Benefits of Ryegrass on Multicontaminated Soils Part 2: A Green Process to Provide Idrocilamide
Abstract
1. Introduction
2. Materials and Methods
2.1. Perennial Ryegrass Shoots Production
2.2. Digestion and Determination of PTE Concentrations in the Biomass of Shoots
2.3. Preparation of Biosourced Catalyst
2.4. Synthesis of Idrocilamide under Green Conditions
2.5. Green Chemistry Metrics
3. Results and Discussion
3.1. Concentration of PTEs in the Ashes of Ryegrass Shoots
3.2. Synthesis of Idrocilamide Using Zn-BioCat
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- USEPA. Cleaning up the Nation’s Hazardous Wastes Sites; USEPA: Washington, DC, USA, 2014. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements, 2nd ed.; Polish Scientific Publishing Company: Warsaw, Poland, 1999. [Google Scholar]
- Douay, F.; Pruvot, C.; Roussel, H.; Ciesielski, H.; Fourrier, H.; Proix, N.; Waterlot, C. Contamination of urban soils in an area of Northern France polluted by dust emissions of two smelters. Water Air Soil Pollut. 2008, 188, 247–260. [Google Scholar] [CrossRef]
- Douay, F.; Pruvot, C.; Waterlot, C.; Fritsch, C.; Fourrier, H.; Loriette, A.; Bidar, G.; Grand, C.; de Vaufleury, A.; Scheifler, R. Contamination of woody habitat soils around a former lead smelter in the North of France. Sci. Total Environ. 2009, 407, 5564–5577. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Waterlot, C.; Douay, F. Influence of land use on human bioaccessibility of metals in smelter-impacted soils. Environ. Pollut. 2013, 178, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Douay, F.; Pelfrêne, A.; Planque, J.; Fourrier, H.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of potential health risk for inhabitants living near a former lead smelter. Part 1: Metal concentrations in soils, agricultural crops, and homegrown vegetables. Environ. Monit. Assess. 2013, 185, 3665–3680. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, A.; Uddh-Söderberg, T.; Hogmalm, K.J.; Filipsson, M.E.M. Metal uptake by homegrown vegetables—The relative importance in human health risk assessments at contaminated sites. Environ. Res. 2015, 138, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, A.; Uddh-Söderberg, T.; Filipsson, M.; Helmfrid, I.; Berglund, M.; Karlsson, H.; Hogmalm, J.; Karlsson, A.; Alriksson, S. Challenges in assessing the health risks of consuming vegetables in metal-contaminated environments. Environ. Int. 2018, 113, 269–280. [Google Scholar] [CrossRef]
- Ávila, P.F.; da Silva, E.F.; Candeias, C. Health risk assessment through consumption of vegetables rich in heavy metals: The case study of the surrounding villages from Panasqueira mine, central Portugal. Environ. Geochem. Health 2017, 39, 565–589. [Google Scholar] [CrossRef]
- Ferri, R.; Hashim, D.; Smith, D.R.; Guazzetti, S.; Donna, F.; Ferretti, E.; Curatolo, M.; Moneta, C.; Beone, G.M.; Lucchini, R.G. Metal contamination of home garden soils and culativated vegetables in the province of Brescia, Italy: Implications for human exposure. Sci. Total Environ. 2015, 518, 507–517. [Google Scholar] [CrossRef]
- Pančeveski, Z.; Stafilov, T.; Bačeva, K. Distribution of heavy metals in the garden soil and vegetables grown in the vicinity of lead and zinc smelter plant. J. Sci. Eng. Res. 2016, 3, 1–11. [Google Scholar]
- Pelfrêne, A.; Douay, F.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of potential health risk for inhabitants living near a former lead smelter. Part2: Site-specific human health risk assessment of Cd and Pb contamination in kitchen gardens. Environ. Monit. Assess. 2013, 185, 2999–3012. [Google Scholar] [CrossRef]
- Pelfrêne, A.; Sahmer, K.; Waterlot, C.; Douay, F. From environmental and social data acquisition to assessment of gardeners’ exposure: Feedback in an urban context highly contaminated with metals. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.C.; Li, X.; Thorthon, I. Urban environmental geochemistry of trace metals. Environ. Pollut. 2006, 142, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.S.; Yu, S.; Zhu, Y.G.; Li, X.D. Trace metal contamination in urban soils of China. Sci. Total Environ. 2012, 421, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Jia, Y.; Tang, S.; Wang, R.; Ju, X.; Ding, Y.; Tu, S.; Smith, D.L. Effects of elevated on growth, photosynthesis, elemental composition, antioxidant level, and phytochelatin concentration in Lolium multiforum and Lolium perenne under Cd stress. J. Hazard. Mater. 2010, 180, 384–394. [Google Scholar] [CrossRef]
- Arienzo, M.; Adamo, P.; Cozzolino, V. The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci. Total Environ. 2004, 319, 13–25. [Google Scholar] [CrossRef]
- Sterckeman, T.; Duquene, L.; Perriguey, J.; Morel, J.L. Quantifying the effect of rhizosphere processes on the availability on soil cadmium and zinc. Plant Soil 2005, 276, 335–345. [Google Scholar] [CrossRef]
- Rees, F.; Germain, C.; Sterckeman, T.; Morel, J.L. Plant growth and metal uptake by a non-hyperaccumulating species (Lolium perenne L.) and a Cd-Zn hyperaccumulator (Noccaea caerulrscens) in contaminated soils amended with biochar. Plant Soil 2015, 395, 57–73. [Google Scholar] [CrossRef]
- Convertini, G.; Ferri, D.; Montemurro, F.; Maiorana, M. Effects of municipal solid waste compost on soils cropped with tomato and sunflower rotation with durum wheat. In Proceedings of the ISCO 2004—13th International Soil Conservation Organisation Conference, Brisbane, Australia, 4–8 July 2004. [Google Scholar]
- Tittarelli, F.; Petruzzelli, G.; Pezzarossa, B.; Civilini, M.; Benedetti, A.; Sequi, P. Quality and agronomic use of compost. Waste Manag. Ser. 2007, 8, 119–157. [Google Scholar]
- Strachel, R.; Wyszkowska, J.; Baémaga, M. The role of compost in stabilizing the microbiological and biochemical properties of zinc-stressed soil. Water Air Soil Pollut. 2017, 228, 349–364. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; He, Z. Monitoring and management. In Managing Soil Health for Sustainable Agriculture; Reicosky, D., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; Volume 2. [Google Scholar]
- Bong, C.P.C.; Lim, L.Y.; Ho, W.S.; Lim, J.S.; Klemes, J.J.; Towprayoon, S.; Ho, C.S.; Lee, C.T. A review on the global warming potential of cleaner composting and mitigation strategies. J. Clean. Prod. 2017, 146, 149–157. [Google Scholar] [CrossRef]
- Harisson, E.Z. Health impact of composting air emission. Biocycle 2007, 48, 44–50. [Google Scholar]
- Smith, S.R. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ. Int. 2009, 35, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, B. Green waste compost as an amendment during induced phytoextraction of mercury-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 3528–3537. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a soil amendment to remediate heavy metal-contaminated agricultural soils: Mechanisms, efficacy, problems, and strategies. Water Air Soil Pollut. 2016, 227, 359–377. [Google Scholar] [CrossRef]
- McBride, M.B.; Shayler, H.A.; Russell-Analli, J.M.; Spliethoff, H.M.; Marquez-Bravo, L.G. Arsenic and lead uptake by vegetable crops grown on an old Orchard site amended with compost. Water Air Soil Pollut. 2015, 226, 265–275. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Paltseva, A.; Cheng, Z.; Deeb, M.; Groffman, P.M.; Shaw, R.K.; Maddaloni, M. Accumulation of arsenic and lead in garden-grown vegetables: Factors and mitigation strategies. Sci. Total Environ. 2018, 640, 273–283. [Google Scholar] [CrossRef]
- Chen, J.H.; Wu, J.T. Benefits and Drawbacks of Composting. Food and Fertilizer Technology Center. 2005. Available online: http://www.fftc.agnet.org/library.php?func=view&id=20110804100401 (accessed on 9 June 2018).
- Grison, C.; Escande, V.; Biton, J. Ecocatalysis: A New Integrated Approach to Scientific Ecology; Elsevier: Amsterdam, The Netherlands, 2015; 100p. [Google Scholar]
- Hechelski, M.; Ghinet, A.; Louvel, B.; Dufrénoy, P.; Rigo, B.; Daïch, A.; Waterlot, C. From Conventional Lewis acids to heterogeneous montmorillonite K10, eco-friendly plant-based catalysts used as green Lewis acids. ChemSusChem 2018, 11, 1249–1277. [Google Scholar] [CrossRef]
- Deyris, P.A.; Bert, V.; Diliberto, S.; Boulanger, C.; Petit, E.; Legrand, Y.M.; Grison, C. Biosourced polymetallic catalysis: A surprising and efficient means to promote the Knoevenagel condensation. Front. Chem. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Deyris, P.A.; Grison, C. Nature, ecology and chemistry: An unusual combination for a new green catalysis, ecocatalysis. Curr. Opin. Green. Sustain. Chem. 2018, 10, 6–10. [Google Scholar] [CrossRef]
- Houzelot, V.; Laubie, B.; Pontvianne, S.; Simonnot, M.O. Effect of up-scaling on the quality of ashes obtained from hyperaccumulator biomass to recover Ni by agromining. Chem. Eng. Res. Des. 2017, 120, 26–33. [Google Scholar] [CrossRef]
- Hazotte, C.; Laubie, B.; Rees, F.; Morel, J.L.; Simonnot, M.O. A novel process to recover cadmium and zinc from the hyperaccumulator plant Noccaea caerulescens. Hydrometallurgy 2017, 174, 56–65. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; 148p. [Google Scholar]
- Anastas, P.T.; Zimmerman, J.B. Peer reviewed: Design through the 12 principles of green engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef] [PubMed]
- Waterlot, C.; Hechelski, M. Benefits of ryegrass on multicontaminated soils Part 1: Effects of fertilizers on bioavailability and accumulation of metals. Sustainability 2019, 11, 5093. [Google Scholar] [CrossRef]
- Savio, M.; Cerutti, S.; Martinez, L.D.; Smichowski, P.; Gil, R.A. Study of matrix and spectral interferences in the determination of lead in sediments, sludges and soils by SR-ETAAS using slurry sampling. Talanta 2010, 82, 523–527. [Google Scholar] [CrossRef]
- Waterlot, C.; Douay, F. The problem of arsenic interference in the analysis of Cd to evaluate its extractability in soils contaminated by arsenic. Talanta 2009, 80, 716–722. [Google Scholar] [CrossRef]
- Waterlot, C.; Bidar, G.; Pruvot, C.; Douay, F. Analysis of cadmium in water extracts from contaminated soils with high arsenic and iron concentration levels. J. Environ. Sci. Eng. 2011, 5, 271–280. [Google Scholar]
- Waterlot, C.; Couturier, D.; Hasiak, B. Friedel-Crafts benzylation of 1,4-dialkoxybenzenes—Cleavage and rearrangement of esters and methoxymethyl ethers in ZnCl2 montmorillonite K10 clays. J. Chem. Res. 2000, 2000, 100–101. [Google Scholar] [CrossRef]
- Waterlot, C.; Ghinet, A.; Hechelski, M.; Dufrénoy, P. Catalyseurs Organo-Minéraux Issus de Plantes Non-Hyper-Accumulatrices Ayant Poussés sur des Supports Contaminés Amendés ou non; Déclaration d’Invention déposée à la SATT Nord, M0488; SATT Nord: Lille, France, 2017. (In French) [Google Scholar]
- Trost, B.M. The atom economy—A research for synthetic efficiency. Science 1191, 254, 1471–1477. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Curzons, A.D.; Constable, D.J.C.; Mortimer, D.N.; Cunningham, V.L. So you think your process is green, how do you know? Using principles of sustainability to determine what is green—A corporate perspective. Green Chem. 2001, 3, 1–6. [Google Scholar] [CrossRef]
- Guan, L.P.; Wei, C.X.; Deng, X.Q.; Sui, X.; Piao, H.R.; Quan, Z.S. Synthesis and anticonvulsant activity of N-(2-hydroxyethyl) cinnamamide derivatives. Eur. J. Med. Chem. 2009, 44, 3654–3657. [Google Scholar] [CrossRef]
- De Sarkar, S.; Grimme, S.; Studer, A. NHC catalysed oxidations of aldehydes to esters: Chemioselective acylation of alcohols in presence of amines. J. Am. Chem. Soc. 2010, 132, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.; Boatman, R.; Bootman, J.; Lewis, C.; Morgott, D.; Rushton, E.; van Rooij, J.; Banton, M. A review of the toxicological and environmental hazards and risks of tetrahydrofuran. Crit. Rev. Toxicol. 2013, 43, 811–828. [Google Scholar] [CrossRef]
- Schlosser, P.M.; Bale, A.S.; Gibbons, C.F.; Wilkins, A.; Cooper, G.S. Human health effects of dichloromethane: Key findings and scientific issues. Environ. Health Perspect. 2015, 123, 114–119. [Google Scholar] [CrossRef]
- Garg, P.; Milton, M.D. Sodium carbonate mediated regioselective synthesis of novel N-(hydroxyalkyl)cinnamamides. Tetrahedron Lett. 2013, 54, 7074–7077. [Google Scholar] [CrossRef]
- Dufrénoy, P.; Rigo, B.; Waterlot, C.; Hechelski, M.; Daïch, A.; Ghinet, A. Valorization of New Eco-Catalysts in the Synthesis of Anti-Inflammatory Compounds; Journées des Jeunes Chercheurs de la Société de Chimie Thérapeutique; Faculté de pharmacie Châtenay-Malabry: Châtenay-Malabry, France, 2017. [Google Scholar]
- Bahmanpour, A.M.; Héroguel, F.; Baranowski, C.J.; Luterbacher, J.S.; Kröcher, O. Selective synthesis of dimethyl ether on eco-friendly K10 montmorillonite clay. Appl. Catal. A 2018, 560, 165–170. [Google Scholar] [CrossRef]
- Grison, C.M.; Velati, A.; Escande, V.; Grison, C. Metallophytes for organic synthesis: Towards new bio-based selective protection/deprotection procedures. Environ Sci. Pollut. Res. 2015, 22, 5686–5698. [Google Scholar] [CrossRef]
- Escande, V.; Renard, B.L.; Grison, C. Lewis acid catalysis and green oxidations: Sequential tandem oxidation process induced by Mn-hyperaccumulating plants. Environ. Sci. Pollut. Res. 2015, 22, 5633–5652. [Google Scholar] [CrossRef]
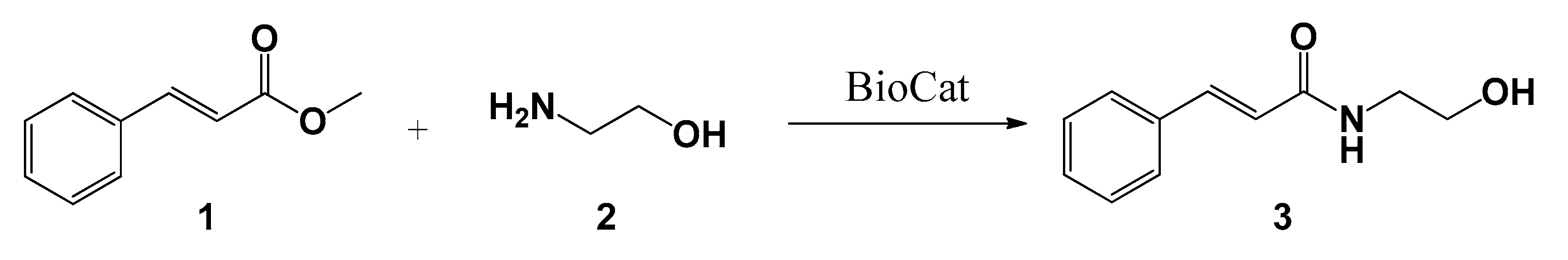
| Element | Cd | Pb | Zn | Cu | Mn | Fe | Al |
|---|---|---|---|---|---|---|---|
| Concentration | 49 ± 1 | 40 ± 1 | 7543 ± 222 | 69 ± 4 | 908 ± 41 | 303 ± 8 | 597 ± 133 |
| Ratio [Zn]/[M] | 152.8 ± 5.5 | 188.3 ± 10.4 | 1 | 109.1 ± 9.4 | 8.3 ± 0.4 | 24.9 ± 1.0 | 13.2 ± 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waterlot, C.; Dufrénoy, P.; Hechelski, M.; Louvel, B.; Daïch, A.; Ghinet, A. Benefits of Ryegrass on Multicontaminated Soils Part 2: A Green Process to Provide Idrocilamide. Sustainability 2019, 11, 6685. https://doi.org/10.3390/su11236685
Waterlot C, Dufrénoy P, Hechelski M, Louvel B, Daïch A, Ghinet A. Benefits of Ryegrass on Multicontaminated Soils Part 2: A Green Process to Provide Idrocilamide. Sustainability. 2019; 11(23):6685. https://doi.org/10.3390/su11236685
Chicago/Turabian StyleWaterlot, Christophe, Pierrick Dufrénoy, Marie Hechelski, Brice Louvel, Adam Daïch, and Alina Ghinet. 2019. "Benefits of Ryegrass on Multicontaminated Soils Part 2: A Green Process to Provide Idrocilamide" Sustainability 11, no. 23: 6685. https://doi.org/10.3390/su11236685
APA StyleWaterlot, C., Dufrénoy, P., Hechelski, M., Louvel, B., Daïch, A., & Ghinet, A. (2019). Benefits of Ryegrass on Multicontaminated Soils Part 2: A Green Process to Provide Idrocilamide. Sustainability, 11(23), 6685. https://doi.org/10.3390/su11236685






