Abstract
Since the advent of flush toilet systems, the aquatic environment has received a massive contaminant flow. Furthermore, the perception of human feces has changed from a useful nutrient source for agriculture to a harmful contaminant. In this study, we compared the nutritional quality of five samples: (1) human manure (HM), (2) human manure from a family mainly eating organic food (HMO), (3) cow manure (CM), (4) poultry manure (PM), and (5) commercial nursery media (CNM). Samples were analyzed in terms of organic and inorganic nutrient contents, molecular composition, seed germination, and chlorophyll concentration. Pyrolysis gas chromatography/mass spectrometry (GC/MS) was used to describe the differences in molecular composition. Three-dimensional excitation and emission matrix fluorescence spectroscopy characterized the organic composition of water extracts. From the results, CNM, PM, and HMO showed humic- and fluvic-like substance peaks, the highest values of potassium and sulfate ions, and of C/N ratios, indicating greater plant growth potential. This was confirmed by their higher chlorophyll concentrations and germination index values. These results contribute knowledge about the positive effects of manure, changing the negative perception of human excreta from waste to resource. This work provides a reference for reducing the wastewater loading rate in society.
1. Introduction
Since the emergence of the flush toilet in conventional sanitation systems, humans have been protected from waterborne diseases, which has resulted in groundbreaking improvements to public health [1]. The ‘drop-and-discharge’ approach is a convenient and comfortable solution for disposing human excreta because it rapidly removes hazardous and unpleasant matter from households [2]. However, the convenient toilet takes the excreta out of sight and out of mind. People now discharge their excreta without considering the method of treatment, thereby forcing our environment to receive a massive contaminant flow. The influent of wastewater treatment plants contains approximately 12% to 31% total nitrogen (TN) originating from human excreta [3]. Residual nutrients are still found in the effluent after treatment; these intensify algal growth and cause the eutrophication of receiving water bodies owing to algal bloom and declining dissolved oxygen concentrations [4]. This recurring problem exists around the world, even in developed countries with well-established wastewater treatment systems [5,6].
Nitrogen (N) is one of most important elements needed for plant growth, and human excreta offers a potential source in the form of manure. However, society has reservations about using human excreta in agriculture owing to the risk of contracting enteric diseases from the pathogens present in human feces as well as psychological reasons [7,8]. Composting is one method presented to eliminate disease-causing microorganisms and reduce the amount of wastewater generated by flush toilets. Bai and Wang [9] previously reported on using an aerobic thermophilic composting reactor for the sanitary disposal of human feces. In their study, they found that thermophilic composting can retain a high organic N content in the compost, which could then be used as a fertilizer. Livestock waste also contributes high concentrations of N and P and has been used as manure for thousands of years [10]. In addition, livestock manure is a good source of potassium (K), sulfur (S), and many trace minerals that are needed for crop growth. Recently, some researchers studied the production of biogas using livestock manure [11,12]. Roubik et al. [11] examined manure management practices in the household for the production of biogas. In their study, they reported the potential of biogas production and some strategies for increasing the production. Biogas also serves as a soil conditioner by improving the porosity and water holding capacity of the soil, and increasing its soil organic matter (SOM) content. Organic matter (OM) provides macro- and micronutrients that improve the microbial activity of the soil [13] and promote a plant growth [14]. SOM contains essential plant nutrients, making it an important factor in sustaining soil fertility and productivity [15,16]. The continuous use of organic materials such as plant or crop residues, green manure, and livestock manure, has a strong impact on the N dynamics of soil-plant systems as well as on soil productivity [17,18,19,20]. Since the usage of organic compost has various advantages, several studies have been published revealing its potential and proposing its possible application in society [21,22]. However, the negative perception of manure prevails mainly because of psychological factors [23].
Therefore, if a positive and valuable perception of manure is created in our society with supporting scientific data, this can help solve aquatic pollution and waste management issues. The main objective of this study was to showcase the efficiency of human feces as a soil amendment and nutrient source. Five samples were assessed based on their physical and chemical properties, seed germination, and chlorophyll concentration. In this study, we do not describe the compositing methods of each sample, as our goal was not to provide an effective method for compositing but to show the potential of human manure as a soil amendment and nutrient source. We believe that the outcome of this research will help to change the general perception of human excreta in our society.
2. Materials and Methods
2.1. Sample Collection
Five sample types were collected: (1) human manure (HM), (2) commercial nursery media (CNM), (3) poultry manure (PM), (4) human manure from a family mainly eating organic food (HMO), and (5) cow manure (CM). HM was obtained from a household located in a small autonomous decentralized community of Namwon City, Jeollanam-do, Republic of Korea. CNM was purchased from Sanglim Inc. in Republic of Korea, and PM was purchased from Olivi Inc. in Republic of Korea. HMO was taken from a family living in Seoul, Republic of Korea, that mainly consumes organic food and cultivated vegetables using homemade manure. CM was obtained from a cow farm in Ulju-gun, Ulsan, Republic of Korea. Since these three samples (HM, HMO, CM) are homemade manures, their respective fermentation and maturity degrees may differ from each other. According to the manufacturer, the CNM contained cocopeat, peatmoss, vermiculite, perlite, zeolite, humic acid, and fertilizer. All solid samples were stored in a refrigerator to minimize microorganism activity. The dissolved organic matter (DOM) in the manure samples was characterized by extracting them using deionized water. A total of 5 g of each sample was mixed with 50 mL of deionized (DI) water for extraction and filtered through a 0.45-μm Whatman filter.
2.2. Ion Chromatography
Ion chromatography was used to measure the ion concentrations in the manure extracts. An ICS-2100 (Thermo Fisher Scientific Corp., Waltham, MA, USA) equipped with a Dionex IonPacTM AS14 column measured the anion concentration. A KOH eluent was used as the mobile phase at a flow rate of 1.0 mL/min, and was generated from an EGC-KOH cartridge. A seven-anion standard solution (Dionex, Sunnyvale, CA, USA) was used for the anion concentration calibration curve. The cation concentration was estimated using an ICS-1600 (Thermo Fisher Scientific Corp., Waltham, MA, USA) equipped with a Dionex IonPacTM CS12A and an MSA eluent generator. The eluent flow rate was 1.2 mL/min. A six-cation standard solution (Dionex, Sunnyvale, CA, USA) was used for the cation concentration calibration curve. The concentration of three anions (chloride, phosphate, sulfate) and four cations (sodium, potassium, magnesium, calcium) was measured in each manure extract. Every sample analysis was triplicated.
2.3. Fluorescence Excitation-Emission Method (FEEM)
The DOM in the manure extracts was characterized using a fluorometer (Cary Eclipse, Varian, Santa Clara, CA, USA). The FEEM has a wavelength range from 220 to 500 nm at 10 nm intervals in both emission and excitation states. Scan speed was 1200 nm/min. DI water was used as a blank to remove the inherent Rayleigh and Raman scattering. The detected organic matter was then classified into four groups: aromatic-proteins-like substances (EX: < 250 nm, EM: < 350); microbial-byproduct-like substances (EX: <250 nm, EM: <380); humic-acid-like substances (EX: >250, EM: >380 nm); and, fulvic-acid-like substances (EX: <250 nm, EM: >380 nm) [24].
2.4. Pyrolysis Gas Chromatography/Mass Spectrometry (GC/MS)
For the pyrolysis GC/MS analysis, 0.1 mg of dried sample was wrap in a ferromagnetic foil (Pyrofoil F590, JAI, Tokyo, Japan). Subsequently, the sample was pyrolyzed at 590 °C using a curie-point pyrolizer (JCI-22, JAI, Tokyo, Japan). A Shimadzu GC/MS equipped with a DB-5MS (30 m, 0.25 mm, i.d., film thickness 0.50 μm) analyzed the injected sample; Helium was used as the carrier gas at 3.0 mL/min. The oven temperature was heated from 40 °C to 300 °C at a rate of 7 °C/min and a 10-min holding time for the final temperature. We used an ion source temperature of 210 °C and an ionizing voltage of 70 eV. Ions were scanned in a mass range of 30–500 amu. The fragments were identified by the NIST 02 library (National Institute of Standards and Technology).
2.5. Seed Germination
For seed sterilization, barley seeds were soaked in a 10% sodium hypochlorite solution for 10 min. The pretreated seeds were then rinsed three times with DI water. Whatman filter paper was placed on a petri dish, wherein 20 mL of manure extract was added. Afterwards, 40 seeds were put on top of the filter paper in each dish. Each seed was carefully placed 1 cm apart from the other seeds. The petri dishes were then covered and incubated in the dark at 30 °C for 5 days. At the end of the 5-day germination period, the number of germinated seeds was counted while the root length of each seed was estimated. The results were used to calculate the germination index (GI) of each manure using the following equation [25]:
2.6. Chlorophyll and Carotenoid Analyses
A total of 50 barley seeds were sown into the dry soil mixed with each manure in a greenhouse at 25 °C. The emerged seedlings were flooded with water for 15 days. Seedling leaves from 15-day old barley were harvested to determine chlorophyll and carotenoid concentrations.
After freeze-drying, the dry weight of the leaf was measured, 5 mg was then transferred to a test-tube. We added 100 μL of DI water to hydrate the samples in the tube. Finally, 8 mL of 96% ethanol was added, and the sample was incubated at room temperature overnight. Ultraviolet (UV) absorbances of the extract at 470, 648, and 664.2 nm were measured using an ultraviolet-visible spectrometer (UV-1601, Shimadzu, Kyoto, Japan). Chlorophyll a (Ca), chlorophyll b (Cb), total chlorophylls (Ca+b), and total carotenoids (Cx+c) concentrations were calculated using the following equations [26]:
where A is the absorbance at a given wavelength and DW is the dry weight of extracted plant tissue (mg).
2.7. Statistical Analysis
A one-way analysis of variance (ANOVA) was used to determine the effect of samples on the growth of plants. Post-hoc comparisons were conducted using Tukey’s tests with a 5% (p = 0.05) significance level in SPSS Statistics Base 22.0 (IBM, Armonk, NY, USA).
3. Results and Discussion
3.1. Nutrient Analysis in Water Extract
Table 1 shows the dissolved organic carbon (DOC) and dissolved total nitrogen (DTN) concentrations of each sample (ANOVA, p < 0.03). Comparing the nutrient values between the samples, CNM contained the largest amounts of DOC and DTN, which were verified by post-hoc tests.

Table 1.
Concentrations of soluble carbon and nitrogen in water extract (n = 3).
Since the values vary based on the sample origin, calculating the ratio between DOC and DTN provides a more informative representation than comparing individual amounts. According to He and Ohno [27], the extraction efficiency of C in manure is lower than that of N, since soluble and low-molecular-mass organic N compounds exist in animal manure [28]. In all water extract samples, however, the C concentration was higher than the N concentration. This is mostly due to the fact that the total mass of carbon in manure is higher than the total mass of nutrients [29]. The C/N ratio of the water extracts followed the following order: CNM (4.41) > PM (3.15) > HMO (2.42) > CM (1.94) > HM (1.000). Chanyasak and Kubota [30] proposed that the C/N ratio in a water extract can be an indicator of compost maturity since soluble C and N concentrations relate to the metabolism rates of microorganisms during composting activity. They suggested that a C/N ratio between 5 and 6 is an appropriate value indicating maturity. On this basis, none of the samples in this study achieved maturity because the C/N ratios in the manures were lower than the suggested range. However, since the C/N ratio decreases with compositing time, the low values indicated that HM and CM had longer compositing time than did the other samples [29]. Tukey’s post-hoc tests for DOC and DTN ratios indicated that HM and CM were not significantly different.
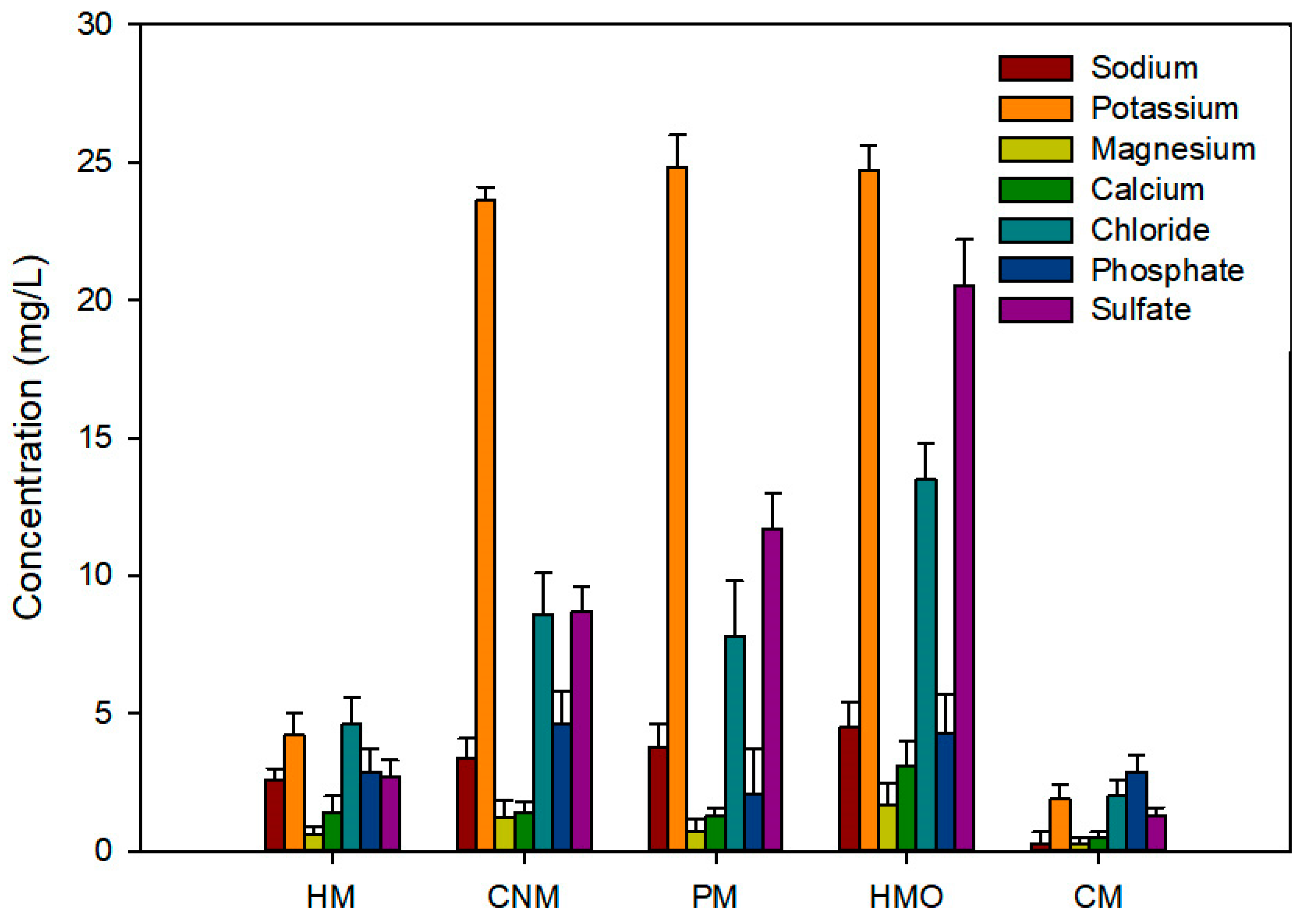
Figure 1 shows the ion concentrations of the manure water extracts. CNM, PM, and HMO showed high concentration of potassium (K+), chloride (Cl-), and sulfate (SO42-), whereas CM and HM had low concentration of the measured ions. The ion concentration of each sample was influenced by their origin and additives to enhance the nutritional efficiency. Yao et al. [31] reported that PM contains sodium, chloride, potassium, magnesium, and sulfate. They suggested using an optimum amount of PM to prevent soil salinization. The high concentration of nutritional ions in CNM was derived from its production, which included the balancing of ions for improving plant growth.
Figure 1.
Ion concentrations in water extracts of five sample types (n = 3). Each sample analysis was duplicated: human manure (HM); commercial nursery media (CNM); poultry manure (PM); human manure from a family eating organic food (HMO); and cow manure (CM). CNM, PM, and HMO showed a high concentration of potassium, chloride, and sulfate.
The results imply that the three manures (CNM, PM, and HMO) are very effective fertilizers that would improve crop produce, since farming reduces the major nutrients in the soil, including phosphorus, potassium, and sulfate [32].
When the results in Table 1 and Figure 1 are compared, the composting periods of HM and CM are longer than those of the other samples. This reflects the low nutrient condition of both samples, as shown in Figure 1, during that period. DOC and DTN tend to decrease with time owing to nutrient decomposition caused by microbial activity [33].
3.2. Organic Matter Characterization
3.2.1. Fluorescence Excitation-Emissions
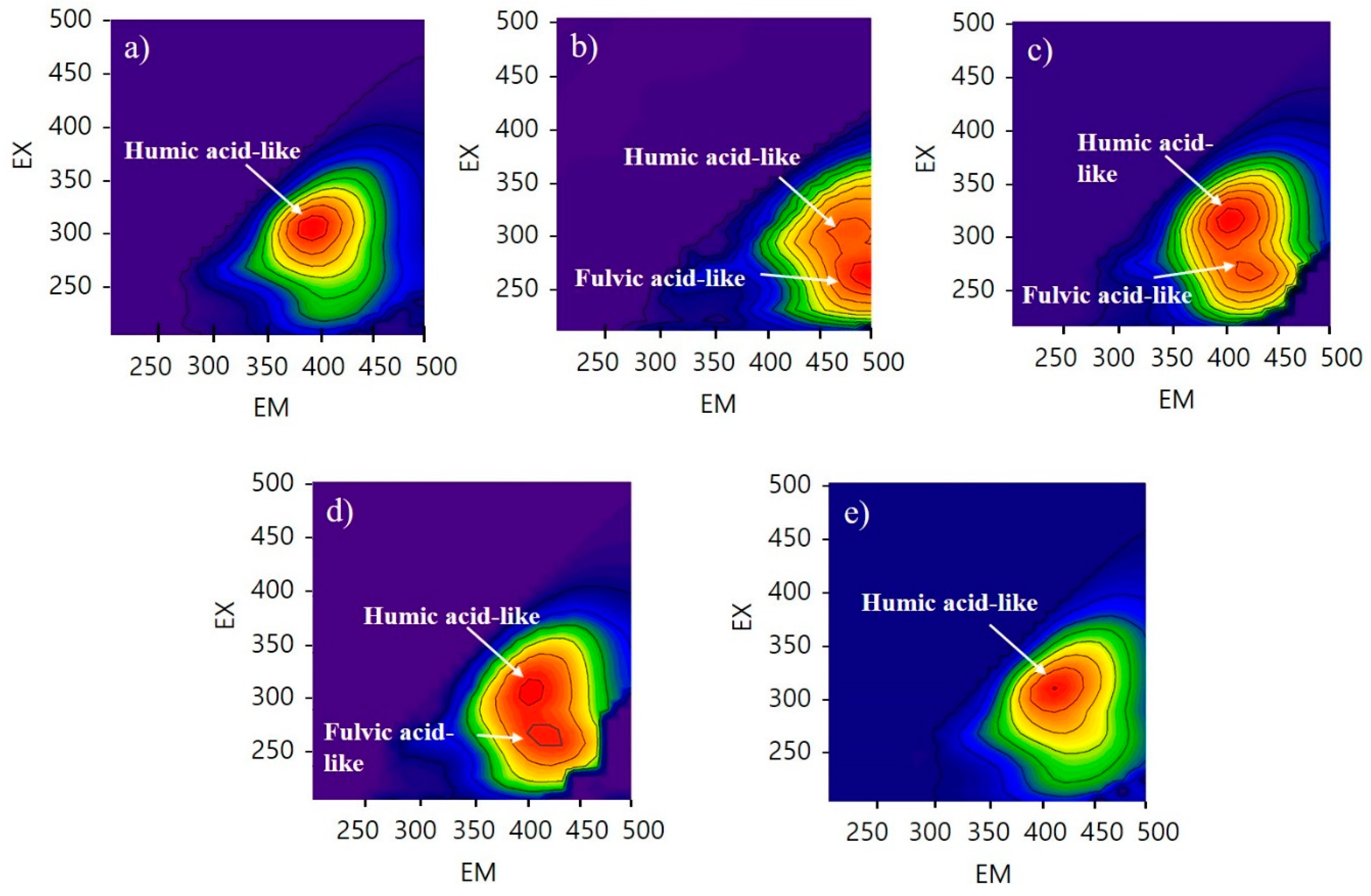
As shown in Figure 2, FEEM results indicate that CNM, PM, and HMO contained humic-acid-like and fulvic-acid-like substances, while HM and CM only contained humic-acid-like substances. The beneficial effects of humic and fulvic acid on plant growth have been investigated in previous studies. Canellas et al. [34] reported that humic acid promotes root growth and proton pump activation in maize vesicles. Jannin et al. [35] found that humic acid induces some genes that encode nitrate transporters and leads to enhanced nitrate uptake in plants. Lulakis and Petsas [36] reported on the beneficial effects of humic and fulvic acid on tomato seedling growth; they suggested that the optimum concentration ranges of humic and fulvic acid are 100–300 ppm and 200–1000 ppm, respectively. Rauthaan and Schnitzer [37] showed the effect of fulvic acid on significant increases in the growth of cucumber plants in terms of height of shoot, length of root, and number of leaves and flowers. Therefore, the three samples (HMO, CNM, PM) containing humic- and fulvic-acid-like substances have higher potential for improving plant growth.
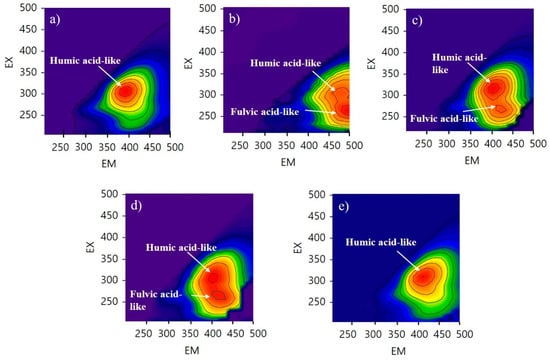
Figure 2.
Fluorescence spectral characteristics of five types of manure; (a) human manure (HM), (b) commercial nursery media (CNM), (c) poultry manure (PM), (d) human manure from a family eating organic food (HMO), and (e) cow manure (CM). The red color means high intensity and the blue color means low intensity. CNM, PM, and HMO have two peaks with high intensity.
Humic- and fulvic-acid-like substances are formed by the humification of manure during composting. Organic matter in the manure increases their aromaticity, molecular weight, and functional groups during the composting process [38]. In addition, humic-acid-like substances increase, while fulvic-acid-like substances decrease, during composting [39]. Since fulvic acid is soluble at all pH values, it is capable of leaching from the stored manure during the composting period. Therefore, the absence of fulvic-acid-like substances in the HM and CM was due to the longer composting periods for these manures. This is supported by the results presented in Section 3.1, wherein the lower nutrient compositions of HM and CM were caused by a longer period of microbial activity as compared with the other samples.
3.2.2. Molecular Composition Analysis by Pyrolysis GC/MS
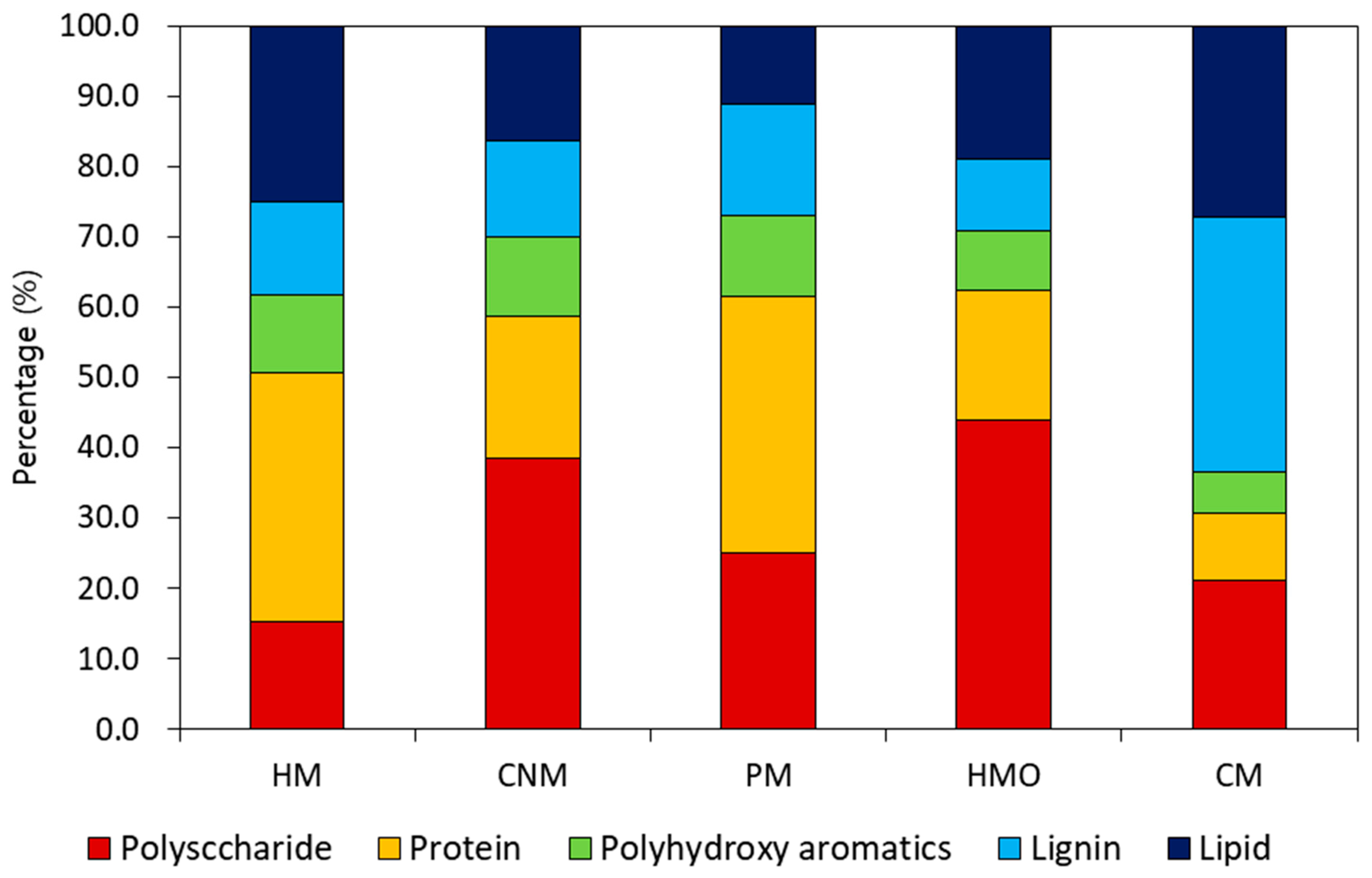
Fragments from the samples after undergoing pyrolysis were classified into five bio-polymer groups. Polysaccharides, protein, and lipid originate from microorganisms [40], while polyhydroxy aromatics and lignin are normally from decomposing plants [41]. The pyrolysis GC/MS results are shown in Figure 3. Since the composition of manure is influenced by diet, the organic composition of each analyzed sample differed from the rest. CM showed a high portion of lignin, since cows are herbivores, whereas HM and PM had large portions of protein. HM and HMO showed different biopolymer compositions even though both manures originated from human excreta. The protein content of HM (35%) was higher than that of HMO (18.5%). However, the percentage of polysaccharide in HMO was significantly higher than that in HM. These differences reflect the varied diet between the human beings that produced the samples. In this regard, the results from this analysis cannot determine the difference between consuming either conventionally grown food or organic products. Conventionally grown food is farmed with the use of synthetic chemicals, while organic foods benefit from low-level chemical contamination from agricultural pesticides. Pyrolysis GC/MS can only quantify the amounts of biopolymers present in the manures but cannot recognize the differences between biopolymer compositions. Therefore, we conducted an ecotoxicity assessment to differentiate HM from HMO; this assessment can provide qualitative and quantitative indicators of the potential impact of some substances [42]. To estimate the ecotoxicity potential, chlorophyll concentrations and germination indices of the seedling samples were compared in the next section.
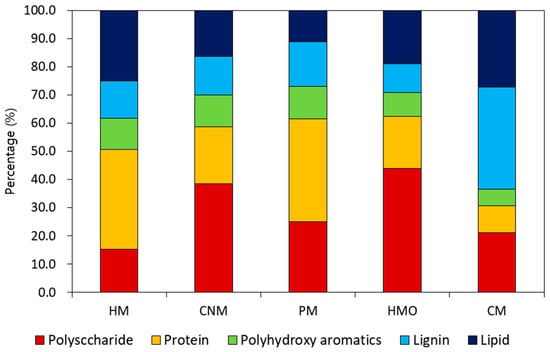
Figure 3.
Biopolymer distribution of five types of manures (HM: human manure, CNM: commercial nursery media, PM: commercial poultry manure, HMO: human manure from a family eating organic food, CM: commercial cow manure).
3.3. Plant Productivity
3.3.1. Chlorophyll Concentration
Chlorophyll is a green pigment widely distributed in the leaves and stems of plants. It is present in chloroplasts with carotenoids bound to proteins or lipoproteins, and plays an important role in plant photosynthesis [43]. The composition of these photosynthetic pigments indicates the physiological state of the plant. In particular, since there is a close correlation between the leaf chlorophyll content and nitrogen availability, it can be used to estimate the nitrogen nutrient status of plants [44]. In this study, the chlorophyll concentrations of barley were compared based on the type of manure applied as fertilizer, as shown in Table 2. The ANOVA results revealed significant differences between the effects of samples on chlorophyll and carotenoids concentrations (p <0.05 in all cases). HMO had the highest chlorophyll a and b concentrations (3.69 mg/g and 1.85 mg/g, respectively). The chlorophyll concentration indicates that the barley cultivated with HMO gained higher nutritional benefits. However, there were only minor differences in chlorophyll and carotenoids concentrations between HM and CNM. The measured concentration of total carotenoids was found to be insignificant. In addition, post hoc results calculated with Tukey’s tests supported the significant difference between HMO and the other samples, while HM and CNM showed no significant differences in any of the cases. The ratio of carotenoids to chlorophylls reflects the effect of stress on the plants or wilting of the plants [45]. Since barley seedlings were grown under identical cultivation conditions (i.e., water, light intensity, and temperature), it is supposed that stress level was not critical to barley growth.

Table 2.
Chlorophylls and carotenoids concentrations of different manures 1 (n = 3).
3.3.2. Germination Index
Table 3 shows the germination parameters of the control seedlings and those grown with the manures as fertilizers. Significant differences between each result were identified using ANOVA (p <0.02). Out of a total seedling count of 40, the number of germinations for the control was 35, while that of CM and HM was 31 and 34, respectively. CNM and HMO showed higher numbers of germination (CNM: 39, HMO: 38). These results are consistent with the nutrient and ion concentration results presented in Section 3.1 (CNM > HMO > PM > HM > CM), but the number of germinations of PM (36) was not significantly higher than that of the control (35), even though it showed high values of DOC and DTN. However, the post-hoc results in Table 3 determined the effects of PM on the increase of the germination number. In addition, CNM, PM, and HMO were classified as samples having positive potential in increasing the number of germinations. The average weight and root length showed a different trend from the number of germinations. The average weight of CM was the highest, although it showed the lowest number of germinations between the samples, while PM showed the shortest length of root despite being second in terms of average weight. According to the post-hoc tests, PM does not have a positive potential in increasing the weight of the seeds, and there were no significant differences in the root lengths in any cases. The variation of the results seems to originate from the different inherent growth potential of the seeds.

Table 3.
Seed germination in terms of the number of germinations, average weight, and average root length (n = 3).
A decreasing trend in the GI values of the five manure samples was as follows (ANOVA, p <0.02, Table 4): CNM (97.5%) > HMO (95.0%) > PM (90.0%) > HM (85.0%) > CM (77.5%). The order is similar to that of the C/N ratio and ion concentration results, indicating that CNM, PM, and HMO have beneficial ions for plant growth (see Section 3.1). Tukey’s post-hoc test detected that the effects of CNM and HMO on the GI value were significantly different from the HM, PM, and CM results. In addition, a GI value higher than 50% indicates that the compost matured well and contained a low level of phytotoxic substances [46]. Although none of the samples in this study achieved maturity according to the results presented in Section 3.1, all of the samples showed GI values higher than 50%. This implies that every sample contained a low level of phytotoxic substances.

Table 4.
Germination index of five manure types 1.
4. Conclusions
Five types of samples (HM, CNM, PM, HMO, and CM) were investigated in terms of nutrient concentration, organic matter composition, chlorophyll concentrations, and germination index. CNM, PM, and HMO showed higher concentrations of potassium and sulfate, and higher C/N ratios than did HM and CM. In addition, the three manures (CNM, PM and HMO) contained humic- and fulvic-acid-like substances, while HM and CM only contained humic-acid-like substances. From the results, CNM, PM, and HMO were considered to have higher plant growth potential than HM and CM. The plant growth potential of the three manures was also proven by their high chlorophyll concentrations and GI compared with the other samples. From the pyrolysis GC/MS results, molecular compositional differences between the manures were related to the diversity of the diets of the organisms from which the manures came from. Overall, the results show that human manure can be an effective nutrient source, and is comparable to commercialized fertilizers. Even though this study had some limitations such as the unclear sample origin and small number of human manure samples, the value of human manure was successfully described. We hope that the results of this study will help to change the perspective on human excreta from an unwanted waste to a useful resource. In addition, this study suggests an alternative way to deal with human waste in our society.
Author Contributions
J.P. and M.-J.C analyzed the samples, and J.P. and M.L. wrote the paper. K.H.C. and M.-J.C. designed the experiments and drew the conclusions.
Funding
This work was funded by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (Grant No. NRF-2015R1A5A7037825).
Acknowledgments
We would like to gratefully acknowledge Prof. Jaeweon Cho for sharing the analytical instruments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Richardson, A. A new world ordure? Thoughts on the use of humanure in developed cities. City 2012, 16, 700–712. [Google Scholar] [CrossRef]
- Drangert, J. Fighting the urine blindness to provide more sanitation options. Water SA-Pretoria 1998, 24, 157–164. [Google Scholar]
- Park, J.; Chon, K.; Cho, J. Science Walden: New horizons of combined ecological sanitation with separated urine/feces and treatment wetlands. Desalin. Water Treat. 2015, 54, 1353–1360. [Google Scholar] [CrossRef]
- Carey, R.O.; Migliaccio, K.W. Contribution of wastewater treatment plant effluents to nutrient dynamics in aquatic systems: A review. Environ. Manag. 2009, 44, 205–217. [Google Scholar] [CrossRef]
- Chapra, S.C.; Boehlert, B.; Fant, C.; Bierman, V.J., Jr.; Henderson, J.; Mills, D.; Mas, D.M.; Rennels, L.; Jantarasami, L.; Martinich, J. Climate change impacts on harmful algal blooms in US freshwaters: A screening-level assessment. Environ. Sci. Technol. 2017, 51, 8933–8943. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; Hall, N.S.; Rossignol, K.L.; Joyner, A.R.; Zhu, G.; Qin, B. Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China: Implications for controlling eutrophication and harmful algal blooms. J. Freshw. Ecol. 2015, 30, 5–24. [Google Scholar] [CrossRef]
- Gerba, C.P.; Smith, J.E. Sources of Pathogenic Microorganisms and Their Fate during Land Application of Wastes the opinions expressed in this article are those of the authors and do not necessarily reflect those of the USEPA. J. Environ. Qual. 2005, 34, 42–48. [Google Scholar]
- Knudsen, L.G.; Phuc, P.D.; Hiep, N.T.; Samuelsen, H.; Jensen, P.K.; Dalsgaard, A.; Raschid-Sally, L.; Konradsen, F. The fear of awful smell: Risk perceptions among farmers in Vietnam using wastewater and human excreta in agriculture. Southeast Asian J. Trop. Med. Public Health 2008, 39, 341. [Google Scholar]
- Bai, F.; Wang, X. Nitrogen-retaining property of compost in an aerobic thermophilic composting reactor for the sanitary disposal of human feces. Front. Environ. Sci. Eng. China 2010, 4, 228–234. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arabian J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Roubík, H.; Mazancová, J.; Banout, J. Current approach to manure management for small-scale Southeast Asian farmers-Using Vietnamese biogas and non-biogas farms as an example. Renew. Energy 2018, 115, 362–370. [Google Scholar] [CrossRef]
- Nasir, I.M.; Ghazi, T.I.M.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Tiwari, S.C.; Tiwari, B.K.; Mishra, R.R. Microbial populations, enzyme activities and nitrogen-phosphorus-potassium enrichment in earthworm casts and in the surrounding soil of a pineapple plantation. Biol. Fert. Soils 1989, 8, 178–182. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Singh, R.G.; Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Prot. 2012, 38, 57–65. [Google Scholar] [CrossRef]
- Kabeerathumma, S.; Mohankumar, C.R.; Nair, G.M.; Nair, P.G. Effect of continuous cropping of cassava with organics and inorganics on the secondary and micronutrient elements status of an Ultisol. J. Indian Soc. Soil Sci. 1993, 41, 710–713. [Google Scholar]
- Bhattacharyya, P.; Chakraborty, A.; Chakrabarti, K.; Tripathy, S.; Powell, M.A. Chromium uptake by rice and accumulation in soil amended with municipal solid waste compost. Chemosphere 2005, 60, 1481–1486. [Google Scholar] [CrossRef]
- Timsina, J.; Connor, D.J. Productivity and management of rice–wheat cropping systems: Issues and challenges. Field Crops Res. 2001, 69, 93–132. [Google Scholar] [CrossRef]
- Schmidt, L.; Merbach, W. Response of soil C and N content to fertilization-results of long-term trials at Halle/S., Germany. Arch. Agron. Soil Sci. 2004, 50, 49–58. [Google Scholar] [CrossRef]
- Leite, L.F.; Madari, B.E. Soil organic matter: Brazilian perspectives. Dyn. Soil Dyn. Plant 5 2011, 1, 1–6. [Google Scholar]
- Singh, S.; Inamdar, S.; Mitchell, M.; McHale, P. Seasonal pattern of dissolved organic matter (DOM) in watershed sources: Influence of hydrologic flow paths and autumn leaf fall. Biogeochemistry 2014, 118, 321–337. [Google Scholar] [CrossRef]
- Jewitt, S. Poo gurus? Researching the threats and opportunities presented by human waste. Appl. Geogr. 2011, 31, 761–769. [Google Scholar] [CrossRef]
- Jenkins, J. The Humanure Handbook; Jenkings Publishing: Grove City, PA, USA, 2005. [Google Scholar]
- Park, J. Movements in perception on human feces for transition sanitation design, using convergence of science and arts. Ph.D. Thesis, Ulsan National Institute of Science and Technology, Ulsan, Korea, 14 February 2017. [Google Scholar]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Zucconi, F. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148C, 350–382. [Google Scholar]
- He, Z.; Ohno, T. Fourier transform infrared and fluorescence spectral features of organic matter in conventional and organic dairy manure. J. Environ. Qual. 2012, 41, 911–919. [Google Scholar] [CrossRef]
- Honeycutt, C.; Hunt, J.; Griffin, T.; He, Z.; Larkin, R. Determinants and Processes of Manure Nitrogen Availability, Environmental Chemistry of Animal Manure; Nova Science Publishers: New York, NY, USA, 2011; pp. 201–224. [Google Scholar]
- Iglesias Jiménez, E.; Pérez García, V. Determination of maturity indices for city refuse composts. Agric. Ecosyst. Environ. 1992, 38, 331–343. [Google Scholar] [CrossRef]
- Chanyasak, V.; Kubota, H. Carbon/organic nitrogen ratio in water extract as measure of composting degradation. J. Ferment. Technol. 1981, 59, 215–219. [Google Scholar]
- Li-Xian, Y.; Guo-Liang, L.; Shi-Hua, T.; Gavin, S.; Zhao-Huan, H. Salinity of animal manure and potential risk of secondary soil salinization through successive manure application. Sci. Total Environ. 2007, 383, 106–114. [Google Scholar] [CrossRef]
- Bhandari, A.; Ladha, J.; Pathak, H.; Padre, A.; Dawe, D.; Gupta, R. Yield and soil nutrient changes in a long-term rice-wheat rotation in India. Soil Sci. Soc. Am. J. 2002, 66, 162–170. [Google Scholar] [CrossRef]
- Huang, G.F.; Wong, J.W.C.; Wu, Q.T.; Nagar, B.B. Effect of C/N on composting of pig manure with sawdust. Waste Manag. 2004, 24, 805–813. [Google Scholar] [CrossRef]
- Canellas, L.P.; Spaccini, R.; Piccolo, A.; Dobbss, L.B.; Okorokova-Façanha, A.L.; de Araújo Santos, G.; Olivares, F.L.; Façanha, A.R. Relationships between chemical characteristics and root growth promotion of humic acids isolated from Brazilian oxisols. Soil Sci. 2009, 174, 611–620. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; San Francisco, S.; Baigorri, R.; Cruz, F. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Lulakis, M.D.; Petsas, S.I. Effect of humic substances from vine-canes mature compost on tomato seedling growth. Bioresour. Technol. 1995, 54, 179–182. [Google Scholar] [CrossRef]
- Rauthan, B.; Schnitzer, M. Effects of a soil fulvic acid on the growth and nutrient content of cucumber (Cucumis sativus) plants. Plant Soil 1981, 63, 491–495. [Google Scholar] [CrossRef]
- Senesi, N. Composted materials as organic fertilizers. Sci. Total Environ. 1989, 81, 521–542. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Molecular characterization of dissolved organic matter (DOM): A critical review. Anal. Bioanal. Chem. 2013, 405, 109–124. [Google Scholar] [CrossRef]
- Jiang, T.; Kaal, J.; Liang, J.; Zhang, Y.; Wei, S.; Wang, D.; Green, N.W. Composition of dissolved organic matter (DOM) from periodically submerged soils in the Three Gorges Reservoir areas as determined by elemental and optical analysis, infrared spectroscopy, pyrolysis-GC–MS and thermally assisted hydrolysis and methylation. Sci. Total Environ. 2017, 603–604, 461–471. [Google Scholar] [CrossRef]
- Nendza, M. Activity and Effects Parameters, Structure—Activity Relationships in Environmental Sciences; Springer: Berlin/Heidelberg, Germany, 1998; pp. 47–61. [Google Scholar]
- Bowers, J. Food Theory and Applications; Pearson: Harlow, UK, 1992. [Google Scholar]
- Filella, I.; Penuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Alscher, R.G.; Cumming, J.R. Stress Responses in Plants: Adaptation and Acclimation Mechanisms; Wiley-Liss: New York, NY, USA, 1990. [Google Scholar]
- Jodice, R. Chemical and biological parameters for evaluating compost quality. In Proceedings of the International Symposium on Compost Production and Use, San Michele all’Adige, Italy, 20–23 June 1989; pp. 20–23. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).