Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Experimental Procedure
2.3. Determination of Number, Size and Composition of Microparticles
2.4. Pollution Prevention
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- PlasticsEurope. PlasticsEurope Operation Clean Sweep ® Report; PlasticsEurope: Bruxelles, Brussels, 2018. [Google Scholar]
- Plastivida Manejo de los Residuos Plásticos en Diferentes Partes del Mundo. Boletín Técnico Informativo N° 5. Entidad Técnica Profesional Especializada en Plásticos y Medio Ambiente Argentina, 2007. Available online: https://ecoplas.org.ar/pdf/5.pdf (accessed on 7 July 2020).
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Rainieri, S.; Barranco, A. Microplastics, a food safety issue? Trends Food Sci. Technol. 2019, 84, 55–57. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. TrAC—Trends Anal. Chem. 2019, 111, 62–72. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants on the Food Chain. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar]
- Hunt, P.K. Los microplásticos en el agua potable “ no parecen representar un riesgo para la salud”, dice la OMS. 2019. Available online: https://cnnespanol.cnn.com/2019/08/22/los-microplasticos-en-el-agua-potable-no-parecen-representar-un-riesgo-para-la-salud-dice-la-oms/ (accessed on 7 July 2020).
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Rist, S.; Carney Almroth, B.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). List of substances subject to POPs Regulation. 2019, p. 1–3. Available online: https://echa.europa.eu/list-of-substances-subject-to-pops-regulation (accessed on 7 July 2020).
- Jemec Kokalj, A.; Kuehnel, D.; Puntar, B.; Gotvajn, A.Ž.; Kalčikova, G. An exploratory ecotoxicity study of primary microplastics versus aged in natural waters and wastewaters. Environ. Pollut. 2019, 254, 112980. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Harrison, J.P.; Ojeda, J.J.; Romero-González, M.E. The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci. Total Environ. 2012, 416, 455–463. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Adaptation of a laboratory protocol to quantity microplastics contamination in estuarine waters. MethodsX 2019, 6, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef]
- Jiang, J.-Q. Occurrence of microplastics and its pollution in the environment: A review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef] [PubMed]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport, a major pathway of microplastics to remote regions. Preprints 2020, 1–32. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 2136–2140. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 1574–1578. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Origin of synthetic particles in honeys. Polish J. Food Nutr. Sci. 2015, 65, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mason, S.A.; Welch, V.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Oliveira, M.; Almeida, M.; Miguel, I. A micro(nano)plastic boomerang tale: A never ending story? TrAC—Trends Anal. Chem. 2019, 112, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [Green Version]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Saez Cifre, E. Análisis de la calidad del aire interior en función de la tipología de ventilación. Universidad Politécnica de Valencia. 2017. Available online: http://hdl.handle.net/10251/85368 (accessed on 7 July 2020).
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Panebianco, A.; Nalbone, L.; Giarratana, F.; Ziino, G. First discoveries of microplastics in terrestrial snails. Food Control 2019, 106, 106722. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Bonet Aracil, M.A. Propiedades geométricas de las fibras. 2018. Available online: https://media.upv.es/player/?id=f4b9d1e0-2e9a-11e8-b43a-51b816915a74 (accessed on 19 June 2020).
- Akdogan, Z.; Guven, B. Microplastics in the Environment: A Critical Review of Current Understanding and Identification of Future Research Needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef] [PubMed]
- Poma, K.; Díaz-Basantes, M. Determinación de la Presencia de Microplásticos en miel Artesanal e Industrial. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2019. [Google Scholar]
- Tituchina, L.; Díaz-Basantes, M. Determinación de la Presencia de Microplasticos en Bebidas Refrescantes. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2019; pp. 1–71. [Google Scholar]
- Criollo, K.; Díaz-Basantes, M. Determinación de la Presencia de Microplásticos en Leche Descremada. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2019; p. 55. [Google Scholar]
- Rengifo, E.; Díaz-Basantes, M. Determinación de la Presencia de Microplásticos en Miel Artesanal e Industrial. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2019; pp. 1–109. [Google Scholar]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. Wiley Interdiscip. Rev. Water 2017, 5, e1268. [Google Scholar] [CrossRef] [Green Version]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks—Future research and environmental considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef]
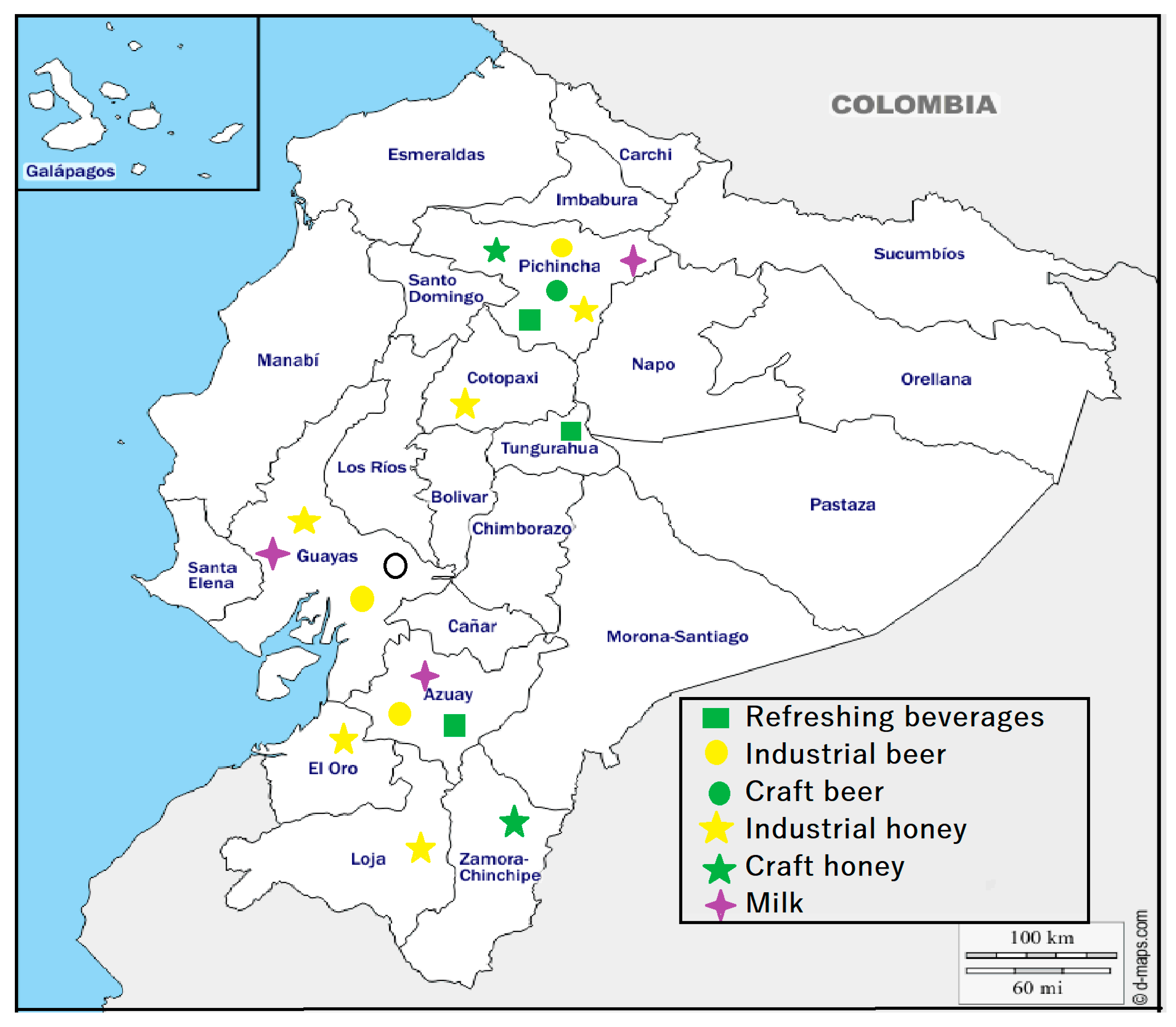






| Food | Analyzed Sample Volume (mL) | Specific Characteristics |
|---|---|---|
| Skim milk | 1000 | Fat < 1% wt, packed in polyethylene covers or Tetra Pak |
| Honey | 700 | Honey packaged by hand or industrially in glass containers |
| Refreshments | 500 | Refreshing drink with citrus flavor to orange or lemon packaged in PET bottle or Tetra Pak |
| Beer | 750 | Lage industrial or artisanal beer bottled in glass |
| Food Sample | Sample No. | Fibers/Liter | * Fiber Particle Size µm | Fragments/Liter | ** Fragment Particle Size µm | Total Microparticles | MPs | City of Producer | Population | Type of Process | Type of Sample | Weather Characteristics | Height (m ASL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geographic Weather | Winds (km/h) | |||||||||||||
| Skim milk | 1 | 116 | 19.94–1447.34 | 212 | 4.48–183.37 | 328 | 39 | Ibarra | 139,721 | Industrial | Urban | Tempered | 8.3 | 2220 |
| 2 | 122 | 28.45–2329.41 | 222 | 3.77–149.11 | 344 | 41 | Quito | 2,690,150 | Industrial | Urban | Subtropical | 7.4 | 2800 | |
| 3 | 74 | 250.53–1175.25 | 206 | 3.10–149.20 | 280 | 34 | Machachi | 27,623 | Industrial | Urban | Cold | 7.8 | 2945 | |
| 4 | 142 | 58.31–2127.49 | 284 | 5.98–169.77 | 426 | 51 | Sangolquí | 81,140 | Industrial | Urban | Subtropical | 8.3 | 2500 | |
| 5 | 144 | 46.33–2410.05 | 194 | 5.52–130.28 | 338 | 41 | Sangolquí | 81,140 | Industrial | Urban | Subtropical | 8.3 | 2500 | |
| 6 | 152 | 119.44–1789.13 | 230 | 4.96–81.49 | 382 | 46 | Tanicuchi | 12,831 | Industrial | Urban | Cold | 9.1 | 2750 | |
| 7 | 196 | 79.97–6742.48 | 236 | 2.48–86.16 | 432 | 52 | Cayambe | 50,829 | Industrial | Urban | Tempered | 15 | 2830 | |
| 8 | 254 | 83.38–2137.91 | 190 | 5.81–101.62 | 444 | 53 | Cuenca | 331,888 | Industrial | Urban | Tempered | 9.7 | 2550 | |
| 9 | 86 | 32.37–1845.67 | 168 | 6.28–99.19 | 254 | 30 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 8.3 | 4 | |
| 10 | 34 | 154.72–3359.87 | 100 | 4.19–87.75 | 134 | 16 | Machachi | 27,623 | Industrial | Urban | Cold | 7.8 | 2945 | |
| Industrial Honey | 11 | 100 | 166.05–966.42 | 530 | 5.63–173.58 | 630 | 76 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 10 | 4 |
| 12 | 56 | 181.65–1388.24 | 190 | 12.88–139 | 246 | 30 | Loja | 180,617 | Industrial | Urban | Hot tempered | 13 | 2065 | |
| 13 | 56 | 208.79–1063.04 | 126 | 14.88–182.96 | 182 | 22 | Tabacundo | 16,403 | Industrial | Urban | Cold | 6 | 2877 | |
| 14 | 20 | 132.12–548.76 | 552 | 7.53–83.99 | 572 | 69 | Machala | 241,606 | Industrial | Urban | Tropical hot | 8 | 6 | |
| 15 | 76 | 140.92–3302.68 | 494 | 13.38–161.0 | 570 | 68 | Quito | 2,690,150 | Industrial | Urban | Subtropical | 2 | 2800 | |
| 16 | 152 | 264.72–2518.02 | 278 | 11.54–88.55 | 430 | 52 | Quito | 2,690,150 | Industrial | Urban | Subtropical | 2 | 2800 | |
| 17 | 166 | 67.18–250.583 | 350 | 9.22–129.02 | 516 | 62 | Tabacundo | 16,403 | Industrial | Urban | Cold | 6 | 2877 | |
| Craft Honey | 18 | 116 | 106.56–1705.28 | 342 | 5.15–175.39 | 458 | 55 | Tambillo | 8319 | Craft | Rural | Humid tropical | 3 | 2800 |
| 19 | 178 | 84.95–5174.01 | 678 | 11.81–139.44 | 856 | 103 | Guayllabamba | 16,213 | Craft | Rural | Dry | 7 | 2171 | |
| 20 | 104 | 394.1–2398.4 | 798 | 12.09–226.01 | 902 | 108 | Tumbaco | 49,944 | Craft | Urban | Hot | 0 | 2355 | |
| 21 | 134 | 169.97–2709.71 | 200 | 26.79–199.16 | 334 | 40 | El Chaupi | 1456 | Craft | Rural | Cold | 7.5 | 3163 | |
| 22 | 82 | 186.09–1936.77 | 246 | 17.54–69.61 | 328 | 39 | Lasso | 1635 | Craft | Rural | Cold | 18 | 3038 | |
| 23 | 98 | 96.44–2566.84 | 202 | 19.44–146.46 | 300 | 36 | Tanicuchi | 12,831 | Craft | Rural | Cold | 18 | 3849 | |
| 24 | 126 | 229.95–1630.12 | 828 | 12.9–213.7 | 954 | 114 | Salcedo | 53,216 | Craft | Urban | Hot tempered | 7 | 2683 | |
| 25 | 106 | 240.4–2248.01 | 254 | 14.86–159.39 | 360 | 43 | Los Encuentros | 3658 | Craft | Rural | Desertic | 8 | 822 | |
| Refreshing beverage | 26 | 144 | 63.85–2224.25 | 350 | 5.94–145.81 | 494 | 59 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 |
| 27 | 68 | 89.64–1015.9 | 290 | 8.44–154.69 | 358 | 43 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| 28 | 36 | 169.5–1049.18 | 272 | 7.57–228.02 | 308 | 37 | Pelileo | 56,573 | Industrial | Urban | Tempered | 14 | 2600 | |
| 29 | 54 | 56.22–2096.24 | 242 | 9.05–127.90 | 296 | 36 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| 30 | 56 | 67.89–848.98 | 190 | 5.62–165.95 | 246 | 30 | Machachi | 27,623 | Industrial | Urban | Cold | 8 | 2945 | |
| 31 | 88 | 15.64–1181.37 | 178 | 5.47–247.54 | 266 | 32 | Quito | 2,690,150 | Industrial | Urban | Subtropical | 3 | 2800 | |
| 32 | 94 | 30.95–1166.96 | 188 | 8.24–145.25 | 282 | 34 | Quito | 2,690,150 | Industrial | Urban | Subtropical | 3 | 2800 | |
| 33 | 32 | 47.2–819.49 | 118 | 7.60–67.56 | 150 | 18 | Cuenca | 331,888 | Industrial | Urban | Tempered | 16 | 2550 | |
| 34 | 62 | 18.16–931.35 | 180 | 6.69–65.88 | 242 | 29 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| 35 | 38 | 59.29–1130.91 | 198 | 8.47–73.25 | 236 | 28 | Cayambe | 50,829 | Industrial | Urban | Tempered | 15 | 2830 | |
| 36 | 10 | 104.6–1446.02 | 58 | 7.35–81.59 | 68 | 8 | Cayambe | 50,829 | Industrial | Urban | Tempered | 15 | 2830 | |
| 37 | 48 | 48.63–717.11 | 140 | 6.84–69.47 | 188 | 23 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| 38 | 96 | 21.92–1174.66 | 152 | 8.60–204.05 | 248 | 30 | Machachi | 27,623 | Industrial | Urban | Cold | 8 | 2945 | |
| 39 | 66 | 105.06–2101.67 | 288 | 7.01–93.47 | 354 | 42 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| Industrial Beer | 40 | 60 | 59.52–1740.24 | 182 | 3.505–186.4 | 242 | 29 | Cumbayá | 31,463 | Industrial | Suburban | Subtropical | 15 | 2200 |
| 41 | 98 | 13.45–1075.55 | 304 | 4.97–202.29 | 402 | 48 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| 42 | 40 | 36.34–1076.31 | 396 | 9.54–131.8 | 436 | 52 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| 43 | 58 | 26.32–1388.43 | 354 | 8.55–140.995 | 412 | 49 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| 44 | 18 | 35.69–588.34 | 310 | 8.445–177.48 | 328 | 39 | Cumbayá | 31,463 | Industrial | Suburban | Subtropical | 15 | 2200 | |
| 45 | 18 | 59.12–427.82 | 496 | 6.75–200.23 | 514 | 62 | Guayaquil | 2,291,158 | Industrial | Urban | Hot | 11 | 4 | |
| Craft Beer | 46 | 12 | 155.01–769.8 | 110 | 6.155–155.8 | 122 | 15 | Puembo | 18,000 | Craft | Rural | Subtropical | 15 | 2400 |
| 47 | 56 | 40.68–500.4 | 920 | 6.5–160.345 | 976 | 117 | Cuenca | 331,888 | Craft | Urban | Tempered | 16 | 2550 | |
| 48 | 22 | 40.28–473.68 | 384 | 11.35–86.705 | 406 | 49 | Chongón | 36,726 | Craft | Rural | Hot | 11 | 10 | |
| 49 | 20 | 37.05–595.52 | 126 | 10.415–72.715 | 146 | 18 | Quito | 2,690,150 | Craft | Urban | Tempered | 3 | 2800 | |
| 50 | 20 | 103.81–1426.3 | 50 | 24.73–128.105 | 70 | 8 | Quito | 2,690,150 | Craft | Urban | Tempered | 3 | 2800 | |
| 51 | 20 | 177.07–737.55 | 96 | 9.37–104.355 | 116 | 14 | Quito | 2,690,150 | Craft | Urban | Tempered | 3 | 2800 | |
| 52 | 52 | 60.71–548.57 | 146 | 10.46–87.655 | 198 | 24 | Quito | 2,690,150 | Craft | Urban | Tempered | 3 | 2800 | |
| 53 | 26 | 95.49–614.89 | 90 | 14.16–73.325 | 116 | 14 | Quito | 2,690,150 | Craft | Rural | Tempered | 3 | 2800 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. https://doi.org/10.3390/su12145514
Diaz-Basantes MF, Conesa JA, Fullana A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability. 2020; 12(14):5514. https://doi.org/10.3390/su12145514
Chicago/Turabian StyleDiaz-Basantes, Milene F., Juan A. Conesa, and Andres Fullana. 2020. "Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants" Sustainability 12, no. 14: 5514. https://doi.org/10.3390/su12145514
APA StyleDiaz-Basantes, M. F., Conesa, J. A., & Fullana, A. (2020). Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability, 12(14), 5514. https://doi.org/10.3390/su12145514






