Properties of Cement Mortar Using Limestone Sludge Powder Modified with Recycled Acetic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
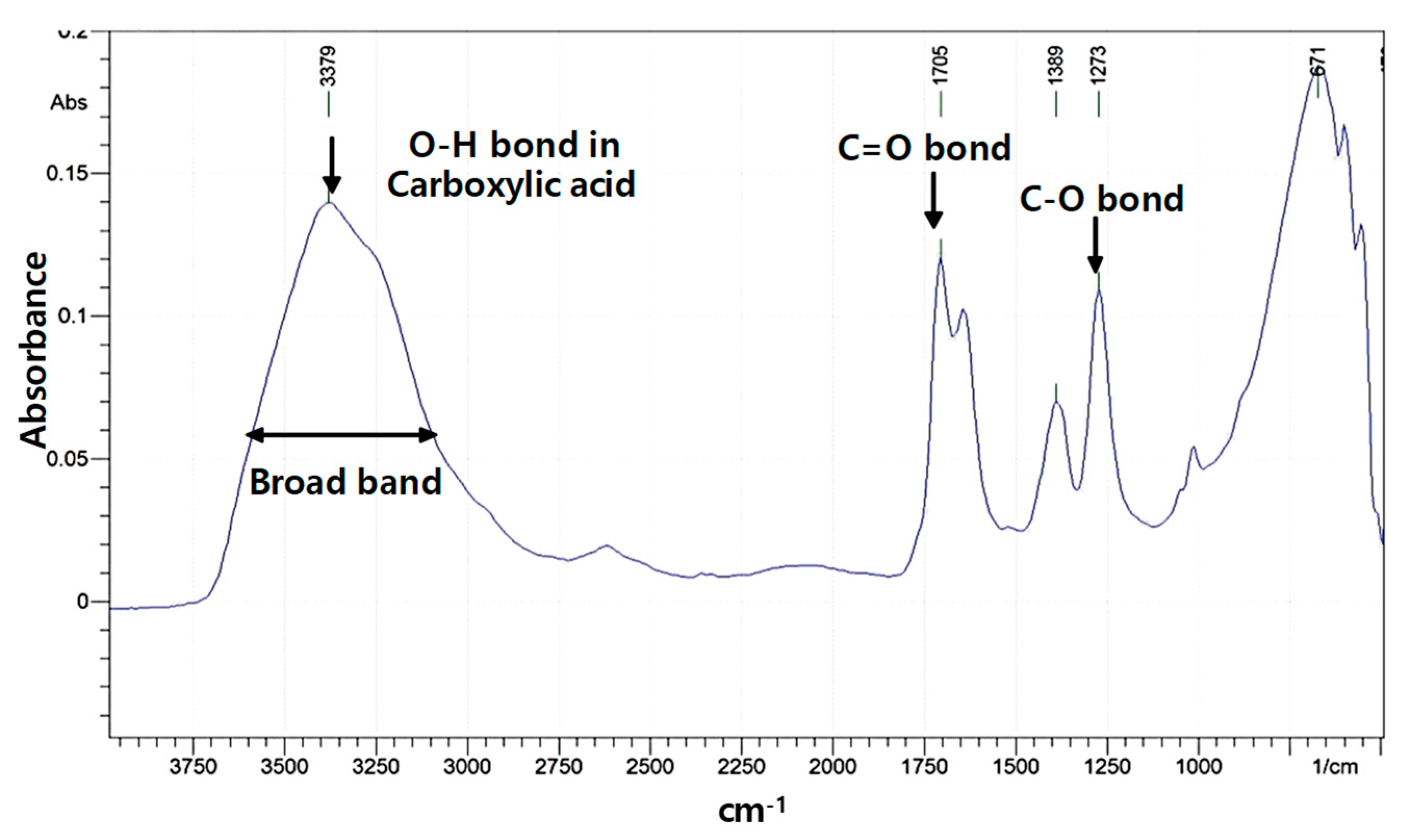
3.1. Characteristics of Modified LSSP
3.2. Mortar Application Characteristics
3.2.1. Setting Time
3.2.2. Compressive Strength
3.3. X-ray Diffraction Analysis
3.4. Thermogravimetric/Differential Thermal Analysis
3.5. Scanning Electron Microscopy Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories. Reference Manual (Revised); Houghton, J.T., Meira Filho, L.G., Lim, B., Treanton, K., Mamaty, I., Bonduki, Y., Griggs, D.J., Callender, B.A., Eds.; IPCC/OECD/IEA: Paris, France, 1997; Volume 3, Available online: https://www.ipcc-nggip.iges.or.jp/public/gl/invs6.html (accessed on 1 October 2018).
- Marland, G.; Boden, T.A.; Griffin, R.C.; Huang, S.F.; Kanciruk, P.; Nelson, T.R. Estimates of CO2 Emissions from Fossil Fuel Burning and Cement Manufacturing, based on the United Nationals Energy Statistics and the U.S. Bureau of Mines Cement Manufacturing Data; Report No. #ORNL/CDIAC-25; Carbon Dioxide Information Analysis Centre, Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1989. Available online: https://cdiac.ess-dive.lbl.gov/epubs/ndp/ndp030/ndp0301.htm (accessed on 1 October 2018).
- Hooton, R.D.; Nkken, M.; Thomas, M.D.A. Portland Limestone Cement: State of the Art Report and Gap Analysis for CSA A 3000. Cem. Assoc. Canada 2007, 3053, 3–5. [Google Scholar]
- Hawkins, P.; Tennis, P.; Detwiler, R. The Use of Limestone in Portland Cement: A State-of-the-Art Review. Portland Cem. Assoc. 2003, 2052b, 27–29. [Google Scholar]
- Caldarone, M.A.; Zemajtis, J.Z. Effect of Use of Limestone on Various Properties of Portland Cement-Part. Portland Cem. Assoc. 2008, 2891a, 1–4. [Google Scholar]
- Paquien, J.N.; Galy, J.; Gerard, J.F.; Pouchelon, A. Rheological studies of fumed silica polydimethylsiloxane suspensions. Colloids Surf. A 2005, 260, 165–172. [Google Scholar] [CrossRef]
- Walberer, J.A.; McHugh, A.J. The linear viscoelastic behavior of highly filled polydimethylsiloxane measured in shear and compression. J. Rheol. 2001, 45, 187–201. [Google Scholar] [CrossRef]
- Khastgir, D.; Adachi, K. Rheological and dielectric studies of aggregation of barium titanate particles suspended in polydimethylsiloxane. Polymer 2000, 41, 6403–6413. [Google Scholar] [CrossRef]
- Aral, B.K.; Kalyon, D.M. Viscoelastic material functions of noncolloidal suspensions with spherical particles. J. Rheol. 1997, 41, 599. [Google Scholar] [CrossRef]
- Aranguren, M.I.; Mora, E.; DeGroot, J.V., Jr.; Macosko, C.W. Effect of reinforcing fillers on the rheology of polymer melts. J. Rheol. 1992, 36, 1165–1181. [Google Scholar] [CrossRef]
- Mishra, S.; Sonawane, S.H.; Singh, R.P. Studies on characterization of nano CaCO3 prepared by the in situ deposition technique and its application in PP-nano CaCO3 composites. J. Polym. Sci. B 2005, 43, 107–113. [Google Scholar] [CrossRef]
- Demjn, Z.; Puknszky, B.; Fldes, E.; Nagy, J. Interaction of silane coupling agents with CaCO3. J. Colloid Interface Sci. 1997, 190, 427–436. [Google Scholar] [CrossRef]
- Na, A.L.; Dong, F.J.; Chen, W.Z. In situ synthesis and modification of calcium carbonate nanoparticles via a bobbling method. Sci. China Ser. B-Chem. 2009, 52, 924–929. [Google Scholar]
- Mishra, S.; Shimpi, N.G.; Mali, A.D. Investigation of photo-oxidative effect on morphology and degradation of mechanical and physical properties of nano CaCO3 silicone rubber composites. Polym. Adv. Technol. 2012, 23, 236–246. [Google Scholar] [CrossRef]
- Mishra, S.; Shimpi, N.G.; Mali, A.D. Influence of stearic acid treated nano-CaCO3 on the properties of silicone nanocomposites. J. Polym. Res. 2011, 18, 1715–1724. [Google Scholar] [CrossRef]
- Hoshino, S.; Ohba, Y.; Sakai, E.; Daimon, M. Relation between the Properties of Inorganic Powders and the Fluidity of Cement Pastes. JCA Pro. Cem. Concr. 1996, 50, 186–191. [Google Scholar]
- Sakai, E.; Ichikawa, M.; Daimon, M. Limestone Powder Application. J. Concr. 1998, 36, 3–9. (In Japanese) [Google Scholar] [CrossRef]
- ACI. Accelerated curing of concrete at atmospheric pressure—state of the art; ACI Committee 517.2R-87; American Concrete Institute: Farmington Hills, MI, USA, 1992. [Google Scholar]
- Lothenbach, B.; Saout, G.L.; Gallucci, E.; Scrivener, K. Influence of Limestone on the Hydration of Portland Cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Heikal, M.; El-Didamony, H.; Morcy, M.S. Limestone Filled Pozzolanic Cement. Cem. Concr. Res. 2000, 30, 1827–1834. [Google Scholar] [CrossRef]
- Bonavetti, V.L.; Rahhal, V.F.; Irassar, E.F. Studies on the Carboaluminate Formation in Limestone Filler-Blended Cements. Cem. Concr. Res. 2001, 31, 853–859. [Google Scholar] [CrossRef]
- ACI. Building Code Requirements for Structural Concrete; ACI Committee 318, ACI 318-08 edition; American Concrete Institute: Farmington Hills, MI, USA, 2008. [Google Scholar]
- Aggoun, S.; Cheikh-Zouaoui, M.; Chikh, N.; Duval, R. Effect of some admixtures on the setting time and strength evolution of cement pastes at early ages. Constr. Build. Mater. 2008, 22, 106–110. [Google Scholar] [CrossRef]
- Hoang, K.; Justnes, H.; Geiker, M. Early age strength increase of fly ash blended cement by a ternary hardening accelerating admixture. Cem. Concr. Res. 2016, 81, 59–69. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Colangelo, F.; Cioffi, R. Low temperature alkaline activation of weathered fly ash: Influence of mineral admixtures on early age performance. Constr. Build. Mater. 2015, 86, 169–177. [Google Scholar] [CrossRef]
- C¸ etin, C.; Erdo˘gan, S.T.; Tokyay, M. Effect of particle size and slag content on the early hydration of interground blended cements. Cem. Concr. Comp. 2016, 67, 39–49. [Google Scholar]
- Le Saoˆut, G.; Lothenbach, B.; Hori, A.; Higuchi, T.; Winnefeld, F. Hydration of Portland cement with additions of calcium sulfoaluminates. Cem. Concr. Res. 2013, 43, 81–94. [Google Scholar] [CrossRef]
- Huang, H.; Shen, X.-D. Interaction effect of triisopropanolamine and glucose on the hydration of Portland cement. Constr. Build. Mater. 2014, 65, 360–366. [Google Scholar] [CrossRef]
- Min, T.-B.; Cho, I.-S.; Park, W.-J.; Choi, H.-K.; Lee, H.-S. Experimental study on the development of compressive strength of early concrete age using calcium-based hardening accelerator and high early strength cement. Constr. Build. Mater. 2014, 64, 208–214. [Google Scholar] [CrossRef]
- ISO. Cement—Test methods—Determination of strength. ISO 679. 2009. Available online: https://www.iso.org/standard/45568.html (accessed on 1 October 2018).
- Farouk, B.; Mohamed, H.; Mostafa, K. Effect of a Carboxylic Acid on Rheological Properties of a High Alumina Cement Mortar. Iran. J. Chem. Chem. Eng. 2013, 32, 49–57. [Google Scholar]
- Choi, W.-H.; Park, C.-W.; Jung, W.-K.; Jeon, B.-J.; Kim, G.-S. Durability Characteristics of Limestone Powder added Concrete for Environment-Friendly Concrete. J. Korea Inst. Struct. Maint. Insp. 2012, 16, 59–67. [Google Scholar]
- Currell, B.R.; Grzeskowlak, R.; Mldgley, H.G.; Parsonage, J.R. The Acceleration and Retardation of Set High Alumina Cement by Additives. Cem. Concr. Res. 1987, 17, 420–432. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Balonis, M.; Glasser, F.P. Calcium nitrite corrosion inhibitor in Portland cement: influence of nitrite on chloride binding and mineralogy. J. Am. Ceram. Soc. 2011, 94, 2230–2241. [Google Scholar] [CrossRef]









| CaO | SiO2 | MgO | Al2O3 | SO3 | Fe2O3 | K2O | Na2O | LOI | |
|---|---|---|---|---|---|---|---|---|---|
| OPC | 63.35 | 21.09 | 3.32 | 4.34 | 3.09 | 2.39 | 1.13 | 0.29 | 1.0 |
| CaCO3 | 52.53 | 1.18 | 2.47 | 0.47 | 0.02 | 0.43 | 0.13 | 0 | 42.77 |
| LSSP | 53.16 | 3.96 | 1.09 | 2.13 | 0.08 | 1.4 | 0.46 | 0.05 | 37.7 |
| Sample | OPC (Ordinary Portland Cement) | LSSP (Limestone Sludge Powder) | Modified LSSP | Silica sand | Water |
|---|---|---|---|---|---|
| OPC | 1000 | - | - | 2000 | 340 |
| OPC-L5 | 950 | 50 | |||
| OPC-L10 | 900 | 100 | |||
| OPC-L15 | 850 | 150 | |||
| OPC-L20 | 800 | 200 | |||
| OPC-ML5 | 950 | 50 | |||
| OPC-ML10 | 900 | 100 | |||
| OPC-ML15 | 850 | 150 | |||
| OPC-ML 20 | 800 | 200 |
| Sample | OPC (Plain) | OPC-L5 | OPC-L10 | OPC-ML5 | OPC-ML10 |
|---|---|---|---|---|---|
| Ca(OH)2 Weight content (%) | 2.33 | 1.01 | 0.985 | 1.771 | 1.876 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.-S.; Kim, D.-M.; Shin, S.-H.; Kim, W.-K.; Lim, S.-M.; Park, W.-J. Properties of Cement Mortar Using Limestone Sludge Powder Modified with Recycled Acetic Acid. Sustainability 2019, 11, 879. https://doi.org/10.3390/su11030879
Ryu H-S, Kim D-M, Shin S-H, Kim W-K, Lim S-M, Park W-J. Properties of Cement Mortar Using Limestone Sludge Powder Modified with Recycled Acetic Acid. Sustainability. 2019; 11(3):879. https://doi.org/10.3390/su11030879
Chicago/Turabian StyleRyu, Hwa-Sung, Deuck-Mo Kim, Sang-Heon Shin, Wan-Ki Kim, Seung-Min Lim, and Won-Jun Park. 2019. "Properties of Cement Mortar Using Limestone Sludge Powder Modified with Recycled Acetic Acid" Sustainability 11, no. 3: 879. https://doi.org/10.3390/su11030879
APA StyleRyu, H.-S., Kim, D.-M., Shin, S.-H., Kim, W.-K., Lim, S.-M., & Park, W.-J. (2019). Properties of Cement Mortar Using Limestone Sludge Powder Modified with Recycled Acetic Acid. Sustainability, 11(3), 879. https://doi.org/10.3390/su11030879





