Alpha-Ketoglutaric Acid Production from a Mixture of Glycerol and Rapeseed Oil by Yarrowia lipolytica Using Different Substrate Feeding Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Media and Culture Conditions
2.3. Analytical Methods
3. Results
3.1. Fed-Batch Culture at a Thiamine Concentration of 0.6 µg·dm−3 and Individual Substrates as Carbon Sources in Batches
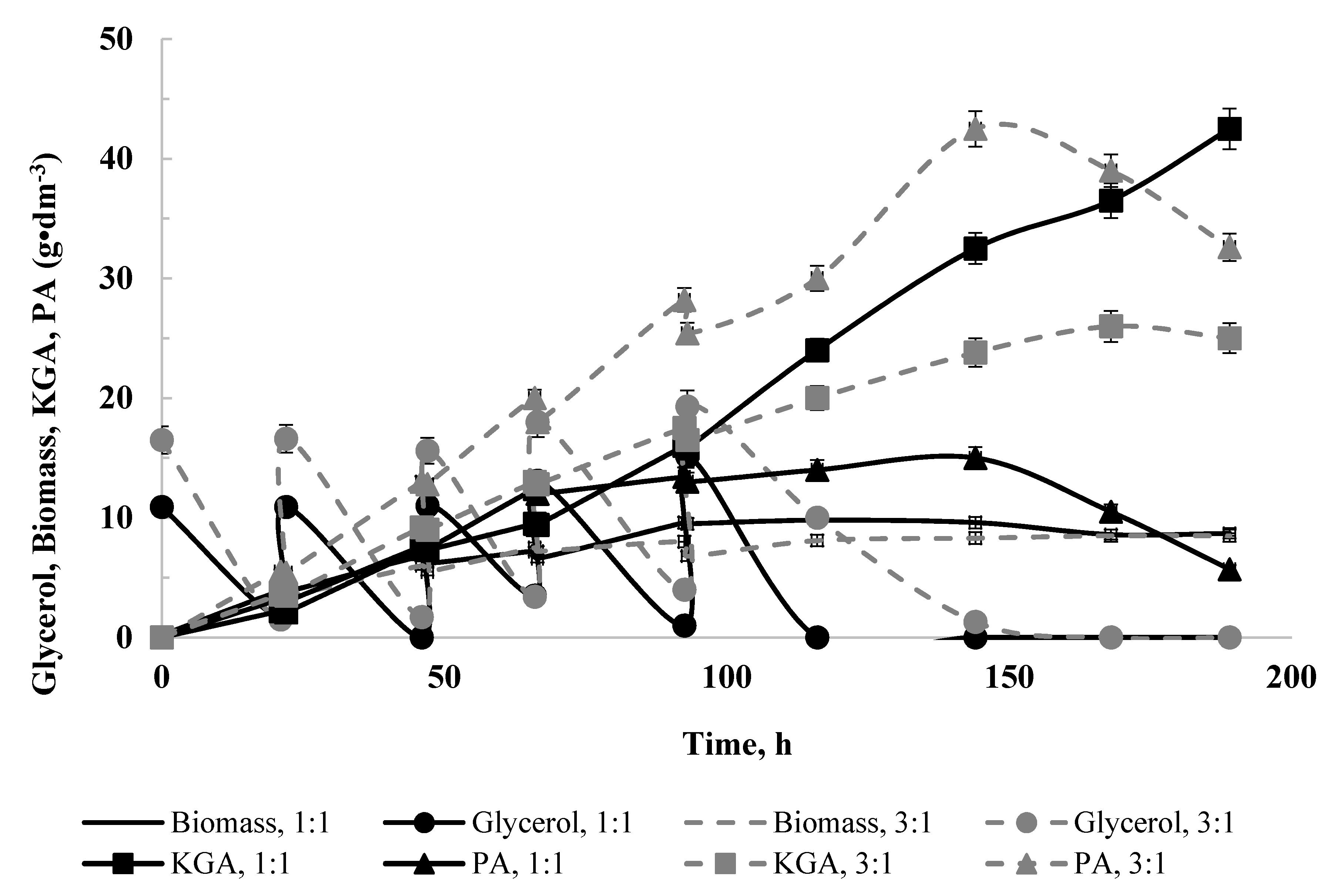
Fed-Batch Culture at a Thiamine Concentration 0.6 µg·dm−3 and with a Mixture of Substrates as a Carbon Source in Batches
3.2. Fed-Batch Culture at a Thiamine Concentration of 3.0 µg·dm−3
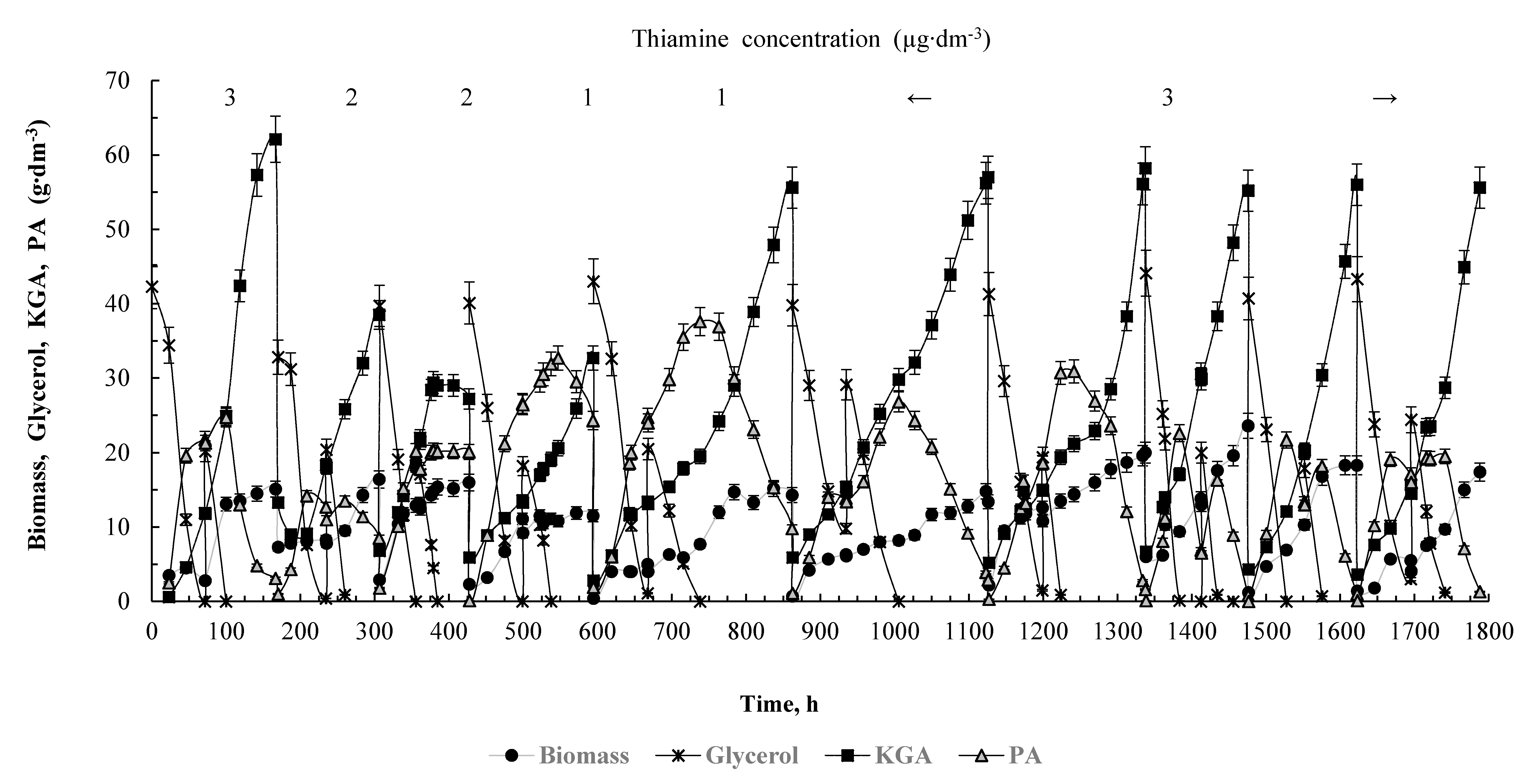
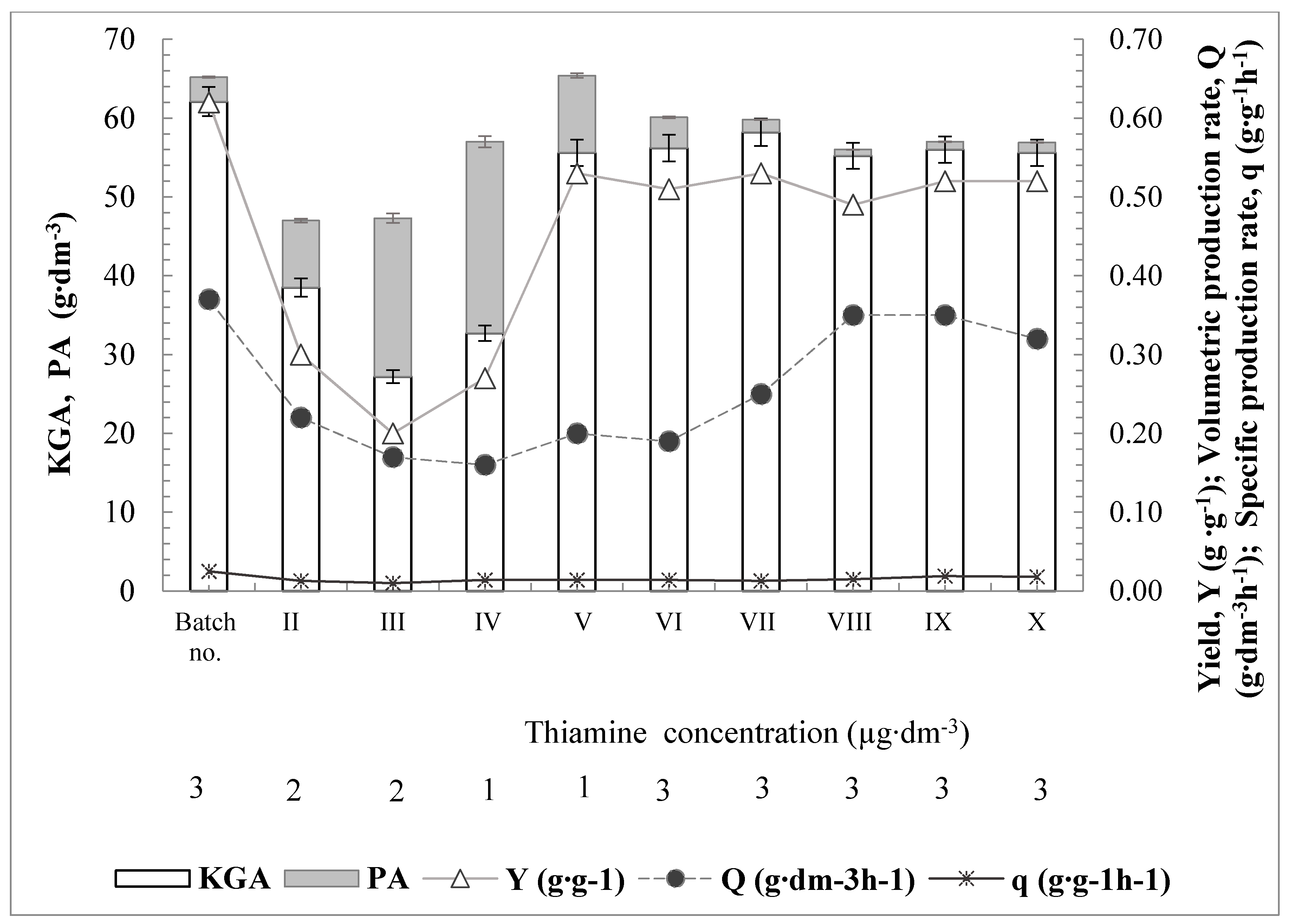
3.3. Repeated-Batch Cultivation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wernerman, J.; Hammarqvist, F. Glutamine: A necessary nutrient for the intensive care patient. Int. J. Colorectal. Dis. 1999, 14, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.K.; Tatara, M.R.; Krupski, W.; Majcher, P.; Harrison, A.P. The long-term effect of alpha-ketoglutarate, given early in postnatal life, on both growth and various bone parameters in pigs. J. Anim. Physiol. Anim. Nutr. 2008, 92, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Tatara, M.; Brodzki, A.; Krupski, W.; Silmanowicz, P.; Majcher, P.; Pierzynowski, S.; Studziński, T. Effects of alpha-ketoglutarate on bone homeostasis and plasma aminoacids in turkeys. Poult. Sci. 2005, 84, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Radzki, R.; Bienko, M.; Puzio, I.; Filip, R.; Pierzynowski, S.; Studzinski, T. The effect of alpha-ketoglutarate(AKG) on mineralization of femur in ovariectomized rats. Acta Orthop. Scand. 2002, 73, 52. [Google Scholar]
- Pierzynowski, S.G.; Filip, R.; Harrison, A. Effect of feed supplementation with alpha-ketoglutarate, combined with vitamin B∼6 or C, on the performance and hemoglobin and aminoacid levels in growing rats. Bull. Vet. Ins. Pulawy 2007, 51, 289–296. [Google Scholar]
- Tatara, M.R.; Tygesen, M.P.; Sawa-Wojtanowicz, B.; Krupski, W.; Majcher, P.; Harrison, A.P. Bonedevelopment: The effect of short-term alpha-ketoglutarate administrationon long-termmechanical properties of ribs in ram lambs. Small Rum. Res. 2007, 67, 179–183. [Google Scholar] [CrossRef]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-Ketoglutarate: Physiological function and applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Filip, R.S.; Pierzynowski, S.G.; Lindegard, B.; Wernerman, J.; Heratym-Maj, A.; Podgurniak, M. Alpha-ketoglutarate decreases serum levels of C-terminal cross-linking telopeptide of type I collagen (CTX) in postmenopausal women with osteopenia: Six-month study. Int. J. Vitam. Nutr. Res. 2007, 77, 89–97. [Google Scholar] [CrossRef]
- Moser, P.M.; Greilberger, J.; Maier, A.; Juan, H.; Bücherl-Harrer, C.; Kager, E. Verwendung von Alpha-Ketoglutarsäure und 5-Hydroxy-Methylfurfural zur Reduktionvon Oxidativem Stress. CYLP GmbH Patent EP1842536A1, 10 October 2007. [Google Scholar]
- Tatara, M.R.; Krupski, W.; Tymczyna, B.; Studziński, T. Effects of combined maternal administration with alpha-ketoglutarate (AKG) and β-hydroxy-β-methylbutyrate (HMB) on prenatal program in gofskeletal properties in the off spring. Nutr. Metab. 2012, 9, 2–12. [Google Scholar] [CrossRef]
- Dąbek, M.; Kruszewska, D.; Filip, R.; Hotowy, A.; Pierzynowski, L.; Wojtasz-Pająk, A.; Szymanczyk, S.; Piedra, J.; Werpachowska, E.; Pierzynowski, S. α-Ketoglutarate (AKG) absorption from pig in testine and plasma pharmacokinetics. J. Anim. Physiol. Anim. Nutr. 2005, 89, 419–426. [Google Scholar] [CrossRef]
- Yu, Z.; Du, G.; Zhou, J.; Chen, J. Enhanced α-ketoglutaric acid productionin Yarrowia lipolytica WSH-Z06 by an improved integrated fed-batch strategy. Bioresour. Technol. 2012, 114, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, S.V.; Monsalve, G.C.; et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Chiglintseva, M.N.; Lunina, J.N.; Morgunov, I.G. Ketoglutaric acid production by Yarrowia lipolytica and its regulation. Appl. Biochem. Microbiol. 2012, 96, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Förster, A.; Mauersberger, S.; Köllner, K.; Barth, G. Verbundvorhaben: Biotechnologische Gewinnung von Carbonsäuren, Teilvorhaben 3: Genetic Engineering Oxocarbonsäure Bildender Hefen. Endbericht; Projektder Fachagenturfürnach Wachsen de Rohstoffee. Gerold Barth Editor Biotechnological Applications No. FZ: 22002505, 30 November 2006. [Google Scholar]
- Barrett, D.G.; Yousaf, M.N. Poly(triolα-ketoglutarate) as biodegradable, chemoselective, and mechanically tunable elastomers. Macromolecules 2008, 41, 6347–6352. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. α-ketoglutaric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl. Microbiol. Biotechnol. 2013, 97, 5517–5525. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Yovkova, V.; Barth, G. Overproduction and secretion of α-ketoglutaric acid by microorganisms. Appl. Microbiol. Biotechnol. 2011, 92, 689–695. [Google Scholar] [CrossRef]
- Barth, G.; Gaillardin, C. Yarrowia Lipolytica Nonconventional Yeasts in Biotechnology; Wolf, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 313–388. [Google Scholar]
- Cybulski, K.; Tomaszewska-Hetman, L.; Rakicka, M.; Juszczyk, P.; Rywińska, A. Production of pyruvic acid from glycerol by Yarrowia lipolytica. Folia Microbiol. 2019, 64, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, R.; Okumura, S. Fermentation of n-paraffins by Yeast. Part II. a-ketoglutarate productivity of Candida lipolytica in various culture media. Agric. Biol. Chem. 1969, 33, 676–682. [Google Scholar]
- Chernyavskaya, O.G.; Shishkanova, N.V.; Il’chenko, A.P.; Finogenova, T.V. Synthesis of alpha ketoglutaric acid by Yarrowia lipolytica yeast grown on ethanol. Appl. Microbiol. Biotechnol. 2000, 53, 152–158. [Google Scholar] [CrossRef]
- Aurich, A.; Stottmeister, U. Verbundvorhaben: Biotechnologische Gewinnungvon Carbonsäuren, Teilvorhaben 1: Biotechnologische Gewinnungvon Oxocarbonsäurenals Synthesebausteine. Präambel, Endbericht; Projektder Fachagenturfürnach Wachsende Rohstoffee. V., BMVEL220010701. Available online: http://www.fnr-server.de/ftp/pdf/berichte/22010701.pdf (accessed on 31 August 2006).
- Zhou, J.; Zhou, H.; Du, G.; Liu, L.; Chen, J. Screeningofathiamine-auxotrophicyeastforα-ketoglutaricacidoverproduction. Lett. Appl. Microbiol. 2010, 51, 264–271. [Google Scholar] [CrossRef]
- Aggelis, G. Microbial Conversion of Raw Glycerol; Nova Science Publishers Inc.: New York, NY, USA, 2009. [Google Scholar]
- Rakicka, M.; Biegalska, A.; Rymowicz, W.; Dobrowolski, A.; Mirończuk, A.M. Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Rymowicz, W.; Żarowska, B.; Skrzypiński, A. Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2010, 26, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Madzak, C.; Du, G.; Zhou, J.; Chen, J. Enhanced alpha-ketoglutaric acid production in Yarrowia lipolytica WSH-Z06 by regulation of the pyruvate carboxylation pathway. Appl. Microbiol. Biotechnol. 2012, 96, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Finogenova, T.V.; Morgunov, I.G.; Kamzolova, S.V.; Chernyavskaya, O.G. Organic acid production by the yeast Yarrowia lipolytica: A review of prospects. Appl. Biochem. Microbiol. 2005, 41, 418–425. [Google Scholar] [CrossRef]
- Otto, C.; Yovkova, V.; Aurich, A.; Mauersberger, S.; Barth, G. Variation of the by-product spectrum during α-ketoglutaric acid production from raw glycerol by overexpression of fumarase and pyruvate carboxylase genes in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2012, 95, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Yovkova, V.; Otto, C.; Aurich, A.; Mauersberger, S.; Barth, G. Engineering the α-ketoglutarate over production from raw glycerol by overexpression of the genes encoding NADP+-dependent isocitrate dehydrogenase and pyruvate carboxylase in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2014, 98, 2003–2013. [Google Scholar] [CrossRef]
- Holz, M.; Otto, C.; Kretzschmar, A.; Yovkova, V.; Aurich, A.; Pötter, M.; Marx, A.; Barth, G. Overexpression of alpha-ketoglutarate dehydrogenase in Yarrowia lipolytica and its effecton production of organic acids. Appl. Microbiol. Biotechnol. 2011, 89, 1519–1526. [Google Scholar] [CrossRef]
- Guo, H.; Madzak, C.; Du, G.; Zhou, J.; Chen, J. Effects of pyruvate dehydrogenase subunits over expressionon the α-ketoglutarate productionin Yarrowia lipolytica WSH-Z06. Appl. Microbiol. Biotechnol. 2014, 98, 7003–7012. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Chen, J.; Wu, Q.; Chen, G. Open and continuous fermentation: Products, conditions, and bioprocess economy. Biotechnol. J. 2014, 9, 1503–1511. [Google Scholar] [CrossRef]
- Benvenuti, G.; Lamers, P.P.; Breuer, G.; Bosma, R.; Cerar, A.; Wijffels, R.H.; Barbosa, M.J. Microalgal TAG production strategies: Why batch beats repeated-batch. Biotechnol. Biofuels 2016, 9, 64. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, C.; Wu, S.; Shen, H.; Zhao, Z.K. Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies. J. Ind. Microbiol. Biotechnol. 2011, 38, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of high titer of citric acid from inulin. BMC Biotechnol. 2019, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Rymowicz, W. High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J. Ind. Microbiol. Biotechnol. 2010, 37, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk, A.M.; Furgała, J.; Rakicka, M.; Rymowicz, W. Enhanced production of erythritol by Yarrowia lipolytica on glycerol in repeated batch cultures. J. Ind. Microbiol. Biotechnol. 2014, 41, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.P.; Odaneth, A.A.; Vadgama, R.N.; Lali, A.M. Simultaneous lipid biosynthesis and recovery for oleaginous yeast Yarrowia lipolytica. Biotechnol. Biofuels 2019, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yin, X.; Madzak, C.; Du, G.; Chen, J. Enhanced α-ketoglutarate productionin Yarrowia lipolytica WSH-Z06 by alteration of the acetyl-CoA metabolism. J. Biotechnol. 2012, 161, 257–264. [Google Scholar] [CrossRef] [PubMed]



| Cultivation Regime | Thiamine (µg·dm−3) | Substrate Feeding Strategy | Y (g·g−1) | Q (g·dm−3·h−1) | q (g·g−1·h−1) | Selectivity (%) |
|---|---|---|---|---|---|---|
| Fed-batch | 0.6 | G(GGGG) | 0.05 | 0.029 | 0.0097 | 21 |
| Fed-batch | 0.6 | G(GGOO) | 0.1 | 0.054 | 0.0174 | 27 |
| Fed-batch | 0.6 | G(OGGO) | 0.3 | 0.16 | 0.017 | 68 |
| Fed-batch | 0.6 | G(OOGG) | 0.13 | 0.069 | 0.0071 | 32 |
| Fed-batch | 0.6 | O(OOOO) | 0.59 | 0.31 | 0.018 | 99 |
| Fed-batch | 0.6 | 10G + 10O(4 × (10G + 10O)) | 0.42 | 0.22 | 0.025 | 88 |
| Fed-batch | 0.6 | 15G + 5O(4 × (15G + 5O)) | 0.25 | 0.13 | 0.016 | 43 |
| Fed-batch | 3.0 | O(GGGO) | 0.38 | 0.2 | 0.019 | 84 |
| Fed-batch | 3.0 | G(GGGO) | 0.19 | 0.1 | 0.01 | 33 |
| Fed-batch | 3.0 | O(4 × (15G + 5O)) | 0.4 | 0.21 | 0.014 | 74 |
| Fed-batch | 3.0 | G(4 × (15G + 5O)) | 0.42 | 0.22 | 0.022 | 79 |
| Repeated fed-batch (no. I) | 3.0 | 2G(_OGO) | 0.62 | 0.37 | 0.025 | 95 |
| Repeated fed-batch (no. II–III) | 2.0 | 2G(_OGO) | 0.2–0.3 | 0.17–0.22 | 0.01–0.013 | 58–82 |
| Repeated fed-batch (no. IV–V) | 1.0 | 2G(_OGO) | 0.27–0.53 | 0.16–0.2 | 0.014 | 57–85 |
| Repeated fed-batch (no. VI–X) | 3.0 | 2G(_OGO) | 0.49–0.53 | 0.19–0.35 | 0.013–0.019 | 94–99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rywińska, A.; Tomaszewska-Hetman, L.; Rakicka-Pustułka, M.; Juszczyk, P.; Rymowicz, W. Alpha-Ketoglutaric Acid Production from a Mixture of Glycerol and Rapeseed Oil by Yarrowia lipolytica Using Different Substrate Feeding Strategies. Sustainability 2020, 12, 6109. https://doi.org/10.3390/su12156109
Rywińska A, Tomaszewska-Hetman L, Rakicka-Pustułka M, Juszczyk P, Rymowicz W. Alpha-Ketoglutaric Acid Production from a Mixture of Glycerol and Rapeseed Oil by Yarrowia lipolytica Using Different Substrate Feeding Strategies. Sustainability. 2020; 12(15):6109. https://doi.org/10.3390/su12156109
Chicago/Turabian StyleRywińska, Anita, Ludwika Tomaszewska-Hetman, Magdalena Rakicka-Pustułka, Piotr Juszczyk, and Waldemar Rymowicz. 2020. "Alpha-Ketoglutaric Acid Production from a Mixture of Glycerol and Rapeseed Oil by Yarrowia lipolytica Using Different Substrate Feeding Strategies" Sustainability 12, no. 15: 6109. https://doi.org/10.3390/su12156109
APA StyleRywińska, A., Tomaszewska-Hetman, L., Rakicka-Pustułka, M., Juszczyk, P., & Rymowicz, W. (2020). Alpha-Ketoglutaric Acid Production from a Mixture of Glycerol and Rapeseed Oil by Yarrowia lipolytica Using Different Substrate Feeding Strategies. Sustainability, 12(15), 6109. https://doi.org/10.3390/su12156109





