Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution
Abstract
1. Introduction
- −
- Growing, harvesting, and pre-processing;
- −
- Primary cocoa processing and production of semi-finished products; and
- −
- Chocolate industry—manufacturing of finished products [6].
2. Cocoa Shell
2.1. Use of Cocoa Shell in Food Production
2.2. Problems with Use of Cocoa Shell in Food Production
2.2.1. Mycotoxins
2.2.2. Heavy Metals
2.2.3. Polycyclic Aromatic Hydrocarbons (PAHs)
2.2.4. Microorganisms
3. High Voltage Electrical Discharge (HVED)
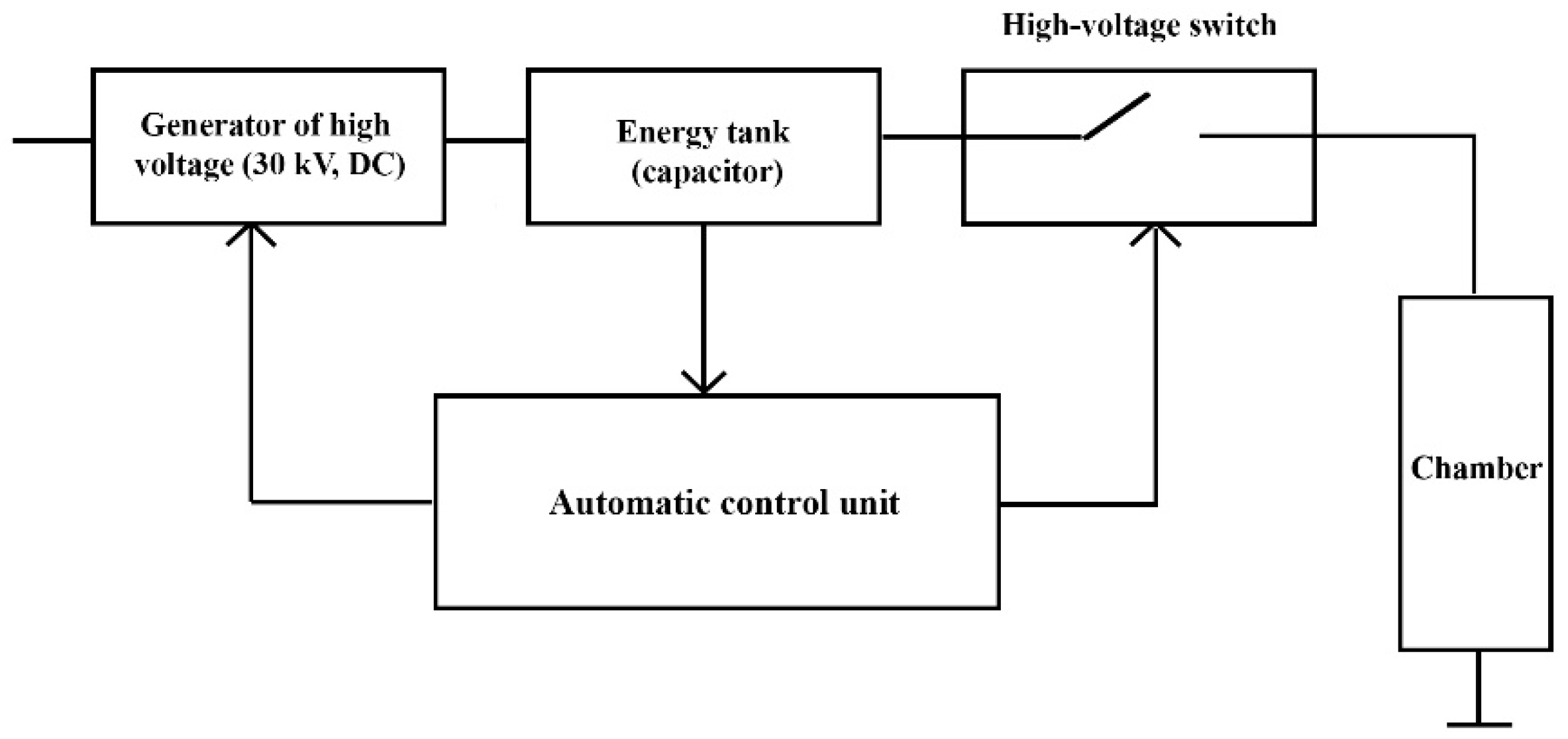
3.1. Work Principle of HVED Device
3.2. Description of HVED Device
3.3. HVED Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The International Cocoa Organization. The World Cocoa Economy: Past and Present; ICCO: London, UK, 2012. [Google Scholar]
- Ntiamoah, A.; Afrane, G. Environmental impacts of cocoa production and processing in Ghana: Life cycle assessment approach. J. Clean. Prod. 2008, 16, 1735–1740. [Google Scholar] [CrossRef]
- Gullón, P.; Gonzáles-Muñoz, M.J.; Parajó, J.C. Manufacture and prebiotic potential of oligosaccharides derived from industrial solid wastes. Bioresour. Technol. 2011, 102, 6112–6119. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef]
- Da Silva do Nascimento, M.; da Silva, N.; da Silva, I.F.; da Silva, J.d.C.; Marques, É.R.; Barbosa Santos, A.R. Eteropathogens in cocoa pre-processing. Food Control 2010, 21, 408–411. [Google Scholar] [CrossRef]
- Grymonpré, D.R.; Sharma, A.K.; Finney, W.C.; Locke, B.R. The role of Fenton’s reaction in aqueous phase pulsed streamer corona reactors. Chem. Eng. J. 2001, 82, 189–207. [Google Scholar] [CrossRef]
- Chen, C.-W.; Lee, H.-M.; Chang, M.-B. Influence of pH on inactivation of aquatic microorganism with a gas–liquid pulsed electrical discharge. J. Electrostat. 2009, 67, 703–708. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological approaches for cocoa waste management; A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Djali, M.; Siti Setiasih, I.; Rindiantika, T.S. Chemical characteristics, phytochemical and cacao shell toxicity changes during the processing of cocoa beans. Asian J. Agri. Biol. 2018, 6, 103–114. [Google Scholar]
- International Cocoa Organization. Production of Cocoa Beans; ICCO Quarterly Bulletin of Cocoa Statistics, XLII; ICCO: London, UK, 2016. [Google Scholar]
- Redgwell, R.; Trovato, V.; Merinat, S.; Curti, D.; Hediger, S.; Manez, A. Dietary fibre in cocoa shell: Characterization of component polysaccharides. Food Chem. 2003, 81, 103–112. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Barišić, V.; Flanjak, I.; Križić, I.; Jozinović, A.; Šubarić, D.; Babić, J.; Miličević, B.; Ačkar, Đ. Impact of high-voltage electric discharge treatment on cocoa shell phenolic components and methylxanthines. J. Food Process Eng. 2020, 43, e13057. [Google Scholar] [CrossRef]
- Rusconi, M.; Conti, A. Theobroma cacao L., the food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef]
- Vojvodić, A.; Komes, D.; Vovk, I.; Belščak-Cvitanović, A.; Bušić, A. Compositional evaluation of selected agro-industrial wastes as valuable sources for the recovery of complex carbohydrates. Food Res. Int. 2016, 89, 565–573. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorization; extraction from lab to pilot-scale cavitation reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluid 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Toda, T.A.; Oliveira, A.L.; Rodrigues, C.E.C. Effect of the temperature on the kinetics of cocoa bean shell fat extraction using pressurized ethanol and evaluation of the lipid fraction and defatted meal. Ind. Crops. Prod. 2019, 130, 96–103. [Google Scholar] [CrossRef]
- Martínez-Cervera, S.; Salvador, A.; Muguerza, B.; Moulay, L.; Fiszman, S.M. Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT 2011, 44, 729–736. [Google Scholar] [CrossRef]
- Collar, C.; Rosell, C.M.; Muguerza, B.; Moulay, L. Breadmaking performance and keeping behavior of cocoa-soluble fiber-enriched wheat breads. Food Sci. Technol. Int. 2009, 15, 79–87. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J. In vitro hypoglycemic and cholesterol lowering effects of dietary fiber prepared from cocoa (Theobroma cacao L.) shells. Food Funct. 2012, 3, 1044. [Google Scholar] [CrossRef]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Coïsson, J.D.; Arlorio, M. Cocoa hulls polyphenols stabilized by microencapsulation as functional ingredient for bakery applications. Food Res. Int. 2019, 115, 511–518. [Google Scholar] [CrossRef]
- Bernaert, H.; Ruysscher, I.D. Process, Use, and Product. U.S. Patent 2016135478A1, 27 January 2016. [Google Scholar]
- Bernaert, H.; Ruysscher, I.D. Process of Producing Cocoa Shell Powder. U.S. Patent 9,375,024B2, 26 June 2013. [Google Scholar]
- Handojo, L.; Triharyogi, H.; Indarto, A. Cocoa bean shell waste as potential raw material for dietary fiber powder. Int. J. Recycl. Org. Waste Agric. 2019, 8, 485–491. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G. Effects of Particle Size and Extraction Methods on Cocoa Bean Shell Functional Beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef]
- Choi, J.; Kim, N.; Choi, H.Y.; Han, Y.S. Effect of Cacao Bean Husk Powder on the Quality Properties of Pork Sausages. Food Sci. Anim. Resour. 2019, 39, 742–755. [Google Scholar] [CrossRef]
- Jozinović, A.; Panak Balentić, J.; Ačkar, Đ.; Babić, J.; Pajin, B.; Miličević, B.; Guberac, S.; Vrdoljak, A.; Šubarić, D. Cocoa husk application in the enrichment of extruded snack products. J. Food Process Preserv. 2019, 43, e13866. [Google Scholar] [CrossRef]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Jokić, S.; Ačkar, Đ. Effect of addition of fibers and polyphenols on properties of chocolate—A review. Food Rev. Int. 2019. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Nester, M.A.; Efraim, P.; Taniwaki, M.H. Occurrence of ochratoxin A in cocoa by-products and determination of its reduction during chocolate manufacture. Food Chem. 2013, 136, 100–104. [Google Scholar] [CrossRef]
- Brera, C.; Debegnach, F.; De Santis, B.; Iafrate, E.; Pannunzi, E.; Berdini, C.; Prantera, E.; Gregori, E.; Miraglia, M. Ochratoxin A in cocoa and chocolate products from the Italian market: Occurrence and exposure assessment. Food Cont. 2011, 22, 1663–1667. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Pereira, J.L.; Lemes, D.P.; Nakano, F.; Taniwaki, M.H. Determination of aflatoxins in by-products of industrial processing of cocoa beans. Food Addit. Contam. Part A 2012, 29, 972–978. [Google Scholar] [CrossRef]
- Bonvehi, J.S. Occurrence of ochratoxin A in cocoa products and chocolate. J. Agric. Food Chem. 2004, 52, 6347–6352. [Google Scholar] [CrossRef]
- Amézqueta, S.; Gonzalez-Penas, E.; Murillo, M.; de Cerain, A.L. Occurrence of ochratoxin A in cocoa beans: Effect of shelling. Food Addit. Contam. 2005, 22, 590–596. [Google Scholar] [CrossRef]
- Assa, A.; Noor, A.; Yunus, M.R.; Djide, M.N. Heavy metal concentrations in cocoa beans (Theobroma cacao L.) originating from East Luwu, South Sulawesi, Indonesia. J. Phys. Conf. Ser. 2018, 979, 12011. [Google Scholar] [CrossRef]
- Rankin, C.W.; Nriagu, J.O.; Aggarwal, J.K.; Arowolo, T.A.; Adebayo, K.; Flegal, A.R. Lead contamination in cocoa and cocoa products: Isotopic evidence of global contamination. Environ. Health Perspect. 2005, 113, 1344–1348. [Google Scholar] [CrossRef]
- Dahiya, S.; Karpe, R.; Hegde, A.; Sharma, R.M. Lead, cadmium, and nickel in chocolates and candies from suburban areas of Mumbai India. J. Food Compost. Anal. 2005, 18, 517–522. [Google Scholar] [CrossRef]
- Aikpopodion, P.E.; Odule, A.; Osobamiro, O.C.; Oduwole, T.; Ademola, S.M. A survey of copper, lead, cadmium and zinc residues in cocoa beans obtained from selected plantations in Nigeria. J. Chem. Pharm. Res. 2013, 56, 88–98. [Google Scholar]
- Kruszewski, B.; Obiedziński, M.W.; Kowalskad, J. Nickel, cadmium and lead levels in raw cocoa and processed chocolate mass materials from three different manufacturers. J. Food Compos. Anal. 2018, 66, 127–135. [Google Scholar] [CrossRef]
- Meunier, N.; Laroulandie, J.; Blais, J.F.; Tyagi, R.D. Lead Removal from Acidic Solutions by Sorption on Cocoa Shells: Effect of Some Parameters. J. Environ. Eng. 2003, 129, 693–698. [Google Scholar] [CrossRef]
- Meunier, N.; Blais, J.F.; Tyagi, R.D. Removal of heavy metals from acid soil leachate using cocoa shells in a batch counter-current sorption process. Hydrometallurgy 2004, 73, 225–235. [Google Scholar] [CrossRef]
- Scientific Committee on Food. Polycyclic Aromatic Hydrocarbons—Occurrence in Foods, Dietary Exposure and Health Effects. Report No. SCF/CS/CNTM/PAH/29 Add1 Final. 4 December 2002. Available online: http://ec.europa.eu.int/comm/food/fs/sc/scf/index_en.html (accessed on 27 February 2020).
- Ciecierska, M. Cocoa beans of different origins and varieties and their derived products contamination with polycyclic aromatic hydrocarbons. Food Chem. 2020, 317, 126408. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, G.; Wang, X.; Liu, X.; Liu, B. Formation of benzo[a]pyrene in sesame seeds during the roasting process for production of sesame seed oil. J. Am. Oil Chem. Soc. 2015, 92, 1725–1733. [Google Scholar] [CrossRef]
- Singh, S.G. Polycyclic Aromatic Hydrocarbons formed during Roasting Process in Arabica Coffee Beans; Thapar University: Punjab, India, 2013. [Google Scholar]
- Misnawi, J. Effect of cocoa bean drying methods on polycyclic aromatic hydrocarbons contamination in cocoa butter. Int. Food Res. J. 2012, 19, 1589–1594. [Google Scholar]
- Agus, B.A.P.; Hussain, N.; Selamat, J. Quantification of PAH4 in Roasted Cocoa Beans Using QuEChERS and Dispersive Liquid-Liquid Micro-extraction (DLLME) Coupled with HPLC-FLD. Food Chem. 2019, 125398. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, W.P.; Komitopoulou, E. Effect of moisture on salmonella spp. heat resistance in cocoa and hazelnut shells. Food Res. Int. 2012, 45, 1087–1092. [Google Scholar] [CrossRef]
- do Nascimento, M.D.S.; Pena, P.O.; Brum, D.M.; Imazaki, F.T.; Tucci, M.L.S.; Efraim, P. Behavior of Salmonella during fermentation, drying and storage of cocoa beans. Int. J. Food Microbiol. 2013, 167, 363–368. [Google Scholar] [CrossRef]
- Barba, F.J.; Puértolas, E.; Brncic, M.; Panchev, I.N.; Dimitrov, D.A.; Athes-Dutour, V.; Mousaa, M.; Souchon, I. Food Waste Recovery Processing Technologies and Industrial Techniques; Galanakis, C.M., Ed.; Elsevier: Cambridge, MA, USA, 2015; pp. 249–272. [Google Scholar]
- Boussetta, N.; Vorobiev, E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Xi, J. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 2019, 277, 246–260. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. High voltage electrical discharges combined with enzymatic hydrolysis for extraction of polyphenols and fermentable sugars from orange peels. Food Res. Int. 2018, 107, 755–762. [Google Scholar] [CrossRef]
- Takaki, K.; Hayashi, N.; Wang, D.; Ohshima, T. High-voltage technologies for agriculture and food processing. J. Phys. D Appl. Phys. 2019, 52. [Google Scholar] [CrossRef]
- Cathignol, D.; Mestas, J.L.; Gomez, F.; Lenz, P. Influence of water conductivity on the efficiency and the reproducibility of electrohydraulic shock wave generation. Ultrasound Med. Biol. 1991, 17, 819–828. [Google Scholar] [CrossRef]
- Boussetta, N.; Lebovka, N.; Vorobiev, E.; Adenier, H.; Bedel-Cloutour, C.; Lanoisellé, J.L. Electrically assisted extraction of soluble matter from chardonnay grape skins for polyphenol recovery. J. Agric. Food Chem. 2009, 57, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Grimi, N.; Vorobiev, E. Evaluating the potential of cell disruption technologies for green selective extraction of antioxidant compounds from Stevia rebaudiana Bertoni leaves. J. Food Eng. 2015, 149, 222–228. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold Plasma Decontamination of Foods. Annu. Rev. Food. Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xia, Y.; Xi, T.; Zhu, D.; Zhang, Q.; Qi, Z.; Liu, D.; Wang, W. Control of pathogenic bacteria on the surface of rolling fruits by an atmospheric pressure air dielectric barrier discharge system. J. Phys. D Appl. Phys. 2020, 53. [Google Scholar] [CrossRef]
- Gavahian, M.; Cullen, P.J. Cold Plasma as an Emerging Technique for Mycotoxin-Free Food: Efficacy, Mechanisms, and Trends. Food Rev. Int. 2020, 36, 1–22. [Google Scholar] [CrossRef]
- Anpilov, A.M.; Barkhudarov, E.M.; Christofi, N.; Kopev, V.A.; Kossyi, I.A.; Taktakishvili, M.I.; Zadiraka, Y. 2002—Pulsed high voltage electric discharge disinfection of microbially contaminated liquids. Lett. Appl. Microbiol. 2002, 35, 90–94. [Google Scholar] [CrossRef]
- Stulić, V.; Vukušić, T.; Jambrak, A.R.; Bačun-Družina, V.; Popović, D.; Mrvčić, J.; Herceg, Z. Quantitative microbial assessment for Escherichia coli after treatment by high voltage gas phase plasma. Innov. Food Sci. Emerg. Technol. 2019, 53, 26–35. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L. Low temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Chen, C.W.; Lee, H.-M.; Chang, M.B. Inactivation of Aquatic Microorganisms by Low-Frequency AC Discharges. IEEE Trans. Plasma Sci. 2008, 36, 215–219. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Ho, P.Q.; Pham, T.V.; Nguyen, T.V.; Kim, L. Treatment of surface water using cold plasma for domestic water supply. Environ. Eng. Res. 2019, 24, 412–417. [Google Scholar] [CrossRef]
- Barišić, V.; Flanjak, I.; Tot, A.; Budeč, M.; Benšić, M.; Jozinović, A.; Babić, J.; Šubarić, D.; Miličević, B.; Ačkar, Đ. 5-Hydroxymethylfurfural and Acrylamide Content of Cocoa Shell Treated with High Voltage Electrical Discharge. Food Cont. 2020, 110, 107043. [Google Scholar] [CrossRef]
- Tessier, D.; Oguic, C.; Pinart, J.; Aaron, J.J. Usefulness of a Technique Based on Negative Corona Discharge for the Degradation of Selected, Condensed PAHs: Application to the Oxidation of Anthracene and Similar Structures. Turk. J. Chem. 2001, 25, 157–164. [Google Scholar]
- Wang, C.Y.; Hsu, C.C. Characterization of plasma in aqueous solution using bipolar pusled power: Tailoring plasma and optical emission with implication for detecting lead. Plasma Process Polym. 2019, e1900159. [Google Scholar] [CrossRef]
- Ceccato, P. Filamentary Plasma Discharge inside Water: Initiation and Propagation of a Plasma in a Dense Medium. Ph.D. Thesis, École Polytechnique, Palaiseau, France, 2009. [Google Scholar]
- Grymonpré, D.R.; Finney, W.C.; Locke, B.R. Aqueous-phase pulsed streamer corona reactor using suspended activated carbon particles for phenol oxidation: Model-data comparison. Chem. Eng. Sci. 1999, 54, 3095–3105. [Google Scholar] [CrossRef]
- Sun, B.; Sato, M.; Clements, J.S. Use of a pulsed high-voltage discharge for removal of organic compounds in aqueous solution. J. Phys. D Appl. Phys. 1999, 32, 1908–1915. [Google Scholar] [CrossRef]
- Du, C.M.; Yan, J.H.; Li, X.D.; Cheron, B.G.; You, X.F.; Chi, Y.; Ni, M.J.; Cen, K.F. Simultaneous Removal of Polycyclic Aromatic Hydrocarbons and Soot Particles from flue Gas by Gliding arc Discharge Treatment. Plasma Chem. Plasma Process. 2006, 26, 517–525. [Google Scholar] [CrossRef]
- Ouf, S.A.; Basher, A.H.; Mohamed, A.A.H. Inhibitory Effect of Double Atmospheric Pressure Argon Cold Plasma on Spores and Mycotoxin Production of Aspergillus Niger Contaminating Date Palm Fruits. J. Sci. Food Agric. 2015, 95, 3204–3210. [Google Scholar] [CrossRef]
- Park, B.J.; Takatori, K.; Sugita-Konishi, Y.; Kim, I.H.; Lee, M.H.; Han, D.W.; Chung, K.H.; Hyun, S.O.; Park, J.C. Degradation of Mycotoxins Using Microwave-Induced Argon Plasma at Atmospheric Pressure. Surf. Coat. Technol. 2007, 201, 5733–5737. [Google Scholar] [CrossRef]
- Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viöl, W.; Karlovsky, P.; Ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef]
- Dasan, B.G.; Mutlu, M.; Boyaci, I.H. Decontamination of Aspergillus Flavus and Aspergillus Parasiticus Spores on Hazelnuts via Atmospheric Pressure Fluidized Bed Plasma Reactor. Int. J. Food Microbiol. 2016, 216, 50–59. [Google Scholar] [CrossRef]
- Grinevich, V.I.; Kvitkova, E.Y.; Plastinina, N.A.; Rybkin, V.V. Application of Dielectric Barrier Discharge for Waste Water Purification. Plasma Chem. Plasma Process. 2011, 201131, 573–583. [Google Scholar] [CrossRef]
- Rincón, G.J.; La Motta, E.J. Simultaneous removal of oil and grease, and heavy metals from artificial bilge water using electro-coagulation/flotation. J. Environ. Manag. 2014, 144, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Pavlović, N.; Jozinović, A.; Ačkar, Đ.; Babić, J.; Šubarić, D. High-Voltage Electric Discharge Extraction of Bioactive Compounds from the Cocoa Bean Shell. Chem. Biochem. Eng. Q. 2019, 33, 271–280. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Fan, Y.; Xi, J. Kinetic modeling for high voltage electrical discharge extraction based on discharge energy input. Food Chem. 2020, 314, 126168. [Google Scholar] [CrossRef]
- Almohammed, F.; Koubaa, M.; Khelfa, A.; Nakaya, M.; Mhemdi, H.; Vorobiev, E. Pectin recovery from sugar beet pulp enhanced by high-voltage electrical discharges. Food Bioprod. Process. 2017, 103, 95–103. [Google Scholar] [CrossRef]
- Žuntar, I.; Putnik, P.; Bursać Kovačević, D.; Nutrizio, M.; Šupljika, F.; Poljanec, A.; Dubrović, I.; Barba, J.F.; Režek Jambrak, A. Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obained by High Voltage Electrical Discharges (HVED). Foods 2019, 8, 248. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Le, L.H.; Cordin-Falcimaigne, A.; Lanoisellé, J.L. Application of electrical treatments in alcoholic solvent for polyphenols extraction from grape seeds. LWT 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-compounds from Fermented Grape Pomace. Food Bioproc. Tech. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Ačkar, Đ. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability 2020, 12, 3981. https://doi.org/10.3390/su12103981
Barišić V, Jozinović A, Flanjak I, Šubarić D, Babić J, Miličević B, Doko K, Ačkar Đ. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability. 2020; 12(10):3981. https://doi.org/10.3390/su12103981
Chicago/Turabian StyleBarišić, Veronika, Antun Jozinović, Ivana Flanjak, Drago Šubarić, Jurislav Babić, Borislav Miličević, Kristina Doko, and Đurđica Ačkar. 2020. "Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution" Sustainability 12, no. 10: 3981. https://doi.org/10.3390/su12103981
APA StyleBarišić, V., Jozinović, A., Flanjak, I., Šubarić, D., Babić, J., Miličević, B., Doko, K., & Ačkar, Đ. (2020). Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability, 12(10), 3981. https://doi.org/10.3390/su12103981








