Design of Appropriate Technology-Assisted Urine Tester Enabling Remote and Long-Term Monitoring of Health Conditions
Abstract
:1. Introduction
2. Materials and Methods
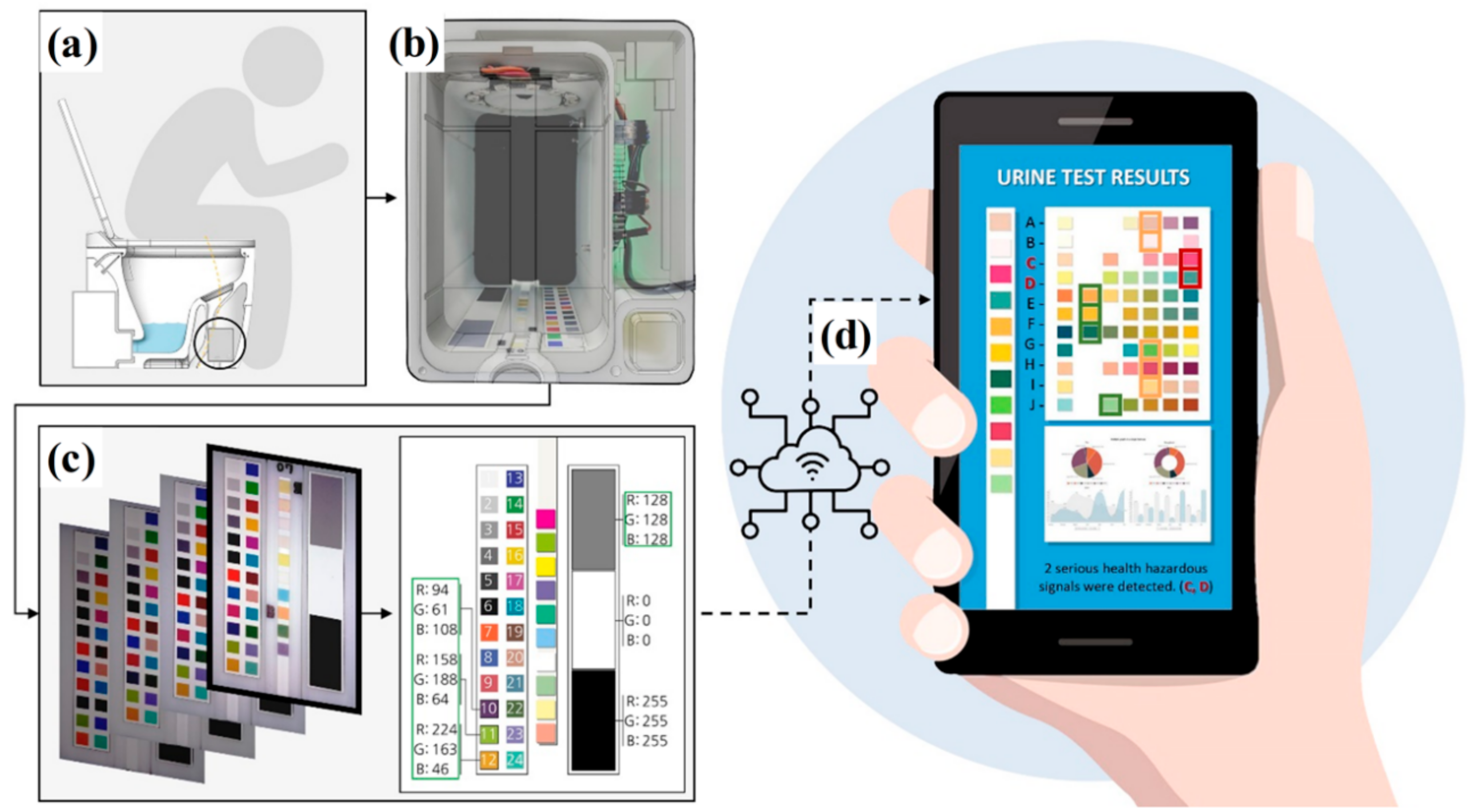
2.1. Conceptual Design of Urine Tester
2.2. Product Development of Urine Tester
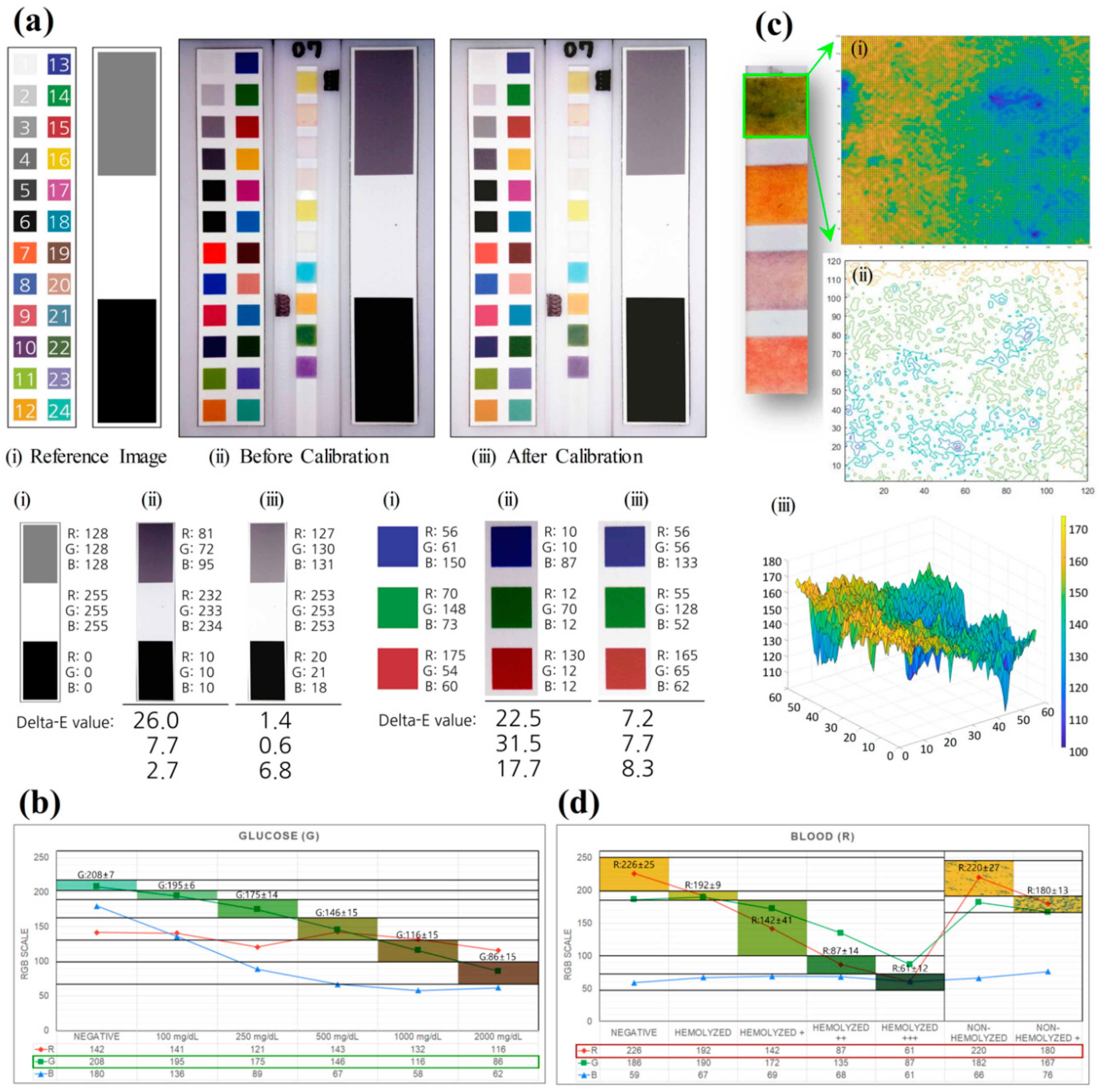
2.3. Color Calibration
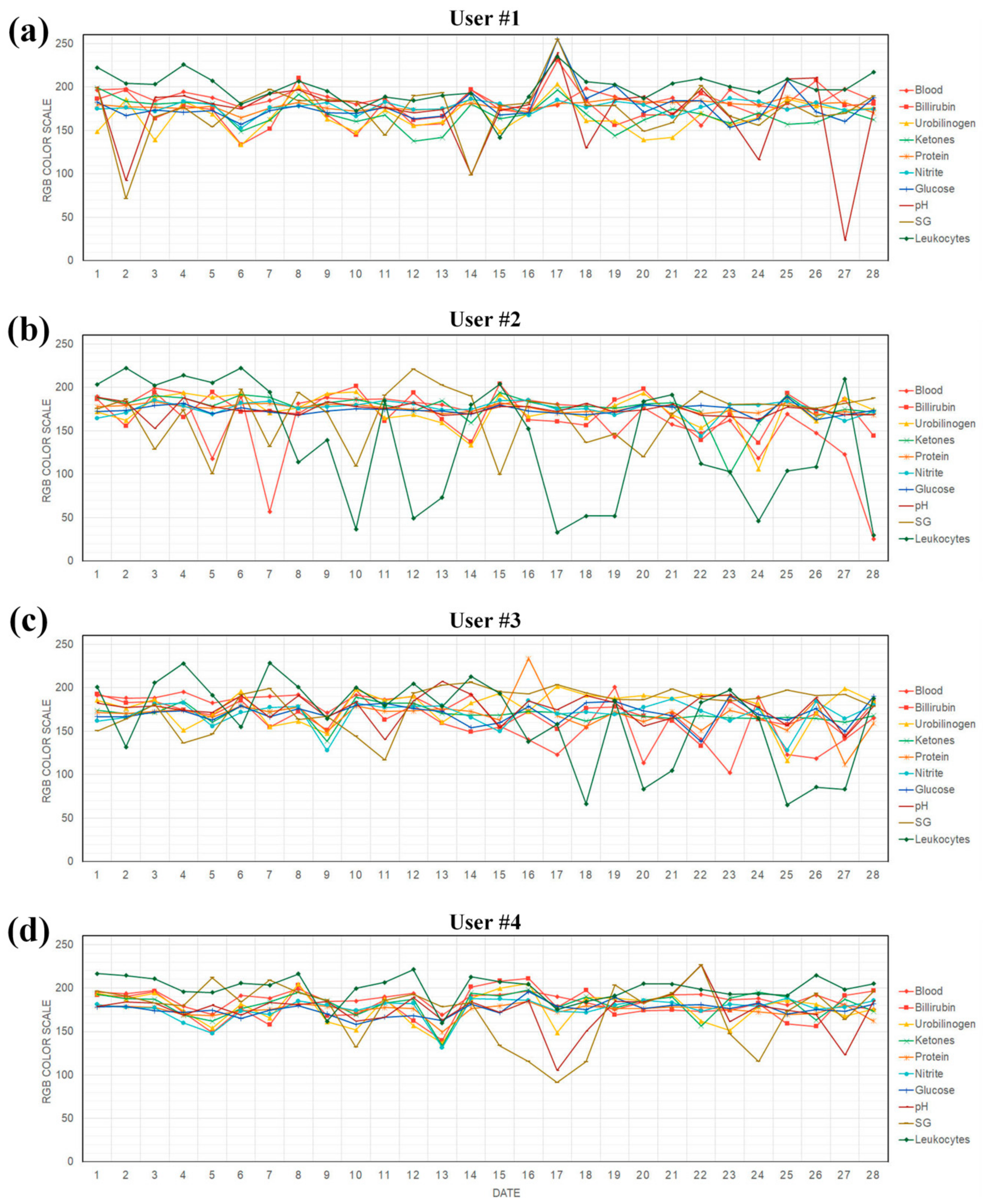
2.4. Test Procedure of Users
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Powell, A. The Costs of Inequality: Money = Quality Health Care = Longer Life; Harvard Gazette: Cambridge, MA, USA, 2016. [Google Scholar]
- Woolf, S.H. How Are Income and Wealth Linked to Health and Longevity? Urban Institute: Washington, DC, USA, 2015. [Google Scholar]
- Wasserman, J.; Palmer, R.C.; Gomez, M.M.; Berzon, R.; Ibrahim, S.A.; Ayanian, J.Z. Advancing Health Services Research to Eliminate Health Care Disparities. Am. J. Public Heal. 2019, 109, S64–S69. [Google Scholar] [CrossRef]
- Marmot, M.; Allen, J.J. Social determinants of health equity. Soc. Determ. Health Equity 2014, S517–S519. [Google Scholar] [CrossRef]
- Ayanian, J.Z.; Landon, B.E.; Newhouse, J.P.; Zaslavsky, A.M. Racial and ethnic disparities among enrollees in Medicare Advantage plans. New Engl. J. Med. 2014, 371, 2288–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glied, S.; Lleras-Muney, A. Health Inequality, Education and Medical Innovation. Health Inequal. Educ. Med. Innov. 2003, 9738, 741–761. [Google Scholar] [CrossRef]
- Zimmerman, F.J.; Anderson, N.W. Trends in Health Equity in the United States by Race/Ethnicity, Sex, and Income, 1993-2017. JAMA Netw. Open 2019, 2, e196386. [Google Scholar] [CrossRef] [PubMed]
- Fiscella, K.A.; Franks, P.; Gold, M.R.; Clancy, C.M. Inequality in quality: Addressing socioeconomic, racial, and ethnic disparities in health care. JAMA 2000, 283, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Ilinca, S.; Di Giorgio, L.; Salari, P.; Chuma, J. Socio-economic inequality and inequity in use of health care services in Kenya: Evidence from the fourth Kenya household health expenditure and utilization survey. Int. J. Equity Heal. 2019, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, O. Access to health care in developing countries: Breaking down demand side barriers. Cad. Saúde Pública 2007, 23, 2820–2834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwatkin, D.R.; Bhuiya, A.; Victora, C.G. Making health systems more equitable. Lancet 2004, 364, 1273–1280. [Google Scholar] [CrossRef]
- Ensor, T.; Cooper, S. Overcoming barriers to health service access: Influencing the demand side. Heal. Policy Plan. 2004, 19, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.; Aghayi, E.; Noferesti, M.; Memarzadeh-Tehran, H.; Mondal, T.K.; Pang, Z.; Deen, J. Smart Homes for Elderly Healthcare—Recent Advances and Research Challenges. Sensors 2017, 17, 2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, E.; Kun, J.; Smieja, M.; Fang, Q. Review—Point-of-Care Urinalysis with Emerging Sensing and Imaging Technologies. J. Electrochem. Soc. 2020, 167, 037518. [Google Scholar] [CrossRef]
- Park, S.-M.; Won, D.D.; Lee, B.J.; Escobedo, D.; Esteva, A.; Aalipour, A.; Ge, T.J.; Kim, J.H.; Suh, S.; Choi, E.H.; et al. A mountable toilet system for personalized health monitoring via the analysis of excreta. Nat. Biomed. Eng. 2020, 4, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Akraa, S.; Tam, A.P.T.; Shen, H.; Tang, Y.; Tang, B.Z.; Li, J.S.J.; Walker, S.; Pham, T.T.A. A smartphone-based point-of-care quantitative urinalysis device for chronic kidney disease patients. J. Netw. Comput. Appl. 2018, 115, 59–69. [Google Scholar] [CrossRef]
- Lepowsky, E.; Ghaderinezhad, F.; Knowlton, S.; Tasoglu, S. Paper-based assays for urine analysis. Biomicrofluidics 2017, 11, 051501. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.D. Intermediate Technology and Appropriate Technology. In Appropriate Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1978; pp. 40–54. [Google Scholar]
- Roberts, J. Supporting medical technology in developing countries—What is appropriate? IEE Semin. Appropr. Med Technol. Dev. Ctries. 2000, 2000, 2. [Google Scholar] [CrossRef]
- Crilly, N. The roles that artefacts play: Technical, social and aesthetic functions. Des. Stud. 2010, 31, 311–344. [Google Scholar] [CrossRef] [Green Version]
- Newton, R.; Mytton, O.T.; Aggarwal, R.; Runciman, W.B.; Free, M.; Fahlgren, B.; Akiyama, M.; Farlow, B.; Yaron, S.; Locke, G.; et al. Making existing technology safer in healthcare. Qual. Saf. Heal. Care 2010, 19, i15–i24. [Google Scholar] [CrossRef] [Green Version]
- Roth, S. The State of Design Research. Des. Issues 1999, 15, 18. [Google Scholar] [CrossRef]
- Desmet, P.M.A.; Hekkert, P. Framework of product experience. Int. J. Des. 2007, 1, 57–66. [Google Scholar]
- Elsanousi, S.; Abdelrahman, S.; Elshiekh, I.; Elhadi, M.; Mohamadani, A.; Habour, A.; Elamin, S.E.; Elnoury, A.; Ahmed, E.A.; Hunter, P.R. A study of the use and impacts of LifeStraw™ in a settlement camp in southern Gezira, Sudan. J. Water Heal. 2009, 7, 478–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhamla, M.S.; Benson, B.; Chai, C.; Katsikis, G.; Johri, A.; Prakash, M. Hand-powered ultralow-cost paper centrifuge. Nat. Biomed. Eng. 2017, 1, 9. [Google Scholar] [CrossRef]
- Wooster, M.M. The Spectacular Failure of One Laptop Per Child; Philanthropy Daily: Washington, DC, USA, 2018. [Google Scholar]
- Taha, A.S.; Grant, V.; Kelly, R.W. Urinalysis for interleukin-8 in the non-invasive diagnosis of acute and chronic inflammatory diseases. Postgrad. Med. J. 2003, 79, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.B.; Owen, J.; Raymond-Barker, P.; Bishop, C.; Elghenzai, S.; Oliver, S.J.; Walsh, N.P. Is This Elderly Patient Dehydrated? Diagnostic Accuracy of Hydration Assessment Using Physical Signs, Urine, and Saliva Markers. J. Am. Med Dir. Assoc. 2015, 16, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, G.D. Basic Urinalysis. JAMA 1979, 242, 2802. [Google Scholar] [CrossRef]
- Tetrault, G.A. Automated Reagent Strip Urinalysis: Utility in Reducing Work Load of Urine Microscopy and Culture. Lab. Med. 1994, 25, 162–167. [Google Scholar] [CrossRef]
- Sreemathy, V.; Dandekar, S.P. Nutrition and Biochemistry for Nurses, 2nd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Chao, M.-R.; Shih, Y.-M.; Hsu, Y.-W.; Liu, H.-H.; Chang, Y.-J.; Lin, B.-H.; Hu, C.-W. Urinary nitrite/nitrate ratio measured by isotope-dilution LC–MS/MS as a tool to screen for urinary tract infections. Free. Radic. Boil. Med. 2016, 93, 77–83. [Google Scholar] [CrossRef]
- He, X.; Pei, Q.; Xu, T.; Zhang, X. Smartphone-based tape sensors for multiplexed rapid urinalysis. Sens. Actuators B Chem. 2020, 304, 127415. [Google Scholar] [CrossRef]
- Kumar, Y.; Sood, K.; Kaul, S.; Vasuja, R. Big Data Analytics and Its Benefits in Healthcare. In Studies in Big Data; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 3–21. [Google Scholar]
- Mohapatra, S.; Mohanty, S.; Mohanty, S. Smart Healthcare: An Approach for Ubiquitous Healthcare Management Using IoT. In Big Data Analytics for Intelligent Healthcare Management; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 175–196. [Google Scholar]
- Bhatt, Y.; Bhatt, C. Internet of things in healthcare. Springer Cham. 2017, 23, 13–33. [Google Scholar]
- Chettri, L.; Bera, R. A Comprehensive Survey on Internet of Things (IoT) Toward 5G Wireless Systems. IEEE Internet Things J. 2020, 7, 16–32. [Google Scholar] [CrossRef]
- Yang, N.; Zhao, X.; Zhang, H. A non-contact health monitoring model based on the Internet of things. Inbiosensors. In Proceedings of the 2012 8th International Conference on Natural Computation, Chongqing, China, 29–31 May 2012; pp. 506–510. [Google Scholar]
- Lee, B.M. Design Requirements for IoT Healthcare Model using an Open IoT Platform. Computer 2014, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, K.-Y.; Kwon, O. Comparison of machine learning algorithms to predict psychological wellness indices for ubiquitous healthcare system design. In Proceedings of the 2014 International Conference on Innovative Design and Manufacturing (ICIDM), Montreal, QC, Canada, 13–15 August 2014; pp. 263–269. [Google Scholar]
- Sahoo, P.K.; Mohapatra, S.K.; Wu, S.-L. Analyzing Healthcare Big Data with Prediction for Future Health Condition. IEEE Access 2016, 4, 9786–9799. [Google Scholar] [CrossRef]
- Ta, V.-D.; Liu, C.-M.; Nkabinde, G.W. Big data stream computing in healthcare real-time analytics. In Proceedings of the 2016 IEEE International Conference on Cloud Computing and Big Data Analysis (ICCCBDA), Chengdu, China, 5–7 July 2016; pp. 37–42. [Google Scholar]
- Bora, D.J. Big Data Analytics in Healthcare: A Critical Analysis. In Big Data Analytics for Intelligent Healthcare Management; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 43–57. [Google Scholar]
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward Flexible Surface-Enhanced Raman Scattering (SERS) Sensors for Point-of-Care Diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Jeong, H.; Cho, K.W.; Lu, N.; Kim, D.-H. Wearable and Implantable Devices for Cardiovascular Healthcare: From Monitoring to Therapy Based on Flexible and Stretchable Electronics. Adv. Funct. Mater. 2019, 29, 1808247. [Google Scholar] [CrossRef]
- Park, S.; Seo, H.; Cha, S.; Noh, J. Auto-calibration of multi-projector displays with a single handheld camera. In Proceedings of the 2015 IEEE Scientific Visualization Conference (SciVis), Chicago, IL, USA, 25–30 October 2015; pp. 65–72. [Google Scholar]
- Kumar, M.; Powduri, P.; Reddy, A. An RGB image encryption using diffusion process associated with chaotic map. J. Inf. Secur. Appl. 2015, 21, 20–30. [Google Scholar] [CrossRef]
- Bui, T.; Vu, T.T.; Hong, K.-S. Extraction of sparse features of color images in recognizing objects. Int. J. Control. Autom. Syst. 2016, 14, 616–627. [Google Scholar] [CrossRef]
- Tyagi, V. Content-Based Image Retrieval Using Integrated Color, Texture, and Shape Features. In Content-Based Image Retrieval; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; pp. 257–271. [Google Scholar]
- Bae, J.-H.; Lee, H.-K. User Health Information Analysis with a Urine and Feces Separable Smart Toilet System. IEEE Access 2018, 6, 78751–78765. [Google Scholar] [CrossRef]
- Murillo-Escobar, M.; Cruz-Hernández, C.; Abundiz-Pérez, F.; Gutierrez, R.M.L.; Del Campo, O.A. A RGB image encryption algorithm based on total plain image characteristics and chaos. Signal Process. 2015, 109, 119–131. [Google Scholar] [CrossRef]
- Komatsu, T.; Mohammadi, S.; Busa, L.S.A.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Image analysis for a microfluidic paper-based analytical device using the CIE L*a*b* color system. Anal. 2016, 141, 6507–6509. [Google Scholar] [CrossRef] [Green Version]
- Zeisl, B.; Pollefeys, M. Structure-based auto-calibration of RGB-D sensors. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–20 May 2016; pp. 5076–5083. [Google Scholar]
- Tan, W.; Zhang, L.; Shen, W. Low-Cost Chemical-Responsive Adhesive Sensing Chips. ACS Appl. Mater. Interfaces 2017, 9, 42366–42371. [Google Scholar] [CrossRef]
- Ma, H.; Xu, Z.; Feng, H.; Li, Q.; Chen, Y.; Huang, J. Texture aware learning-based image fusion method for fixed focal-length cameras. Opt. Sens. Imaging Technol. Appl. 2017, 10462, 104624V. [Google Scholar] [CrossRef]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yang, L.; Liu, R.; Kadir, A. A chaotic image encryption algorithm based on perceptron model. Nonlinear Dyn. 2010, 62, 615–621. [Google Scholar] [CrossRef]
- Dong, C. Color image encryption using one-time keys and coupled chaotic systems. Signal Process. Image Commun. 2014, 29, 628–640. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, D.; Sharma, R.K. A first approach on an RGB image encryption. Opt. Lasers Eng. 2014, 52, 27–34. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, T. Design of a wearable device for real-time screening of urinary tract infection and kidney disease based on smartphone. Analyst 2018, 143, 2812–2818. [Google Scholar] [CrossRef]
- Suresh, V.; Qunya, O.; Kanta, B.L.; Yuh, L.Y.; Chong, K.S.L. Non-invasive paper-based microfluidic device for ultra-low detection of urea through enzyme catalysis. R. Soc. Open Sci. 2018, 5, 171980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kafeza, E.; Chiu, D.K.W.; Cheung, S.C.; Kafeza, M. Alerts in mobile healthcare applications: Requirements and pilot study. IEEE Trans. Inf. Technol. Biomed. 2004, 8, 173–181. [Google Scholar] [CrossRef]
- Aragues, A.; Escayola, J.; Martinez, I.; Valle, P.; Muñoz, P.; Trigo, J.D.; Garcia, J. Trends and challenges of the emerging technologies toward interoperability and standardization in e-health communications. IEEE Commun. Mag. 2011, 49, 182–188. [Google Scholar] [CrossRef]
- Demiris, G.; Rantz, M.; Aud, M.A.; Marek, K.D.; Tyrer, H.W.; Skubic, M.; Hussam, A.A. Older adults’ attitudes towards and perceptions of ‘smart home’ technologies: A pilot study. Med Inform. Internet Med. 2004, 29, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hensel, B.K.; Demiris, G. Technologies for an Aging Society: A Systematic Review of “Smart Home” Applications. Yearb. Med. Inform. 2008, 17, 33–40. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-K.; Bae, J.-H. Design of Appropriate Technology-Assisted Urine Tester Enabling Remote and Long-Term Monitoring of Health Conditions. Sustainability 2020, 12, 5165. https://doi.org/10.3390/su12125165
Lee H-K, Bae J-H. Design of Appropriate Technology-Assisted Urine Tester Enabling Remote and Long-Term Monitoring of Health Conditions. Sustainability. 2020; 12(12):5165. https://doi.org/10.3390/su12125165
Chicago/Turabian StyleLee, Hyun-Kyung, and Jeong-Hyeon Bae. 2020. "Design of Appropriate Technology-Assisted Urine Tester Enabling Remote and Long-Term Monitoring of Health Conditions" Sustainability 12, no. 12: 5165. https://doi.org/10.3390/su12125165
APA StyleLee, H.-K., & Bae, J.-H. (2020). Design of Appropriate Technology-Assisted Urine Tester Enabling Remote and Long-Term Monitoring of Health Conditions. Sustainability, 12(12), 5165. https://doi.org/10.3390/su12125165




