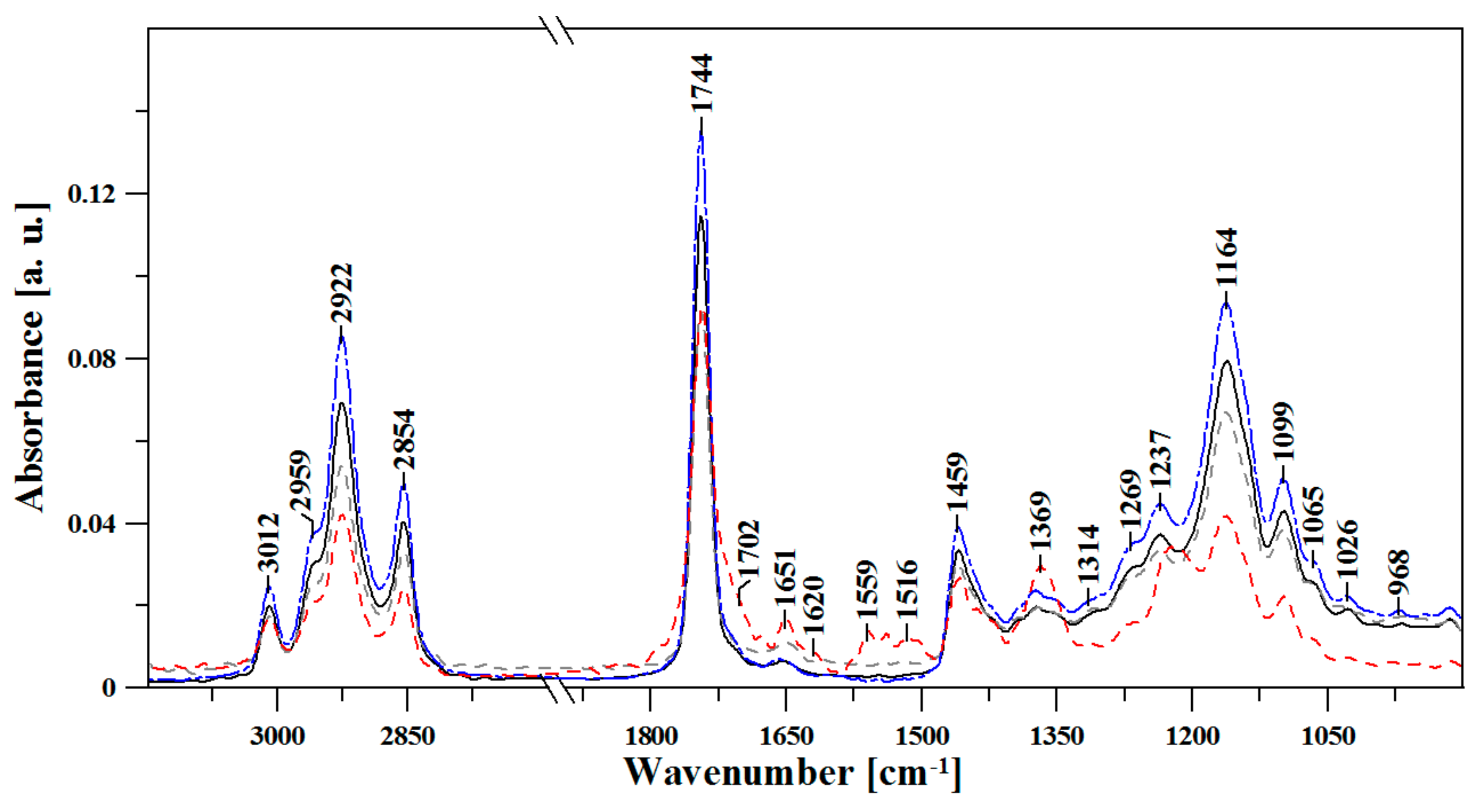



| FTIR | Type and Origin of Vibrations | |||
|---|---|---|---|---|
| Position of Bands (cm−1) | ||||
| a | b | c | d | |
| 3010 | 3008 | 3007 | 3013 | ν(=C-Hm, cis-) |
| 2956 | 2963 | 2965 | 2961 | νas(-C-Hvst, -CHa) and νs(-C-Hvst, -CHa) (aliphatic groups in triglycerides) |
| 2926 | 2926 | 2925 | 2928 | |
| 2853 | 2853 | 2852 | 2854 | |
| 1743 | 1742 | 1743 | 1743 | ν(-C=Ovst) in esters |
| 1708 | 1701 | 1709 | 1701 | ν(-C=Ovw) in acids |
| 1656 | 1647 | 1656 | 1651 | νvw(-C=C-, cis-) |
| 1588 | 591 | 1600 | 1613 | |
| - | - | 1589 | 1557 | |
| - | - | - | 1540 | |
| - | - | - | 1515 | |
| 1459 | 1460 | 1460 | 1457 | δvw(-C-H) w CH2 and in CH3, groups, deformation (scissoring) νvw(-C-H, cis-) deformation (ring) |
| - | - | 1429 | 1428 | |
| 1373 | 1375 | 1375 | 1369/1393 | νw, m, vw (-C-H, -CH3) and deformation |
| 1302 | 1318 | 1304/1348 | - | δm(-C-H, -CH3) |
| 1271 | 1262 | 1268 | 1267 | νm(-C-O) or δm(-CH2-) |
| 1236 | 1240 | 1237 | - | νm(-C-O) or δm(-CH2-) |
| 1161 | 1159 | 1160 | 1161 | νm(-C-O) |
| 1097 | 1099 | 1100 | 1098 | νm,vw(-C-O) |
| 1067 | 1025 | 1065 | 1028 | |
| 1027 | - | 1030 | - | |
| 966 | 968 | 964 | 964 | δw(-HC=CH-, trans-) out-of-plain deformation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matwijczuk, A.; Oniszczuk, T.; Matwijczuk, A.; Chruściel, E.; Kocira, A.; Niemczynowicz, A.; Wójtowicz, A.; Combrzyński, M.; Wiącek, D. Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil. Sustainability 2019, 11, 6414. https://doi.org/10.3390/su11226414
Matwijczuk A, Oniszczuk T, Matwijczuk A, Chruściel E, Kocira A, Niemczynowicz A, Wójtowicz A, Combrzyński M, Wiącek D. Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil. Sustainability. 2019; 11(22):6414. https://doi.org/10.3390/su11226414
Chicago/Turabian StyleMatwijczuk, Arkadiusz, Tomasz Oniszczuk, Alicja Matwijczuk, Edyta Chruściel, Anna Kocira, Agnieszka Niemczynowicz, Agnieszka Wójtowicz, Maciej Combrzyński, and Dariusz Wiącek. 2019. "Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil" Sustainability 11, no. 22: 6414. https://doi.org/10.3390/su11226414
APA StyleMatwijczuk, A., Oniszczuk, T., Matwijczuk, A., Chruściel, E., Kocira, A., Niemczynowicz, A., Wójtowicz, A., Combrzyński, M., & Wiącek, D. (2019). Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil. Sustainability, 11(22), 6414. https://doi.org/10.3390/su11226414








