Use of Calcite Mud from Paper Factories in Phosphorus Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-Treatment Calcite Mud
2.2. Batch Experiments
2.3. Adsorption Kinetics
2.4. Characterizations
3. Results and Discussion
3.1. Characteristics of Muds
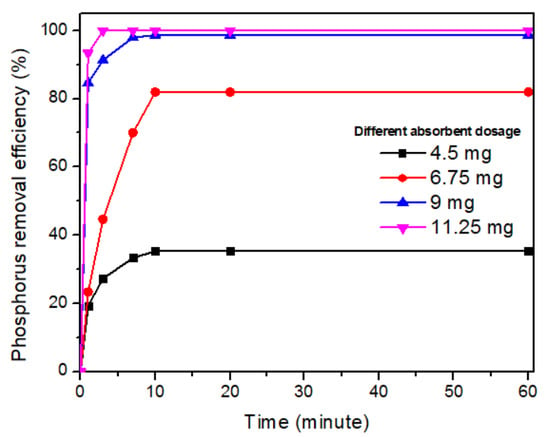
3.2. Phosphorus Removal
3.3. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ott, C.; Degg, A.; Walke, P.; Reiter, F.; Nilges, T. Influence of copper on the capacity of phosphorus-anode in sodium-ion-batteries. J. Solid State Chem. 2019, 270, 636–641. [Google Scholar] [CrossRef]
- Puijenbroek, P.J.T.M.V.; Beusen, A.H.W.; Bouwman, A.F. Datasets of the phosphorus content in laundry and dishwasher detergents. Data Brief 2018, 21, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.R.B.; Verhagen, H.; Klungel, O.H.; Garssen, J.; Loveren, H.V.; Henk, J.V.K.; Rompelberg, C.J.M. Functional foods and dietary supplements: Products at the interface between pharma and nutrition. Eur. J. Pharmacol. 2011, 668, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.M. Pollution: Causes, Effects, and Control, 3rd ed.; The Royal Society of Chemistry: London, UK, 1996. [Google Scholar]
- Kiely, G. Environmental Engineering; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]
- Chen, M.; Chen, J.; Sun, F. Agricultural phosphorus flow and its environmental impacts in China. Sci. Total Environ. 2008, 405, 140–152. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Elser, J.J.; Hilton, J.; Ohtake, H.; Schipper, W.J.; Dijk, K.C. Greening the global phosphorus cycle: How green chemistry can help achieve planetary P sustainability. Green Chem. 2015, 17, 2087–2099. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Xiao, J.; Zheng, T.; Liu, M.; Wu, H.; Liu, Z. Highly selective and sensitive detection of PO43—Ions in aqueous solution by a luminescent terbium metal-organic framework. ACS Omega 2019, 4, 16378–16384. [Google Scholar] [CrossRef] [Green Version]
- Blaas, H.; Kroeze, C. Excessive nitrogen and phosphorus in European rivers: 2000–2050. Ecol. Indic. 2016, 67, 328–337. [Google Scholar] [CrossRef]
- Ding, S.; Chen, M.; Gong, M.; Fan, X.; Qin, B.; Xu, H.; Gao, S.S.; Jin, Z.; Tsang, D.C.W.; Zhang, C. Internal phosphorus loading from sediments causes seasonal nitrogen limitation for harmful algal blooms. Sci. Total Environ. 2018, 625, 872–884. [Google Scholar] [CrossRef]
- Nandakumar, S.; Pipil, H.; Ray, S.; Haritash, A.K. Removal of phosphorus and nitrogen from wastewater in brachiaria-based constructed wetland. Chemosphere 2019, 233, 216–222. [Google Scholar] [CrossRef]
- Troesch, S.; Esser, D.; Molle, P. Nature rock phosphate: A sustainable solution for phosphorus removal from wastewater. Procedia Eng. 2019, 138, 119–126. [Google Scholar] [CrossRef]
- Monetti, J.; Ledezma, P.; Virdis, B.; Freguia, S. Nutrient recovery by bio-electro concentration is limited by wastewater conductivity. ACS Omega 2019, 4, 2152–2159. [Google Scholar] [CrossRef]
- Dai, H.; Lu, X.; Peng, Y.; Yang, Z.; Zhu, H. Effects of supersaturation control strategies on hydroxyapatite (HAP) crystallization for phosphorus recovery from wastewater. Environ. Sci. Pollut. Res. 2017, 24, 5791–5799. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Nan, J.; Zu, X.; Zhang, X.; Huang, W.; Wang, W. La-based-adsorbents for efficient biological phosphorus treatment of wastewater: Synergistically strengthen of chemical and biological removal. Chemosphere 2020, 255, 127010. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, Y. Phosphorus removal and recovery from domestic wastewater in a novel process of enhanced biological phosphorus removal coupled with crystallization. Bioresour. Technol. 2016, 211, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mbamba, C.K.; Lindblom, E.; Alsina, X.F.; Tait, S.; Anderson, S.; Saagi, R.; Batstone, D.J.; Gernaey, K.V.; Jeppsson, U. Plant-wide model-based analysis of iron dosage strategies for chemical phosphorus removal in wastewater treatment systems. Water Res. 2019, 155, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Sun, P.D.; Han, J.Y.; Song, Y.Q.; Hu, Z.R.; Fan, H.Q.; Lv, S.Y. Inhibitory factors affecting the process of enhanced biological phosphorus removal (EBPR)—A mini-review. Proc. Biochem. 2014, 49, 2207–2213. [Google Scholar] [CrossRef]
- Dai, H.L.; Lu, X.W.; Peng, Y.H.; Zou, H.M.; Shi, J. An efficient approach for phosphorus recovery from wastewater using series-coupled air-agitated crystallization reactors. Chemosphere 2016, 165, 211–220. [Google Scholar] [CrossRef]
- Qiu, L.P.; Wang, G.W.; Zhang, S.B.; Yang, Z.X.; Li, Y.B. An approach for phosphate removal with quartz sand, ceramsite, blast furnace slag and steel slag as seed crystal. Water Sci. Technol. 2012, 65, 1048–1053. [Google Scholar] [CrossRef]
- Bi, W.; Li, Y.Y.; Hu, Y.Y. Recovery of phosphorus and nitrogen from alkaline hydrolysis supernatant of excess sludge by magnesium ammonium phosphate. Bioresour. Technol. 2014, 166, 1–8. [Google Scholar] [CrossRef]
- Ye, Z.L.; Shen, Y.; Ye, X.; Zhang, Z.J.; Chen, S.H.; Shi, J.W. Phosphorus recovery from wastewater by struvite crystallization: Property of aggregates. J. Environ. Sci. 2014, 26, 991–1000. [Google Scholar] [CrossRef]
- Yang, M.; Shi, J.; Xu, Z.; Zhu, S.; Cui, Y. Phosphorus removal and recovery from Fosfomycin pharmaceutical wastewater by the induced crystallization process. J. Environ. Manag. 2019, 231, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bligh, M.W.; Liang, P.; Waite, T.D.; Huang, X. Phosphorus removal by in situ generated Fe (II): Efficacy, kinetics, and mechanism. Water Res. 2018, 136, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Quin, Y.; Islam, E.; Yue, M.; Wang, W. Phosphate removal from solution using powdered freshwater mussel shells. Desalination 2011, 276, 317–321. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sakoda, A.; Suzuki, M. Technical Note: Removal of phosphate by adsorption onto oyster shell powder-kinetic studies. J. Chem. Technol. Biotechnol. 2011, 80, 356–358. [Google Scholar] [CrossRef]
- Wirojanagud, W.; Tantemsapya, N.; Tantriratna, P. Precipitation of heavy metals by lime mud waste of pulp and paper mill. Songklanakarin J. Sci. Technol. 2004, 26, 45–53. [Google Scholar]
- Zhang, J.; Zheng, P.; Wang, Q. Lime mud from papermaking process as a potential ameliorant for pollutants at ambient conditions: A review. J. Clean. Prod. 2015, 103, 828–836. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.Y.; Hussain, A.; Yang, C.M. Preparation and characterization of ceramsite from lime mud and coal fly ash. Constr. Build. Mater. 2015, 95, 10–17. [Google Scholar] [CrossRef]
- Vu, H.H.T.; Khan, M.D.; Chilakala, R.; Lai, T.Q.; Thenepalli, T.; Ahn, J.W.; Park, D.U.; Kim, J. Utilization of lime mud waste from paper mills for efficient phosphorus removal. Sustainability 2019, 11, 1524. [Google Scholar] [CrossRef] [Green Version]
- Habte, L.; Shiferaw, N.; Khan, M.D.; Thriveni, T.; Ahn, J.W. Sorption of Cd2+ and Pb2+ on aragonite synthesized from eggshell. Sustainability 2020, 12, 1174. [Google Scholar] [CrossRef] [Green Version]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multiwalled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.V.; Quang, D.V.; Nguyen Thi, H.P.; Truong, T.N.; La, D.D. Effective removal of Pb (II) from aqueous media by a new design of Cu-Mg binary ferrite. ACS Omega 2020, 5, 7298–7306. [Google Scholar] [CrossRef] [PubMed]
- Makreski, P.; Jovanovski, G. Minerals from Macedonia IX. Distinction between some rhombohedral carbonates by FT IR spectroscopy. Bull. Chem. Technol. Macedonia 2003, 22, 25–32. [Google Scholar]
- Miguel, G.-R.; Juan, H.; Leticia, B.; Joaquin, N.-M.; Mario, E.R.-G. Characterization of calcium carbonate, calcium oxide, and calcium hydroxide as starting point to the improvement of lime for their use in construction. J. Mater. Civ. Eng. 2009, 22, 625–708. [Google Scholar]
- Mohadi, R.; Sueb, A.; Anggraini, K.; Lesbani, A. Calcium oxide catalyst base on quail eggshell for biodiesel systhesis from waste palm oil. J. Pure Appl. Chem. Res. 2018, 7, 130–139. [Google Scholar] [CrossRef]
- Torit, J.; Phihusut, D. Phosphorus removal from wastewater using eggshell ash. Environ. Sci. Pollut. Res. 2019, 26, 34101–34109. [Google Scholar] [CrossRef]
- Krishna, K.C.B.; Niaz, M.E.; Sarker, D.C.; Jansen, T. Phosphorus removal from aqueous solution can be enhance through the calcination of lime sludge. J. Environ. Manag. 2017, 2000, 359–365. [Google Scholar] [CrossRef]
- Ronkanen, A.K.; Marttila, H.; Celebi, A.; Klove, B. The role of aluminum and iron in phosphorus removal by treatment peatlands. Ecol. Eng. 2016, 86, 190–201. [Google Scholar] [CrossRef]
- Sima, T.V.; Letshwenyo, M.W.; Lebogang, L. Efficiency of waste clinker ash and iron oxide tailings for phosphorus removal from tertiary wastewater: Batch studies. Environ. Technol. Innov. 2018, 11, 49–63. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, L.; He, Y.; Zhang, B.; Kirumba, G.; Xie, J. Adsorptive removal of phosphorus from aqueous solution using sponge iron and zeolite. J. Colloid Interface Sci. 2013, 402, 246–252. [Google Scholar] [CrossRef]
- Guo, G.; Ekama, G.A.; Wang, Y.; Dai, J.; Biswal, B.K.; Chen, G.; Wu, D. Advances in sulfur conversion associated enhanced biological phosphorus removal in sulfate-rich wastewater treatment: A review. Bioresour. Techol. 2019, 285, 121303. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 8. [Google Scholar] [CrossRef]










| Parameter | Value | Experiment Conditions | |
|---|---|---|---|
| Calcination temperature (°C) | 0 | Phosphorus concentration = 15 mg TP/L, Sorbent dosage = 10 mg | 200 rpm of mixing speed, room temperature (25 °C), 150 mL volume of phosphorus solution |
| 800 | |||
| 1000 | |||
| Sorbent dosage (mg) | 4.5 | Phosphorus concentration = 15 mg TP/L, Calcite mud calcined at 1000 °C | |
| 6.75 | |||
| 9 | |||
| 11.25 | |||
| Phosphorus concentration (mg TP/L) | 5 | Sorbent dosage = 11.25 mg, Calcite mud calcined at 1000 °C | |
| 15 | |||
| 30 | |||
| 60 | |||
| Kinetic Models | Fitting Parameters | Unit | Values |
|---|---|---|---|
| Pseudo-first-order model (PFO) | qe | mg/g | 98.79 |
| kPFO | min−1 | 4.234 | |
| R2 | 0.614 | ||
| Pseudo-second-order model (PSO) | qe | mg/g | 342.47 |
| kPSO | g∙mg−1∙ min−1 | 0.0055 | |
| R2 | 0.9995 | ||
| Intraparticle diffusion model (IPD) | c | 275.15 | |
| kIPD | mg∙ g−1∙min−2 | 9.751 | |
| R2 | 0.7837 |
| Experimental Conditions | qe (Experiment) (mg/g) | qe (Model) (mg/g) | kPSO (g∙mg−1∙min−1) | R2 | |
|---|---|---|---|---|---|
| Co = 5 (mg TP/L) | W = 11.25 (mg) | 66.67 | 66.67 | 0.5625 | 1.0000 |
| Co = 15 (mg TP/L) | 200.00 | 200.00 | 0.3125 | 1.0000 | |
| Co = 30 (mg TP/L) | 341.33 | 344.83 | 0.0057 | 0.9995 | |
| Co = 60 (mg TP/L) | 570.67 | 588.24 | 0.0006 | 0.9998 | |
| W = 4.50 (mg) | Co = 15 (mg TP/L) | 176.67 | 178.57 | 0.0128 | 0.9997 |
| W = 6.75 (mg) | 273.33 | 277.7778 | 0.0029 | 0.9974 | |
| W = 9.00 (mg) | 246.67 | 250.00 | 0.0041 | 1.0000 | |
| W = 11.25 (mg) | 200.00 | 200.00 | 0.3125 | 1.0000 | |
| Kinetic Models | Fitting Parameters | Values |
|---|---|---|
| Langmuir’s isotherm model | qmax | 851.42 mg/g |
| kLang | 0.9820 | |
| R2 | 0.9545 | |
| Freundlich’s isotherm model | n | 0.57967 |
| kFreu | 387.11 | |
| R2 | 0.9474 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, H.H.T.; Khan, M.D.; Tran, V.T.; Quang, D.V.; Dao, V.-D.; Lee, S.; Ahn, J.W.; Jung, S.-h. Use of Calcite Mud from Paper Factories in Phosphorus Treatment. Sustainability 2020, 12, 5982. https://doi.org/10.3390/su12155982
Vu HHT, Khan MD, Tran VT, Quang DV, Dao V-D, Lee S, Ahn JW, Jung S-h. Use of Calcite Mud from Paper Factories in Phosphorus Treatment. Sustainability. 2020; 12(15):5982. https://doi.org/10.3390/su12155982
Chicago/Turabian StyleVu, Hong Ha Thi, Mohd Danish Khan, Van Tan Tran, Dang Viet Quang, Van-Duong Dao, Seongho Lee, Ji Whan Ahn, and Seok-ho Jung. 2020. "Use of Calcite Mud from Paper Factories in Phosphorus Treatment" Sustainability 12, no. 15: 5982. https://doi.org/10.3390/su12155982