A Social-Ecological System Framework for Marine Aquaculture Research
Abstract
1. Introduction
2. Materials and Methods
3. Results
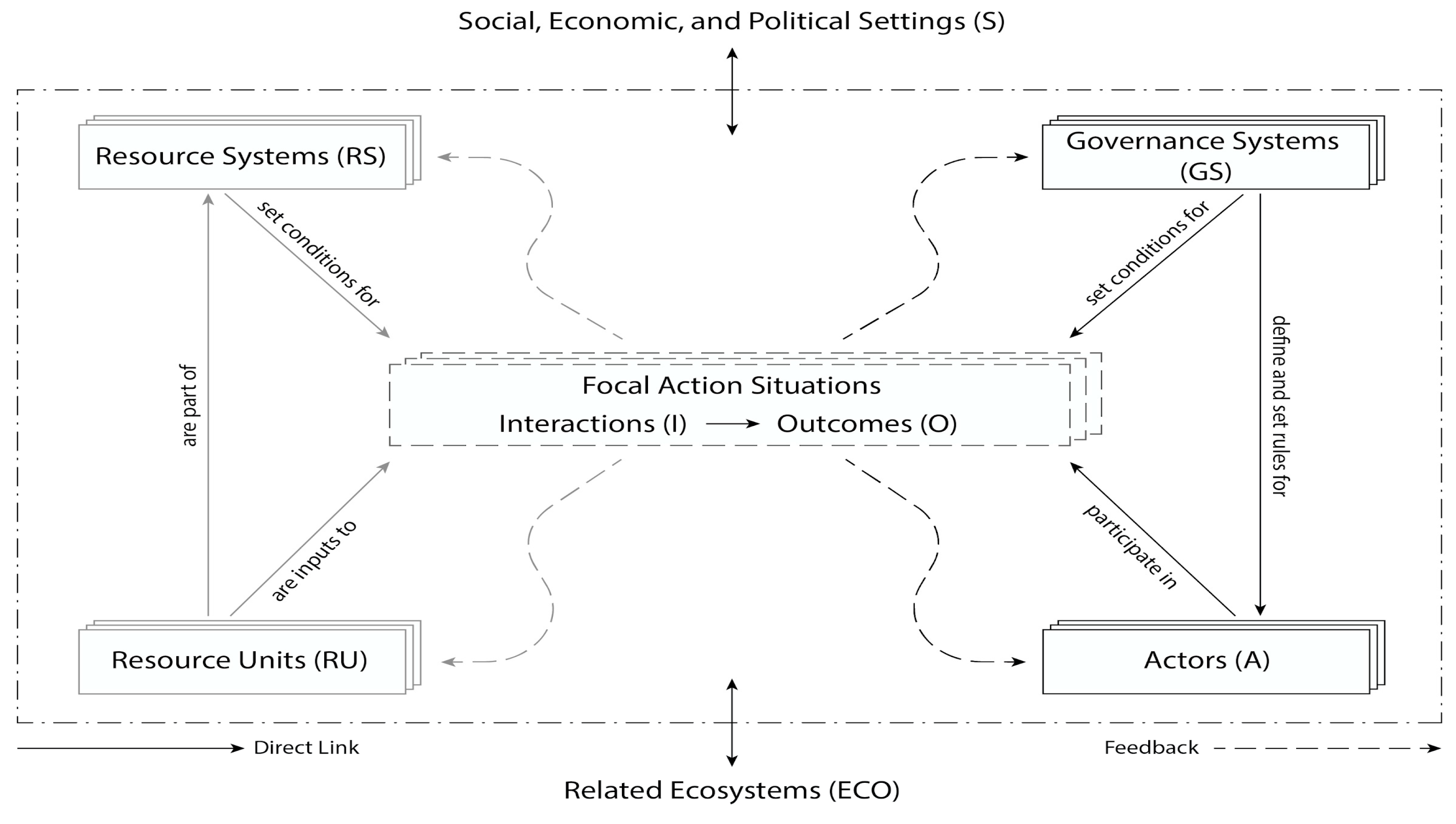
3.1. The SES Framework Applied to Marine Aquaculture
3.1.1. Four Subsystems: Resource System, Resource Units, Actors, and the Governance System
3.1.2. Exogenous Influences: The Social, Political, and Economic Setting and Related Ecosystems
3.1.3. Focal Action Situation: Interactions and Outcomes
3.2. Application: Marine Aquaculture in Maine (USA)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Hilborn, R.; Banobi, J.; Hall, S.J.; Pucylowski, T.; Walsworth, T.E. The Environmental Cost of Animal Source Foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef]
- Lester, S.E.; Gentry, R.R.; Kappel, C.V.; White, C.; Gaines, S.D. Opinion: Offshore Aquaculture in the United States: Untapped Potential in Need of Smart Policy. Proc. Natl. Acad. Sci. USA 2018, 115, 7162–7165. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pierce, B.A. Sustainable Ecological Aquaculture Systems: The Need for a New Social Contract for Aquaculture Development. Mar. Technol. Soc. J. 2010, 44, 88–112. [Google Scholar] [CrossRef]
- Krause, G.; Brugere, C.; Diedrich, A.; Ebeling, M.W.; Ferse, S.C.A.; Mikkelsen, E.; Pérez Agúndez, J.A.; Stead, S.M.; Stybel, N.; Troell, M. A Revolution without People? Closing the People-Policy Gap in Aquaculture Development. Aquaculture 2015, 447, 44–55. [Google Scholar] [CrossRef]
- Costa-Pierce, B. Ecological Aquaculture: The Evolution of the Blue Revolution; Blackwell: Oxford, UK, 2002. [Google Scholar]
- Nunes, J.P.; Ferreira, J.G.; Bricker, S.B.; O’Loan, B.; Dabrowski, T.; Dallaghan, B.; Hawkins, A.J.S.; O’Connor, B.; O’Carroll, T. Towards an Ecosystem Approach to Aquaculture: Assessment of Sustainable Shellfish Cultivation at Different Scales of Space, Time and Complexity. Aquaculture 2011, 315, 369–383. [Google Scholar] [CrossRef]
- Brugère, C.; Aguilar-Manjarrez, J.; Beveridge, M.C.M.; Soto, D. The Ecosystem Approach to Aquaculture 10 Years on—A Critical Review and Consideration of Its Future Role in Blue Growth. Rev. Aquac. 2018, 10. [Google Scholar] [CrossRef]
- Folke, C.; Kautsky, N. Aquaculture with Its Environment: Prospects for Sustainability. Ocean Coast. Manag. 1992, 17, 5–24. [Google Scholar] [CrossRef]
- Costa-Pierce, B.A.; Page, G.G. Sustainability Science in Aquaculture. In Sustainable Food Production; Springer: New York, NY, USA, 2013; pp. 206–222. [Google Scholar] [CrossRef]
- Pennington, D. A Conceptual Model for Knowledge Integration in Interdisciplinary Teams: Orchestrating Individual Learning and Group Processes. J. Environ. Stud. Sci. 2016, 6, 300–312. [Google Scholar] [CrossRef]
- Palmer, M.A.; Kramer, J.G.; Boyd, J.; Hawthorne, D. Practices for Facilitating Interdisciplinary Synthetic Research: The National Socio-Environmental Synthesis Center (SESYNC). Curr. Opin. Environ. Sustain. 2016, 19, 111–122. [Google Scholar] [CrossRef]
- McGinnis, M.D.; Ostrom, E. Social-Ecological System Framework: Initial Changes and Continuing Challenges. Ecol. Soc. 2014, 19. [Google Scholar] [CrossRef]
- Ostrom, E. A General Framework for Analyzing Sustainability of Social-Ecological Systems. Science 2009, 325, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P. A Systems Approach for the Promotion of Integrated Aquaculture. Aquac. Econ. Manag. 1998, 2, 1–12. [Google Scholar] [CrossRef]
- Caroppo, C.; Giordano, L.; Palmieri, N.; Bellio, G.; Bisci, A.P.; Portacci, G.; Sclafani, P.; Hopkins, T.S. Progress Toward Sustainable Mussel Aquaculture in Mar Piccolo, Italy. Ecol. Soc. 2012, 17. [Google Scholar] [CrossRef]
- Byron, C.; Link, J.; Costa-Pierce, B.; Bengtson, D. Calculating Ecological Carrying Capacity of Shellfish Aquaculture Using Mass-Balance Modeling: Narragansett Bay, Rhode Island. Ecol. Model. 2011, 222. [Google Scholar] [CrossRef]
- Binder, C.R.; Hinkel, J.; Bots, P.W.G.; Pahl-Wostl, C. Comparison of Frameworks for Analyzing Social-Ecological Systems. Ecol. Soc. 2013, 18. [Google Scholar] [CrossRef]
- Pulver, S.; Ulibarri, N.; Sobocinski, K.L.; Alexander, S.M.; Johnson, M.L.; McCord, P.F.; Dell’Angelo, J. Frontiers in Socio-Environmental Research: Components, Connections, Scale, and Context. Ecol. Soc. 2018, 23. [Google Scholar] [CrossRef]
- Partelow, S. A Review of the Social-Ecological Systems Framework: Applications, Methods, Modifications, and Challenges. Ecol. Soc. 2018, 23. [Google Scholar] [CrossRef]
- Agrawal, A.; Chhatre, A. Against Mono-Consequentialism: Multiple Outcomes and Their Drivers in Social–Ecological Systems. Glob. Environ. Chang. 2011, 21. [Google Scholar] [CrossRef]
- Schlüter, A.; Wise, S.; Mánez, K.S.; de Morais, G.W.; Glaser, M. Institutional Change, Sustainability and the Sea. Sustainability 2013, 5, 5373–5390. [Google Scholar] [CrossRef]
- Ban, N.C.; Mills, M.; Tam, J.; Hicks, C.C.; Klain, S.; Stoeckl, N.; Bottrill, M.C.; Levine, J.; Pressey, R.L.; Satterfield, T.; et al. A Social–Ecological Approach to Conservation Planning: Embedding Social Considerations. Front. Ecol. Environ. 2013, 11, 194–202. [Google Scholar] [CrossRef]
- Basurto, X.; Gelcich, S.; Ostrom, E. The Social-Ecological System Framework as a Knowledge Classificatory System for Benthic Small-Scale Fisheries. Glob. Environ. Chang. 2013, 23, 1366–1380. [Google Scholar] [CrossRef]
- Partelow, S.; Boda, C. A Modified Diagnostic Social-Ecological System Framework for Lobster Fisheries: Case Implementation and Sustainability Assessment in Southern California. Ocean Coast. Manag. 2015, 114, 204–217. [Google Scholar] [CrossRef]
- Partelow, S.; Senff, P.; Buhari, N.; Schlüter, A. Operationalizing the Social-Ecological Systems Framework in Pond Aquaculture. Int. J. Commons 2018, 12, 485–518. [Google Scholar] [CrossRef]
- Hinkel, J.; Cox, M.E.; Schlüter, M.; Binder, C.R.; Falk, T. A Diagnostic Procedure for Applying the Social-Ecological Systems Framework in Diverse Cases. Ecol. Soc. 2015, 20. [Google Scholar] [CrossRef]
- McKindsey, C.W.; Thetmeyer, H.; Landry, T.; Silvert, W. Review of Recent Carrying Capacity Models for Bivalve Culture and Recommendations for Research and Management. Aquaculture 2006, 261, 451–462. [Google Scholar] [CrossRef]
- Von Brand, E.; Abarca, A.; Merino, G.E.; Stotz, W. Scallop Fishery and Aquaculture in Chile: A History of Developments and Declines. Dev. Aquac. Fish. Sci. 2016, 40, 1047–1072. [Google Scholar] [CrossRef]
- Von Brand, E.; Merino, G.E.; Abarca, A.; Stotz, W. Chapter 27 Scallop Fishery and Aquaculture in Chile. Dev. Aquac. Fish. Sci. 2006, 35, 1293–1314. [Google Scholar] [CrossRef]
- Vita, R.; Marin, A. Environmental Impact of Capture-Based Bluefin Tuna Aquaculture on Benthic Communities in the Western Mediterranean. Aquac. Res. 2007, 38, 331–339. [Google Scholar] [CrossRef]
- Boynton, W.R.; Kemp, W.M.; Keefe, C.W. A Comparative Analysis of Nutrients and Other Factors Influencing Estuarine Phytoplankton Production; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Cloern, J.E.; Foster, S.Q.; Kleckner, A.E. Phytoplankton Primary Production in the World’s Estuarine-Coastal Ecosystems. Biogeosciences 2014, 11, 2477–2501. [Google Scholar] [CrossRef]
- Shumway, S.E. A Review of the Effects of Algal Blooms on Shellfish and Aquaculture. J. World Aquac. Soc. 1990, 21, 65–104. [Google Scholar] [CrossRef]
- MacCready, P.; Geyer, W.R. Advances in Estuarine Physics. Ann. Rev. Mar. Sci. 2010, 2, 35–58. [Google Scholar] [CrossRef]
- Adams, T.; Black, K.; MacIntyre, C.; MacIntyre, I.; Dean, R. Connectivity Modelling and Network Analysis of Sea Lice Infection in Loch Fyne, West Coast of Scotland. Aquac. Environ. Interact. 2012, 3, 51–63. [Google Scholar] [CrossRef]
- Newell, C.R.; Brady, D.C.; Richardson, J. Farm-Scale Production Models. In Goods and Services of Marine Bivalves; Springer International: Cham, Switzerland, 2019; pp. 485–506. [Google Scholar]
- Puniwai, N.; Canale, L.; Haws, M.; Potemra, J.; Lepczyk, C.; Gray, S.; Puniwai, N.; Canale, L.; Haws, M.; Potemra, J.; et al. Development of a GIS-Based Tool for Aquaculture Siting. ISPRS Int. J. Geoinf. 2014, 3, 800–816. [Google Scholar] [CrossRef]
- DePiper, G.S.; Lipton, D.W.; Lipcius, R.N. Valuing Ecosystem Services: Oysters, Denitrification, and Nutrient Trading Programs. Mar. Resour. Econ. 2017, 32, 1–20. [Google Scholar] [CrossRef]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed Aquaculture: Cultivation Technologies, Challenges and Its Ecosystem Services. Algae 2017, 32. [Google Scholar] [CrossRef]
- Lee, R.; Lovatelli, A.; Ababouch, L. Bivalve Depuration: Fundamental and Practical Aspects; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Yund, P.O.; Tilburg, C.E.; McCartney, M.A. Across-Shelf Distribution of Blue Mussel Larvae in the Northern Gulf of Maine: Consequences for Population Connectivity and a Species Range Boundary. R. Soc. Open Sci. 2015, 2, 150513. [Google Scholar] [CrossRef]
- Naylor, R.; Hindar, K.; Fleming, I.A.; Goldburg, R.; Williams, S.; Volpe, J.; Whoriskey, F.; Eagle, J.; Kelso, D.; Mangel, M. Fugitive Salmon: Assessing the Risks of Escaped Fish from Net-Pen Aquaculture. Bioscience 2005, 55, 427–437. [Google Scholar] [CrossRef]
- Rust, M.B.; Amos, K.H.; Bagwill, A.L.; Dickhoff, W.W.; Juarez, L.M.; Price, C.S.; Morris, J.A.; Rubino, M.C. Environmental Performance of Marine Net-Pen Aquaculture in the United States. Fisheries 2014, 39, 508–524. [Google Scholar] [CrossRef]
- Secor, D.H.; Arefjev, V.; Nikolaev, A.; Sharov, A. Restoration of Sturgeons: Lessons from the Caspian Sea Sturgeon Ranching Programme. Fish Fish. 2008, 1, 215–230. [Google Scholar] [CrossRef]
- Lorenzen, K.; Agnalt, A.L.; Blankenship, H.L.; Hines, A.H.; Leber, K.M.; Loneragan, N.R.; Taylor, M.D. Evolving Context and Maturing Science: Aquaculture-Based Enhancement and Restoration Enter the Marine Fisheries Management Toolbox. Rev. Fish. Sci. 2013, 21, 213–221. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological Engineering in Aquaculture—Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Gibbs, M.T. Implementation Barriers to Establishing a Sustainable Coastal Aquaculture Sector. Mar. Policy 2009, 33, 83–89. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Hawkins, A.J.S.; Bricker, S.B. Management of Productivity, Environmental Effects and Profitability of Shellfish Aquaculture—The Farm Aquaculture Resource Management (FARM) Model. Aquaculture 2007, 264, 160–174. [Google Scholar] [CrossRef]
- Hanes, S.P. Aquaculture and the Postproductive Transition on the Maine Coast. Geogr. Rev. 2018, 108, 185–202. [Google Scholar] [CrossRef]
- D’Anna, L.M.; Murray, G.D. Perceptions of Shellfish Aquaculture in British Columbia and Implications for Well-Being in Marine Social-Ecological Systems. Ecol. Soc. 2015, 20. [Google Scholar] [CrossRef]
- Weeks, P. Fish and People: Aquaculture and the Social Sciences. Soc. Nat. Resour. 1992, 5, 345–357. [Google Scholar] [CrossRef]
- Kroehn, M.; Maude, A.; Beer, A. Leadership of Place in the Rural Periphery: Lessons from Australia’s Agricultural Margins. Policy Stud. 2010, 31, 491–504. [Google Scholar] [CrossRef]
- Benessaiah, K.; Sengupta, R. How Is Shrimp Aquaculture Transforming Coastal Livelihoods and Lagoons in Estero Real, Nicaragua: The Need to Integrate Social–Ecological Research and Ecosystem-Based Approaches. Environ. Manag. 2014, 54, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.; D’Anna, L. Seeing Shellfish from the Seashore: The Importance of Values and Place in Perceptions of Aquaculture and Marine Social–Ecological System Interactions. Mar. Policy 2015, 62, 125–133. [Google Scholar] [CrossRef]
- Dalton, T.; Jin, D.; Thompson, R.; Katzanek, A. Using Normative Evaluations to Plan for and Manage Shellfish Aquaculture Development in Rhode Island Coastal Waters. Mar. Policy 2017, 83, 194–203. [Google Scholar] [CrossRef]
- Dempster, T.; Ecosystem, P. Aquaculture and Coastal Space Management in Europe: An Ecological Perspective; Holmer, K.B., Duarte, C.M., Marbà, N., Karakassis, I., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 47–85. [Google Scholar]
- Olsen, Y.; Otterstad, O.; Duarte, C.M. Status and Future Perspectives of Marine Aquaculture. In Aquaculture in the Ecosystem; Springer: Dordrecht, The Netherlands, 2008; pp. 293–319. [Google Scholar]
- Knapp, G.; Rubino, M.C. The Political Economics of Marine Aquaculture in the United States. Rev. Fish. Sci. Aquac. 2016, 24, 213–229. [Google Scholar] [CrossRef]
- Schlager, E.; Ostrom, E.; Schlager, E.; Ostrom, E. Property-Rights Regimes and Natural Resources: A Conceptual Analysis. Land Econ. 1992, 68, 249–262. [Google Scholar] [CrossRef]
- Anderson, J.A. Aquaculture and the Future: Why Fisheries Economists Should Care. Mar. Resour. Econ. 2002, 17, 133–157. [Google Scholar] [CrossRef]
- Guevara, L.E.T.; Schlüter, A.; Lopez, M.C. Collective Action in a Tropical Estuarine Lagoon: Adapting Ostrom’s SES Framework to Ciénaga Grande de Santa Marta, Colombia. Int. J. Commons 2016, 10, 334–362. [Google Scholar] [CrossRef]
- Johnson, T.R.; Hanes, S.P. Considering Social Carrying Capacity in the Context of Sustainable Ecological Aquaculture. In Towards Coastal Resilience and Sustainability; Routeledge: New York, NY, USA, 2018. [Google Scholar]
- Bostick, K. NGO Approaches to Minimizing the Impacts of Aquaculture: A Review. In Aquaculture in the Ecosystem; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 227–249. [Google Scholar] [CrossRef]
- Marshall, J. Landlords, Leaseholders and Sweat Equity: Changing Property Regimes in Aquaculture. Mar. Policy 2001, 25, 335–352. [Google Scholar] [CrossRef]
- Whitmarsh, D.; Palmieri, M.G. Consumer Behaviour and Environmental Preferences: A Case Study of Scottish Salmon Aquaculture. Aquac. Res. 2011, 42, 142–147. [Google Scholar] [CrossRef]
- Bush, S.R.; Belton, B.; Little, D.C.; Islam, M.S. Emerging Trends in Aquaculture Value Chain Research. Aquaculture 2019, 498, 428–434. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Dickson, M.W.; El-Naggar, G.O. Value Chain Analysis of the Aquaculture Feed Sector in Egypt. Aquaculture 2015, 437, 92–101. [Google Scholar] [CrossRef]
- Rickard, L.N.; Noblet, C.L.; Duffy, K.; Christian Brayden, W. Cultivating Benefit and Risk: Aquaculture Representation and Interpretation in New England. Soc. Nat. Resour. 2018, 31, 1358–1378. [Google Scholar] [CrossRef]
- Dalton, T.M.; Jin, D. Attitudinal Factors and Personal Characteristics Influence Support for Shellfish Aquaculture in Rhode Island (US) Coastal Waters. Environ. Manag. 2018, 61, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, H.E.; Gentry, R.R.; Rust, M.B.; Grimm, D.; Halpern, B.S. Public Perceptions of Aquaculture: Evaluating Spatiotemporal Patterns of Sentiment around the World. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.A.; Curtis, A.L. Understanding Community Perceptions of Aquaculture: Lessons from Australia. Aquac. Int. 2008, 16, 601–621. [Google Scholar] [CrossRef]
- Paget, N.; Daniell, K.A.; Rubio Zuazo, A.; Barreteau, O. Environmental Information Sharing: A Means to Support the Legitimization of Oyster Farmers’ Stewardship over Water Quality Management in NSW, Australia. Nat. Resour. Forum 2016, 40, 21–36. [Google Scholar] [CrossRef]
- Callaway, R.; Shinn, A.P.; Grenfell, S.E.; Bron, J.E.; Burnell, G.; Cook, E.J.; Crumlish, M.; Culloty, S.; Davidson, K.; Ellis, R.P.; et al. Review of Climate Change Impacts on Marine Aquaculture in the UK and Ireland. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 389–421. [Google Scholar] [CrossRef]
- Clements, J.C.; Chopin, T. Ocean Acidification and Marine Aquaculture in North America: Potential Impacts and Mitigation Strategies. Rev. Aquac. 2017, 9, 326–341. [Google Scholar] [CrossRef]
- Waldbusser, G.G.; Salisbury, J.E. Ocean Acidification in the Coastal Zone from an Organism’s Perspective: Multiple System Parameters, Frequency Domains, and Habitats. Ann. Rev. Mar. Sci. 2014, 6, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, J.; Green, M.; Hunt, C.; Campbell, J. Coastal Acidification by Rivers: A Threat to Shellfish? Eos Trans. Am. Geophys. Union 2008, 89, 513. [Google Scholar] [CrossRef]
- Testa, J.M.; Murphy, R.R.; Brady, D.C.; Kemp, W.M. Nutrient- and Climate-Induced Shifts in the Phenology of Linked Biogeochemical Cycles in a Temperate Estuary. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Jacobs, M.N.; Covaci, A.; Schepens, P. Investigation of Selected Persistent Organic Pollutants in Farmed Atlantic Salmon (Salmo Salar), Salmon Aquaculture Feed, and Fish Oil Components of the Feed. Environ. Sci. Technol. 2002, 36, 2797–2805. [Google Scholar] [CrossRef]
- Amlund, H.; Berntssen, M.H.G.; Lunestad, B.T.; Lundebye, A.K. Aquaculture Feed Contamination by Persistent Organic Pollutants, Heavy Metals, Additives and Drug Residues. In Animal Feed Contamination: Effects on Livestock and Food Safety; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 205–229. [Google Scholar] [CrossRef]
- Burreson, E.M.; Stokes, N.A.; Friedman, C.S. Increased Virulence in an Introduced Pathogen: Haplosporidium Nelsoni (MSX) in the Eastern Oyster Crassostrea Virginica. J. Aquat. Anim. Health 2000, 12. [Google Scholar] [CrossRef]
- Lee, D.J.; Gordon, R.M. Economics of aquaculture and invasive aquatic species—An overview. Aquac. Econ. Manag. 2006, 10, 83–96. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The Impact and Control of Biofouling in Marine Aquaculture: A Review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Bridger, C.; Garber, A. Aquaculture Escapement, Implications and Mitigation: The Salmonid Case Study. In Ecological Aquaculture: The Evolution of the Blue Revolution; Blackwell: Oxford, UK, 2002; pp. 77–102. [Google Scholar]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of Aquaculture on World Fish Supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Refstie, S.; Storebakken, T.; Roem, A. Feed Consumption and Conversion in Atlantic Salmon (Salmo Salar) Fed Diets with Fish Meal, Extracted Soybean Meal or Soybean Meal with Reduced Content Of. Aquaculture 1998, 162, 301–312. [Google Scholar] [CrossRef]
- Cleaver, C.; Johnson, T.R.; Hanes, S.P.; Pianka, K. From Fishers to Farmers: Assessing Aquaculture Adoption in a Training Program for Commercial Fishers. Bull. Mar. Sci. 2018, 94, 1215–1222. [Google Scholar] [CrossRef]
- Lester, S.E.; Stevens, J.M.; Gentry, R.R.; Kappel, C.V.; Bell, T.W.; Costello, C.J.; Gaines, S.D.; Kiefer, D.A.; Maue, C.C.; Rensel, J.E.; et al. Marine Spatial Planning Makes Room for Offshore Aquaculture in Crowded Coastal Waters. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.M.; McDonald, P.S.; Feinberg, D.S.; Hall, L.W.; Hamerly, J.G.; Wright, C.W. Digging Deep: Managing Social and Policy Dimensions of Geoduck Aquaculture Conflict in Puget Sound, Washington. Coast. Manag. 2017, 45, 73–89. [Google Scholar] [CrossRef]
- Hempel, E. Constraints and Possibilities for Developing Aquaculture. Aquac. Int. 1993, 1, 2–19. [Google Scholar] [CrossRef]
- Meltzoff, S.K.; Lemons, M.; Asfour, L.; Guardia-Montoya, G.; Gonzales, R. Sustaining El Nino-Induced Scallop Booms with Aquaculture. Cult. Agric. 2005, 27. [Google Scholar] [CrossRef]
- Galappaththi, E.K.; Kodithuwakku, S.S.; Galappaththi, I.M. Can Environment Management Integrate into Supply Chain Management? Information Sharing via Shrimp Aquaculture Cooperatives in Northwestern Sri Lanka. Mar. Policy 2016, 68, 187–194. [Google Scholar] [CrossRef]
- Ackefors, H. Review of Swedish Regulation and Monitoring of Aquaculture. J. Appl. Ichthyol. 2000, 16, 214–223. [Google Scholar] [CrossRef]
- Heaslip, R. Monitoring Salmon Aquaculture Waste: The Contribution of First Nations’ Rights, Knowledge, and Practices in British Columbia, Canada. Mar. Policy 2008, 32, 988–996. [Google Scholar] [CrossRef]
- Townsend, R.E.; Young, M.D. Evergreen Leasing of Aquaculture Sites. Mar. Resour. Econ. 2005, 20, 203–210. [Google Scholar] [CrossRef][Green Version]
- Byron, C.; Bengtson, D.; Costa-Pierce, B.; Calanni, J. Integrating Science into Management: Ecological Carrying Capacity of Bivalve Shellfish Aquaculture. Mar. Policy 2011, 35, 363–370. [Google Scholar] [CrossRef]
- Testa, J.M.; Brady, D.C.; Cornwell, J.C.; Owens, M.S.; Sanford, L.P.; Newell, C.R.; Suttles, S.E.; Newell, R.I.E. Modeling the Impact of Floating Oyster (Crassostrea Virginica) Aquaculture on Sediment-Water Nutrient and Oxygen Fluxes. Aquac. Environ. Interact. 2015, 7, 205–222. [Google Scholar] [CrossRef]
- Byron, C.J.; Jin, D.; Dalton, T.M. An Integrated Ecological–Economic Modeling Framework for the Sustainable Management of Oyster Farming. Aquaculture 2015, 447, 15–22. [Google Scholar] [CrossRef]
- Filgueira, R.; Comeaua, L.A.; Guyondeta, T.; McKindsey, C.W.; Byron, C.J. Modelling Carrying Capacity of Bivalve Aquaculture: A Review of Definitions and Methods. Encycl. Sustain. Sci. Technol. 2015, 33. [Google Scholar] [CrossRef]
- Folke, C. Resilience: The Emergence of a Perspective for Social–Ecological Systems Analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Ann. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Wang, X.; Olsen, L.M.; Reitan, K.I.; Olsen, Y. Discharge of Nutrient Wastes from Salmon Farms: Environmental Effects, and Potential for Integrated Multi-Trophic Aquaculture. Aquac. Environ. Interact. 2012, 2, 267–283. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Allison, E.H.; Andrew, N.L.; Oliver, J. Enhancing the Resilience of Inland Fisheries and Aquaculture Systems to Climate Change. J. SAT Agric. Res. 2007, 4, 1–35. [Google Scholar]
- Frank-Lawale, A.; Allen, S.K.; Dégremont, L. Breeding and Domestication of Eastern Oyster (Crassostrea Virginica) Lines for Culture in the Mid-Atlantic, Usa: Line Development and Mass Selection for Disease Resistance. J. Shellfish Res. 2014. [Google Scholar] [CrossRef]
- Rist, L.; Felton, A.; Nyström, M.; Troell, M.; Sponseller, R.A.; Bengtsson, J.; Österblom, H.; Lindborg, R.; Tidåker, P.; Angeler, D.G.; et al. Applying Resilience Thinking to Production Ecosystems. Ecosphere 2014. [Google Scholar] [CrossRef]
- Bush, S.R.; van Zwieten, P.A.M.; Visser, L.; van Dijk, H.; Bosma, R.; de Boer, W.F.; Verdegem, M. Scenarios for Resilient Shrimp Aquaculture in Tropical Coastal Areas. Ecol. Soc. 2010, 15, 26. [Google Scholar] [CrossRef]
- Adger, W.N. Social and Ecological Resilience: Are They Related? Prog. Hum. Geogr. 2000, 24, 347–364. [Google Scholar] [CrossRef]
- Cote, M.; Nightingale, A.J. Resilience Thinking Meets Social Theory. Prog. Hum. Geogr. 2012, 36, 475–489. [Google Scholar] [CrossRef]
- Marshall, N.A.; Marshall, P.A. Conceptualizing and Operationalizing Social Resilience within Commercial Fisheries in Northern Australia. Ecol. Soc. 2007, 12, 1. [Google Scholar] [CrossRef]
- Adger, W.N.; Kelly, P.M.; Winkels, A.; Huy, L.Q.; Locke, C. Migration, Remittances, Livelihood Trajectories, and Social Resilience. AMBIO J. Hum. Environ. 2002, 31, 358–366. [Google Scholar] [CrossRef]
- Irz, X.; Mckenzie, V. Profitability and Technical Efficiency of Aquaculture Systems in Pampaanga, Philippines. Aquac. Econ. Manag. 2003, 7, 195–211. [Google Scholar] [CrossRef]
- Iliyasu, A.; Mohamed, Z.A.; Ismail, M.M.; Abdullah, A.M.; Kamarudin, S.M.; Mazuki, H. A Review of Production Frontier Research in Aquaculture (2001-2011). Aquac. Econ. Manag. 2014, 18, 221–247. [Google Scholar] [CrossRef]
- Scuderi, B.; Chen, X. Production Efficiency in New England’s Oyster Aquaculture Industry. Aquac. Econ. Manag. 2018, 23. [Google Scholar] [CrossRef]
- Shumway, S.E.; Davis, C.; Downey, R.; Karney, R.; Kraeuter, J.; Parsons, J.; Rheault, R.; Wikfors, G. Shellfish Aquaculture—In Praise of Sustainable Economies and Environments. World Aquac. 2003, 34, 15–17. [Google Scholar] [CrossRef]
- Tveteras, R.; Battese, G.E. Agglomeration Externalities, Productivity, and Technical Inefficiency. J. Reg. Sci. 2006, 46, 605–625. [Google Scholar] [CrossRef]
- Bush, S.; Belton, B.; Hall, D.; Vandergeest, P.; Murray, F.J.; Oosterveer, P.; Islam, M.; Mol, A.; Hatanaka, M.; et al. Certify Sustainable Aquaculture? Science 2013, 341, 1067–1068. [Google Scholar] [CrossRef]
- Snyder, J.; Boss, E.; Weatherbee, R.; Thomas, A.C.; Brady, D.; Newell, C. Oyster Aquaculture Site Selection Using Landsat 8-Derived Sea Surface Temperature, Turbidity, and Chlorophyll A. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Krause, G.; Buck, B.H.; Breckwoldt, A. Socio-Economic Aspects of Marine Bivalve Production. In Goods and Services of Marine Bivalves; Springer International: Cham, Switzerland, 2019; pp. 317–334. [Google Scholar]
- Hertz, T.; Schlüter, M. The SES-Framework as Boundary Object to Address Theory Orientation in Social–Ecological System Research: The SES-TheOr Approach. Ecol. Econ. 2015, 116, 12–24. [Google Scholar] [CrossRef]
- Partelow, S. Coevolving Ostrom’s Social–Ecological Systems (SES) Framework and Sustainability Science: Four Key Co-Benefits. Sustain. Sci. 2016, 11, 399–410. [Google Scholar] [CrossRef]



| Resource System (RS) | Actors (A) |
| RS1—Resource Sector: Marine Aquaculture | A1—Number of relevant actors |
| RS2—Clarity of system boundaries | A2—Socioeconomic attributes |
| RS3—Size of resource system | A3—History or past experiences (of actors) |
| RS4—Human constructed facilities | A4—Location in relation to resource & market |
| RS5—Productivity | A5—Leadership/entrepreneurship |
| RS5.1 Stock status | A6—Norms/Social capital |
| RS5.2 Biophysical factors | A6.1 Trust and reciprocity |
| RS6—Predictability of the system | A7—Knowledge of SES/mental models |
| RS7—Connectivity | A8—Importance of resource |
| RS8—Location | A8.1—Economic importance of resource |
| A8.2—Cultural importance of resource | |
| A9—Characteristics of the technologies used | |
| Resource Units (RU) | Governance Systems (GS) |
| RU1—Mobility of the resource units | GS1—Policy area |
| RU2—Growth or replacement rate of RUs | GS2—Geographic range |
| RU3—Interaction among resource units | GS3—Size of Population |
| RU4—Economic value of the resource | GS4—Regime type |
| RU5—Number or size of units produced | GS5—Organizations |
| RU6—Distinctive characteristics | GS5.1 Government organizations |
| RU7—Spatial and temporal distribution | GS5.2 Non-government organizations |
| GS6—Rules-in-use | |
| GS6.1 Operational rules | |
| GS6.2 Collective choice rules | |
| GS6.3 Constitutional Rules | |
| GS7—Property rights | |
| GS8—Norms and strategies | |
| GS9—Network structure | |
| GS10—Historical continuity |
| Exogenous Influences | Focal Action Situation |
|---|---|
| Social, Economic, and Political Setting (S) | Interactions (I) |
| S1—Economic development trends | I1—Farming (Harvesting) |
| S2—Demographic trends | I2—Information sharing |
| S2.1 Urbanization | I3—Deliberative processes |
| S2.2 Gentrification | I4—Conflicts between/among actors |
| S3—Political stability | I5—Investment activities |
| S4—Non-local govt. org mandates | I6—Lobbying activities |
| S5—Markets | I7—Self-organizing activities |
| S5.1 Demand | I8—Networking activities |
| S5.2 Suppliers of industry inputs | I9—Monitoring and sanctioning activities |
| S6—Media | I10—Evaluation activities |
| S7—Technology available | |
| S8—Perceptions of other marine users | |
| Related Ecosystems (ECO) | Outcomes (O) |
| ECO1—Climate patterns | O1—Social performance measures |
| ECO1.1 Ocean acidification | O1.1.1 Economic carrying capacity |
| ECO2—Pollution Patterns | O1.2 Social resilience |
| ECO3—Flows into/out of focal system | O1.3 Efficiency |
| O1.3.1 Technical efficiency | |
| O1.3.2 Economic efficiency | |
| O1.3.3 Social efficiency | |
| O2—Ecological performance measures | |
| O2.1 Ecological carrying capacity | |
| O2.2 Ecological resilience | |
| O3—Externalities to other SESs |
| System Component | Variable Name | Variable Metric | Damariscotta | Bagaduce |
|---|---|---|---|---|
| Resource System | ||||
| RS1 | Resource Sector | Oyster aquaculture | ||
| RS3 | Size of RS/Physical carrying capacity | Area within RS optimal for oyster aquaculture | Higher 7.2 sq km2 | Lower 4.3 sq km2 |
| RS5 | Productivity | Chlorophyll levels | Higher m(3.1) med(2.7) | Lower m(2.2), med(1.9) |
| RS5.2 | Biophysical factors | Water Temperature | Warmer M(16.9) med(18.3) | Cooler M(15.6), med(15.8) |
| Actors | ||||
| A1 | # of Actors | # Standard Leases # of LPAs Population | 30 49 12,977 | 2 45 7820 |
| A3 | Actor history | # Years experience with aquaculture | <40 Years | <10 Years |
| Governance System | ||||
| GS1 | Policy Arena | Oyster Aquaculture | ||
| GS5.2 | NGOs | Nature of NGOs involvement in aquaculture | Supportive NGOs impacting discourse | Opposing NGOs impacting discourse |
| Social, Economic, and Political Setting | ||||
| S2.2 | Gentrification | Metrics from US Census (Hanes, 2018; Johnson & Hanes, 2018) | Similarly gentrified | Similarly gentrified |
| Interactions | ||||
| I1 | Farming | Standard Lease Area LPA Lease Area | More farming 177.83 0.49 | Less farming 9.5 0.45 |
| I4 | Conflicts | Level of conflicts at lease hearings (Hanes 2018) | Low | High |
| Outcomes | ||||
| O1.1 | Social carrying capacity | Inferred from conflicts and development patterns | Not yet exceeded | At or exceeded |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, T.R.; Beard, K.; Brady, D.C.; Byron, C.J.; Cleaver, C.; Duffy, K.; Keeney, N.; Kimble, M.; Miller, M.; Moeykens, S.; et al. A Social-Ecological System Framework for Marine Aquaculture Research. Sustainability 2019, 11, 2522. https://doi.org/10.3390/su11092522
Johnson TR, Beard K, Brady DC, Byron CJ, Cleaver C, Duffy K, Keeney N, Kimble M, Miller M, Moeykens S, et al. A Social-Ecological System Framework for Marine Aquaculture Research. Sustainability. 2019; 11(9):2522. https://doi.org/10.3390/su11092522
Chicago/Turabian StyleJohnson, Teresa R., Kate Beard, Damian C. Brady, Carrie J. Byron, Caitlin Cleaver, Kevin Duffy, Nicholas Keeney, Melissa Kimble, Molly Miller, Shane Moeykens, and et al. 2019. "A Social-Ecological System Framework for Marine Aquaculture Research" Sustainability 11, no. 9: 2522. https://doi.org/10.3390/su11092522
APA StyleJohnson, T. R., Beard, K., Brady, D. C., Byron, C. J., Cleaver, C., Duffy, K., Keeney, N., Kimble, M., Miller, M., Moeykens, S., Teisl, M., van Walsum, G. P., & Yuan, J. (2019). A Social-Ecological System Framework for Marine Aquaculture Research. Sustainability, 11(9), 2522. https://doi.org/10.3390/su11092522





