Effect of Seed Meals on Weed Control and Soil Physical Properties in Direct-Seeded Pumpkin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Description
2.2. Measurements and Data Collection
2.3. Soil Sampling and Determination of Soil Properties
2.4. Statistical Analysis
3. Results and Discussion
3.1. Crop Response
3.2. Weed Density and Biomass
3.3. Pumpkin Yield
3.4. Soil Physical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture Economic Research Service. Pumpkins: Background and Statistics. Retrieved, 18 October 2019. Available online: http://www.ers.usda.gov/newsroom/trending-topics/pumpkins-background-statistics/ (accessed on 22 June 2020).
- Smith, D.; Anciso, J. Vegetable Resources: Pumpkins in Texas. Texas A&M AgriLife Extention. Texas A&M University: College Station, TX, USA, 2000. Available online: https://aggie-horticulture.tamu.edu/vegetable/guides/crop-briefs/pumpkins-in-texas/ (accessed on 18 May 2020).
- Marek, G.W.; Gowda, P.H.; Marek, T.H.; Porter, D.O.; Baumhardt, R.L.; Brauer, D.K. Modeling long-term water use of irrigated cropping rotations in the Texas High Plains using SWAT. Irrig. Sci. 2017, 35, 111–123. [Google Scholar] [CrossRef]
- Singh, S.; Angadi, S.V.; Hilaire, R.S.; Grover, K.; VanLeeuwen, D.M. Spring safflower performance under growth stage based irrigation management practices. Crop. Sci. 2016, 56, 1878–1889. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Allen, V.G.; Brown, C.P.; Kellison, R.; Green, P.; Zilverberg, C.J.; Johnson, P.; Zobeck, T.M. Integrating cotton and beef production in the Texas Southern High Plains: I. Water use and measures of productivity. Agron. J. 2016, 104, 1625–1642. [Google Scholar] [CrossRef]
- Pimental, D.; McNair, S.; Janecka, J. Economic and environmental threats of alien plant, animal and microbe invasions. Agric. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- McDade, M.C.; Christians, N.E. Corn gluten meal—A natural pre-emergence herbicide: Effect on vegetable seedling survival and weed cover. Am. J. Altern. Agric. 2000, 15, 189–191. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Fundamentals of Weed Science, 4th ed.; Academic Press: San Diego, CA, USA, 2013; p. 31. [Google Scholar]
- Cano, E.; Musarella, C.M.; Cano-Ortiz, A.; Piñar Fuentes, J.C.; Rodríguez Torres, A.; Del Río González, S.; Spampinato, G. Geobotanical study of the microforests of Juniperus oxycedrus subsp. badia in the Central and Southern Iberian Peninsula. Sustainability 2019, 11, 1111. [Google Scholar] [CrossRef] [Green Version]
- Fanfarillo, E.; Latini, M.; Iberite, M.; Bonari, G.; Nicolella, G.; Rosati, L.; Salerno, G.; Abbate, G. The segetal flora of winter cereals and allied crops in Italy: Species inventory with chorological, structural and ecological features. Plant Biosyst. 2020. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Pimentel, D.; McLaughlin, L.; Zepp, A.; Lakitan, B.; Kraus, T.; Kleinman, P.; Vancini, F.; Roach, W.J.; Graap, E.; Keeton, W.S.; et al. Environmental and economic impacts of reducing U.S. agricultural pesticide use. In Handbook of Pest Management in Agriculture; Pimentel, D., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 679–720. [Google Scholar]
- Brown, B.; Hoshide, A.K.; Gallandt, E.R. An economic comparison of weed management systems used in small-scale organic vegetable production. Org. Agric. 2019, 9, 53–63. [Google Scholar] [CrossRef]
- Swanton, C.J.; Weaver, S.; Cowan, P.; Acker, R.V.; Deen, W.; Shreshta, A. Weed thresholds: Theory and applicability. J. Crop Prod. 1999, 2, 9–29. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, A.; Kahlon, C.S.; Brar, A.S.; Grover, K.K.; Dia, M.; Steiner, R.L. The role of cover crops towards sustainable soil health and agriculture—A review paper. Am. J. Plant Sci. 2018, 9, 1935–1951. [Google Scholar] [CrossRef] [Green Version]
- Peters, K.; Breitsameter, L.; Gerowitt, B. Impact of climate change on weeds in agriculture: A review. Agron. Sustain. Dev. 2014, 34, 707–721. [Google Scholar] [CrossRef] [Green Version]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Naik, S.; Prasad, R. Pesticide residue in organic and conventional food-risk analysis. Chem. Health Saf. 2006, 13, 12–19. [Google Scholar]
- Wolf, T. Best management practices for herbicide application technology. Prairie Soils Crop. 2009, 2, 24–30. [Google Scholar]
- Duke, S.O.; Dayan, F.E.; Rimando, A.M.; Schrader, K.K.; Aliotta, G.; Oliva, A.; Romagni, J.G. Chemicals from nature for weed management. Weed Sci. 2002, 50, 138–151. [Google Scholar] [CrossRef]
- Walz, E. Final Results of the Third Biennial National Organic Farmers’ Survey; Organic Farming Research Foundation (OFRF): Santa Cruz, CA, USA, 1999; Available online: http://www.ofrf:publications/survey/Final.Results.Third.NOF.Survey.pdf (accessed on 22 April 2018).
- Rice, A.; Johnson-Maynard, J.; Thill, D.; Morra, M. Vegetable crop emergence and weed control following amendment with different Brassicaceae seed meals. Renew. Agric. Food Syst. 2007, 22, 204–212. [Google Scholar] [CrossRef]
- Brown, J. Oil Crop Potential for Biodiesel Production: Summary of Three Years of Spring Mustard Research: Methodologies, Results, and Recommendations; SR-510-36309; National Renewable Energy Laboratory: Golden, CO, USA, 2006. Available online: http://www.nrel.gov/docs/fy05osti/36309.pdf (accessed on 13 November 2017).
- Maceiras, R.; Rivero, J.J.; Cancela, M.A.; Urrejola, S.; Sanchez, A. Development and modeling of production of biodiesel from sunflower oil. Chem. Technol. Fuels Oils 2010, 46, 154–159. [Google Scholar] [CrossRef]
- Boydston, R.A.; Vaughn, S.F.; Webber, C.L., III; Chaves-Cordoba, B. Evaluating mustard seed meal for weed suppression in potato (Solanum tuberosum). J. Agric. Sci. 2018, 10, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Rawat, L.S.; Maikhuri, R.K.; Bahuguna, Y.M.; Jha, N.K.; Phondani, P.C. Sunflower allelopathy for weed control in agriculture systems. J. Crop. Sci. Biotechnol. 2017, 20, 45–60. [Google Scholar] [CrossRef]
- Varela, R.M. Allelopathic Studies on Cultivars of Sunflower. Master’s Thesis, University of Cadiz, Puerto Real, Spain, 1982. [Google Scholar]
- Webber, C.L.I.; White, P.M.J.; Boydston, R.A.; Shrefler, J.W. Impact of mustard seed meal applications on direct-seeded cucurbits and weed control. J. Agric. Sci. 2017, 9, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Mithen, R.F. Glucosinolates and their degradation products. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2001; pp. 213–262. [Google Scholar]
- Borek, V.; Morra, M.J. Ionic thiocyanate (SCN-) production from 4-hydroxybenzyl glucosinolate contained in Sinapis alba seed meal. J. Agric. Food Chem. 2005, 53, 8650–8654. [Google Scholar] [CrossRef] [PubMed]
- Gimsing, A.L.; Kirkegaard, J.A.; Bruun Hansen, H.C. Extraction and determination of glucosinolates from soil. J. Agric. Food Chem. 2005, 53, 9663–9667. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Malik, M.S.; Jha, P.; Oliveira, M.J. Effects of isothiocyanates on purple (Cyperus rotundus L.) and yellow nutsedge (Cyperus esculentus L.). Weed Biol. Manag. 2006, 6, 131–138. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Palmquist, D.E.; Duval, S.M.; Berhow, M.A. Herbicidal activity of glucosinolate-containing seedmeals. Weed Sci. 2006, 54, 743–748. [Google Scholar] [CrossRef]
- Banuelos, G.S.; Hanson, B.D. Use of selenium-enriched mustard and canola seed meals as potential bioherbicides and green fertilizer in strawberry production. HortScience 2010, 45, 1567–1572. [Google Scholar] [CrossRef] [Green Version]
- Boydston, R.A.; Morra, M.J.; Borek, V.; Clayton, L.; Vaughn, S.F. Onion and weed response to mustard (Sinapis alba) seed meal. Weed Sci. 2011, 59, 546–552. [Google Scholar] [CrossRef]
- Yu, J.; Morishita, D.W. Response of seven weed species to corn gluten meal and white mustard (Sinapis alba) seed meal rates. Weed Technol. 2014, 28, 259–265. [Google Scholar] [CrossRef]
- Handiseni, M.; Brown, J.; Zemetra, R.; Mazzola, M. Herbicidal activity of Brassicaceae seed meal on wild oat (Avena fatua), Italian ryegrass (Lolium multiflorum), redroot pigweed (Amaranthus retroflexus), and prickly lettuce (Lactuca serriola). Weed Technol. 2011, 25, 127–134. [Google Scholar] [CrossRef]
- Miller, T.W. Natural herbicides and amendments for organic weed control. In Crop Protection Products for Organic Agriculture; 174175 ACS Symposium Series, 947; Felsot, A.S., Racke, K.D., Eds.; American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]
- Earlywine, D.; Teuton, T.; Sorochan, J.C.; Fresenburg, B.; Smeda, R.; Sweets, L. Evaluation of Yellow Mustard Seed Meal for Weed Control ASA, CSSA, and SSSA International Meetings New Orleans 13 February 2008. Available online: http://a-c-s.confex.com/a-c-s/2007am/techprogram/P36683.HTM (accessed on 8 July 2020).
- Gerald, R.L. Sunflowers (Helianthus annuus) are allelopathic to weeds. Weed Sci. 1983, 31, 37–42. [Google Scholar]
- Oracz, K.; Bailly, C.; Gniazdowska, A.; Côme, D.; Corbineau, F.; Bogatek, R. Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds. J. Chem. Ecol. 2007, 33, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini, C. Short term effects of bioenergy by-products on soil C and N dynamics nutrient availability and biochemical properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Wang, A.S.; Hu, P.; Hollister, E.B.; Rothlisberger, K.L.; Somenahally, A.; Provin, T.L.; Hons, F.M.; Gentry, T.J. Impact of Indian mustard (Brassica juncea) and flax (Linum usitatissimum) seed meal applications on soil carbon, nitrogen, and microbial dynamics. Appl. Environ. Soil Sci. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zaccardelli, M.; Villecco, D.; Celano, G.; Scotti, R. Soil amendment with seed meals: Short-term effects on soil respiration and biochemical properties. Appl. Soil Ecol. 2013, 72, 225–231. [Google Scholar] [CrossRef]
- Agassi, A.; Hadas, A.; Benyamini, Y.; Levy, G.J.; Kautsky, L.; Avrahamov, L.; Zhevelev, H. Mulching effects of composted MSW on water percolation and compost degradation rate. Compost Sci. Util. 1998, 6, 34–41. [Google Scholar] [CrossRef]
- Zinati, G.M.; Li, Y.C.; Bryan, H.H. Accumulation and distribution of copper, iron, manganese and zinc in calcareous soil amended with compost. J. Environ. Sci. Health Part B 2001, 36, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Zinati, G.M.; Li, Y.C.; Bryan, H.H.; Mylavarapu, R.S.; Codallo, M. Distribution and fractionation of phosphorus, cadmium, nickel, and lead in calcareous soils amended with compost. J. Environ. Sci. Health Part B 2004, 39, 209–223. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The ‘Terra Preta’ phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.; Thies, J.; Luizao, F.; Petersen, J. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Orzolek, M.; Elkner, T.; Lamont, W.; Kime, L.; Harper, J. Agricultural Alternatives: Pumpkin Production; Penn State Extension, Pennsylvania State University: State College, PA, USA, 2012. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size Analysis. In Methods of Soil Analysis: Part 1, 2nd ed.; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 383–409. [Google Scholar]
- Klute, A.; Dirksen, C. Hydraulic conductivity and diffusivity. Laboratory methods. In Methods of Soil Analysis—Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 687–734. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. In Methods of Soil Analysis: Part 1, 2nd ed.; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 635–660. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis: Part 1, 2nd ed.; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 336–375. [Google Scholar]
- Shrestha, A.; Rodriguez, A.; Pasakdee, S.; Bañuelos, G. Comparative efficacy of white mustard (Sinapis alba L.) and soybean (Glycine max L. Merr.) seed meals as bioherbicides in organic broccoli (Brassica oleracea Var. Botrytis) and spinach (Spinacea oleracea) production. Commun. Soil Sci. Plant 2015, 46, 33–46. [Google Scholar] [CrossRef]
- Darby, H.; Hills, K.; Cummings, E.; Madden, R. Assessing the Value of Oilseed Meals for Soil Fertility and Weed Suppression; University of Vermont Extension: Burlington, VT, USA, 2010. [Google Scholar]
- Fine, K.E.; Cole, J.C.; Penn, C.J. Nitrogen mineralization from canola meal or cottonseed meal with or without soapstock. HortScience 2013, 48, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Tejada, M.; Gonzalez, J.L.; García-Martínez, A.M.; Parrado, J. Application of a green manure and green manure composted with beet vinasse on soil restoration: Effects on soil properties. Bioresour. Technol. 2008, 99, 4949–4957. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Gunal, H.; Budak, M.; Akpinar, C. Effects of long-term organic and mineral fertilizers on bulk density and penetration resistance in semi-arid Mediterranean soil conditions. Geoderma 2010, 160, 236–243. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, H.; White, E. Surface soil hydraulic properties in four soil series under different land uses and their temporal changes. Catena 2008, 73, 180–188. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; McLaughlin, N.B.; Mi, J.; Chen, Q.; Liu, J. Effect of synthetic and natural water absorbing soil amendment soil physical properties under potato production in a semi-arid region. Soil Tillage Res. 2015, 148, 31–39. [Google Scholar] [CrossRef]
- Yu, Y.; Peng, R.; Yang, C.; Tang, Y. Eco-friendly and cost-effective superabsorbent sodium polyacrylate composites for environmental remediation. J. Mater. Sci. 2015, 50, 5799–5808. [Google Scholar] [CrossRef]
- Agaba, H.; Baguma Orikiriza, L.J.; Osoto Esegu, J.F.; Obua, J.; Kabasa, J.D.; Hüttermann, A. Effects of hydrogel amendment to different soils on plant available water and survival of trees under drought conditions. Clean-Soil Air Water 2010, 38, 328–335. [Google Scholar] [CrossRef]
- Farrell, C.; Ang, X.Q.; Rayner, J.P. Water-retention additives increase plant available water in green roof substrates. Ecol. Eng. 2013, 52, 112–118. [Google Scholar] [CrossRef]

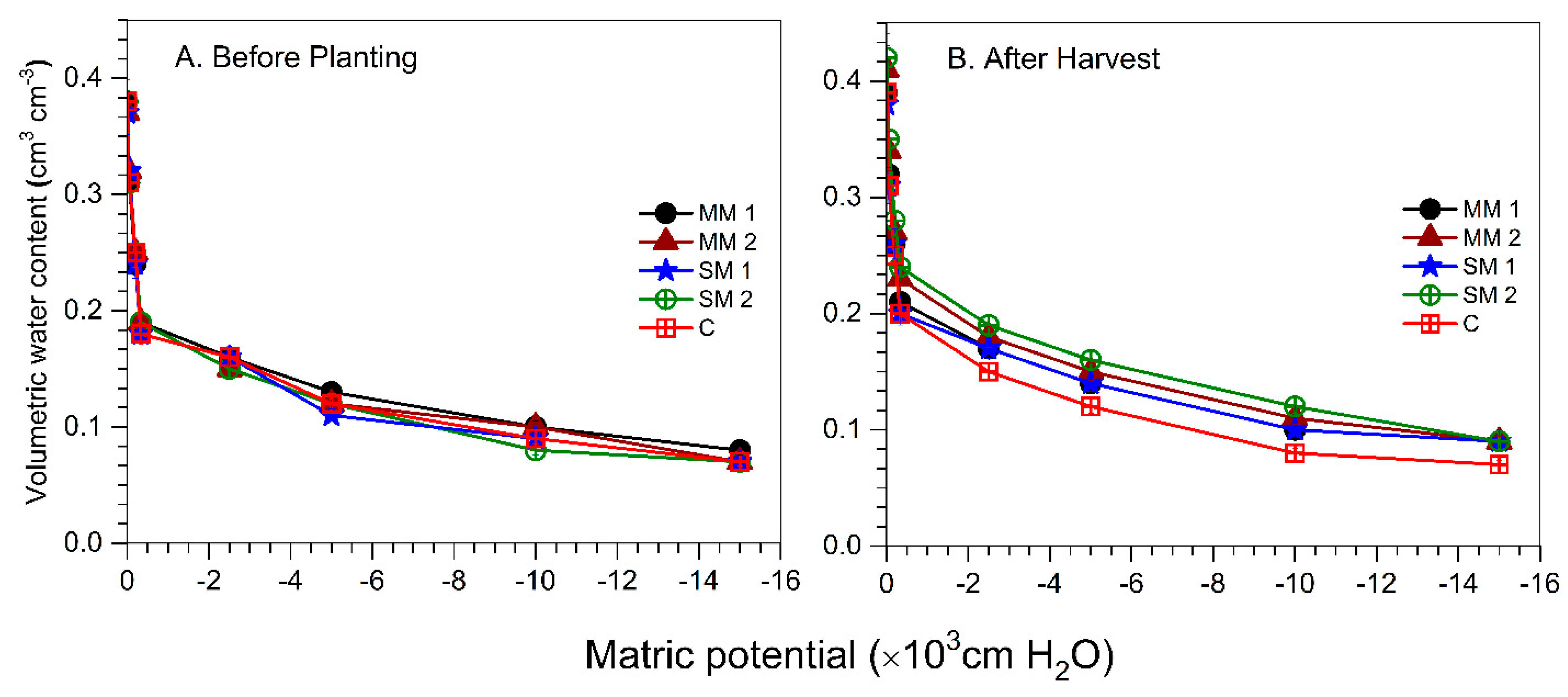
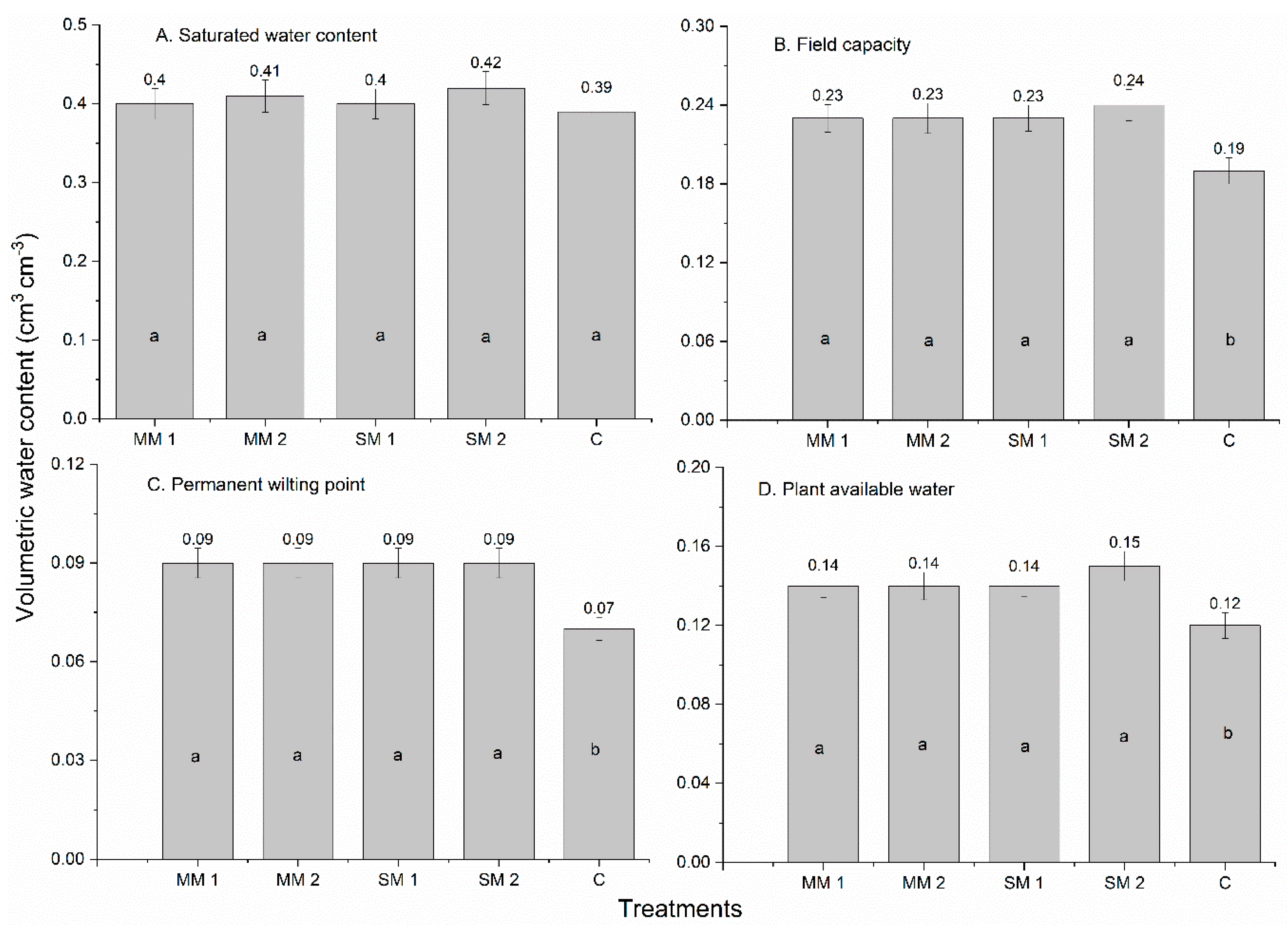
| Treatments | Rate (Kg ha−1) | Visual Weed Control (%) | Crop Toxicity (%) | |
|---|---|---|---|---|
| Narrow Leaf Weeds | Broadleaf Weeds | |||
| Mustard Seed Meal (MSM) | ||||
| MM 1 MM 2 | 1150 | 75.00 b | 68.75 b | 3.75 b |
| 2250 | 82.50 a | 76.25 a | 7.50 a | |
| Sunflower Seed Meal (SSM) | ||||
| SM 1 SM 2 | 1150 | 58.75 d | 58.75 c | 3.75 b |
| 2250 | 65.00 c | 53.75 d | 3.75 b | |
| Untreated control | - | 0.00 | 0.00 | 0.00 |
| Treatments | Rate (Kg ha−1) | Narrow leaf Weed Density (plant m−2) | Broadleaf Weed Density (plant m−2) | Leaf Area Index |
|---|---|---|---|---|
| Mustard Seed Meal (MSM) | ||||
| MM 1 MM 2 | 1150 | 10.0b c | 7.00 c | 2.26 a |
| 2250 | 8.00 c | 5.67 c | 2.19 a | |
| Sunflower Seed Meal (SSM) | ||||
| SM 1 SM 2 | 1150 | 10.7b c | 11.3 b | 2.24 a |
| 2250 | 12.4 b | 7.33 c | 2.15 a | |
| Untreated control | - | 20.3 a | 16.3 a | 2.36 a |
| Treatments | Rate (Kg ha−1) | Narrow Leaf Weed Density (plant m−2) | Broadleaf Weed Density (plant m−2) | Narrow Leaf Weed Biomass (g m−2) | Broadleaf Weed Biomass (g m−2) | Pumpkin Yield (Kg ha−1) |
|---|---|---|---|---|---|---|
| Mustard Seed Meal (MSM) | ||||||
| MM 1 MM 2 | 1150 | 9.50 b | 11.5 b | 46.3 b | 42.9 b | 11093 a |
| 2250 | 8.50 b | 2.50 c | 48.3 b | 9.45 c | 10849 a | |
| Sunflower Seed Meal (SSM) | ||||||
| SM 1 SM 2 | 1150 | 10.0 b | 9.50 b | 53.6 b | 54.9 b | 10496 a |
| 2250 | 9.00 b | 12.0 b | 50.1 b | 49.9 b | 9980 a | |
| Untreated control | - | 22.0 a | 26.0 a | 110.8 a | 92.2 a | 6858 b |
| Soil Depths (cm) | Particle Size Distribution (%) | Soil Texture | ||
|---|---|---|---|---|
| Sand | Silt | Clay | ||
| 0–10 | 73.21 ± 0.68 * | 10.07 ± 2.21 | 16.72 ± 0.19 | Sandy loam |
| 10–20 | 61.28 ± 1.19 | 20.07 ± 1.83 | 18.65 ± 0.34 | Sandy loam |
| 20–30 | 73.21 ± 2.41 | 8.14 ± 1.58 | 18.65 ± 0.43 | Sandy loam |
| 30–40 | 64.99 ± 1.92 | 16.36 ± 1.34 | 18.65 ± 0.37 | Sandy loam |
| Treatments | Rate (Kg ha−1) | Soil Bulk Density (g cm−3) | Saturated Hydraulic Conductivity (Ks, cm d −1) | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Mustard Seed Meal (MSM) | |||||
| MM 1 | 1150 | 1.66 ± 0.06* | 1.64± 0.10 b | 104.4 ± 0.008 | 107.5 ± 0.0037 a |
| MM 2 | 2250 | 1.64 ± 0.05 | 1.63 ± 0.08 b | 105.5 ± 0.0024 | 108.6 ± 0.0012 a |
| Sunflower Seed Meal (SSM) | |||||
| SM 1 | 1150 | 1.67 ± 0.08 | 1.64 ± 0.07 b | 106.9 ± 0.0015 | 107.3 ± 0.0044 a |
| SM2 | 2250 | 1.65 ± 0.08 | 1.64 ± 0.11 b | 105.1 ± 0.0026 | 108.9 ± 0.0032 a |
| Untreated control | - | 1.66 ± 0.07 | 1.67 ± 0.09 a | 107.7 ± 0.0019 | 107.2 ± 0.0029 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, R.; Singh, A.; Deb, S.K. Effect of Seed Meals on Weed Control and Soil Physical Properties in Direct-Seeded Pumpkin. Sustainability 2020, 12, 5811. https://doi.org/10.3390/su12145811
Saini R, Singh A, Deb SK. Effect of Seed Meals on Weed Control and Soil Physical Properties in Direct-Seeded Pumpkin. Sustainability. 2020; 12(14):5811. https://doi.org/10.3390/su12145811
Chicago/Turabian StyleSaini, Rupinder, Atinderpal Singh, and Sanjit K. Deb. 2020. "Effect of Seed Meals on Weed Control and Soil Physical Properties in Direct-Seeded Pumpkin" Sustainability 12, no. 14: 5811. https://doi.org/10.3390/su12145811





