MPN Drop Agar Method for Determination of Heterotrophic Microorganisms in Soil and Water Samples Using Tissue Plate as a Carrier
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
- -
- Providing a modified method that may be applied to microbiological examination of water and soil samples in simplified arrangement and at lower cost, thus preventing soil degradation and encouraging sustainable agricultural development;
- -
- Reducing the amount of single-use plastic consumable material used within a laboratory microbiological examination;
- -
- Reducing the amount of biohazardous waste, whose handling is typically related to complicated and expensive disposal procedures.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barberán, A.; Ramirez, K.S.; Leff, J.W.; Bradford, M.A.; Wall, D.H.; Fierer, N. Why are some microbes more ubiquitous than others? Predicting the habitat breadth of soil bacteria. Ecol. Lett. 2014, 17, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Furtak, K.; Grządziel, J.; Gałązka, A.; Niedźwiecki, J. Analysis of soil properties, bacterial community composition, and metabolic diversity in fluvisols of a floodplain area. Sustainability 2019, 11, 3929. [Google Scholar] [CrossRef] [Green Version]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Faber, J.; Bloem, J. Applying soil health indicators to encourage sustainable soil use: the transition from scientific study to practical application. Sustainability 2018, 10, 3021. [Google Scholar] [CrossRef] [Green Version]
- Chumchalová, J.; Kubal, M. Laboratory tests for aerobic bioremediation of the contaminated sites in the Czech Republic. Plant. Soil Environ. 2020, 66, 191–199. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Seo, C.; Ji, M.; Paik, M.-J.; Myung, S.; Kim, J. Effective soil extraction method for cultivating previously uncultured soil bacteria. Appl. Environ. Microbiol. 2018, 84, e01145-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torsvik, V.; Øvreås, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Hedderich, R.; Müller, R.; Greulich, Y.; Bannert, N.; Holland, G.; Kaiser, P.; Reissbrodt, R. Mechanical damage to Gram-negative bacteria by surface plating with the Drigalski-spatula technique. Int. J. Food Microbiol. 2011, 146, 105–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M. A modified spread plate technique for the determinations of concentrations of viable heterotrophic bacteria. In Methodology for Biomass Determinations and Microbial Activities in Sediments; ASTM International: West Conshohocken, PA, USA, 2009; p. 40. [Google Scholar]
- Thomas, P.; Sekhar, A.; Mujawar, M. Nonrecovery of varying proportions of viable bacteria during spread plating governed by the extent of spreader usage and proposal for an alternate spotting-spreading approach to maximize the CFU. J. Appl. Microbiol. 2012, 113, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Carvalhal, M.; Oliveira, M.; Alterthum, F. An economical and time saving alternative to the most-probable-number method for the enumeration of microorganisms. J. Microbiol. Methods 1991, 14, 165–170. [Google Scholar] [CrossRef]
- Woomer, P.L. Most probable number counts. In Methods of Soil Analysis, Part 2. Microbiological and Biochemical Properties; Bottomley, P.J., Angle, J.S., Weaver, R.W., Eds.; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1994; pp. 59–79. [Google Scholar]
- Harrigan, W.F. Laboratory Methods in Food Microbiology; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Maťátková, O.; Pospíšilová, D.; Michailidu, J.; Jaroš, P.; Masák, J. Effect of subinhibitory concentration of antibiotics on Rhodococcus erythropolis and Pseudomonas fluorescens biofilm formation. Chem. Pap. 2018, 73, 1113–1119. [Google Scholar] [CrossRef]
- Overhage, J.; Bains, M.; Brazas, M.D.; Hancock, R.E. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 2008, 190, 2671–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Elsas, J.D.; Hartmann, A.; Schloter, M.; Trevors, J.T.; Jansson, J.K. The bacteria and archaea in soil. In Modern Soil Microbiology, 2nd ed.; Informa UK Limited: Colchester, UK, 2019; pp. 49–64. [Google Scholar]
- Maier, R.M.; Pepper, I.L. Earth Environments. In Environmental Microbiology; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 57–82. [Google Scholar]
- Arenskötter, M.; Bröker, D.; Steinbüchel, A. Biology of the metabolically diverse genus Gordonia. Appl. Environ. Microbiol. 2004, 70, 3195–3204. [Google Scholar] [CrossRef] [Green Version]
- Darnton, N.C.; Turner, L.; Rojevsky, S.; Berg, H.C. Dynamics of bacterial swarming. Biophys. J. 2010, 98, 2082–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covelli, J.M.; Althabegoiti, M.J.; López, M.F.; Lodeiro, A.R. Swarming motility in Bradyrhizobium japonicum. Res. Microbiol. 2013, 164, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, D. Significance and roles of Proteus spp. bacteria in natural environments. Microb. Ecol. 2016, 72, 741–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, I.A.; León, N.; Izquierdo, P. Microtiter technique for enumeration of mesophiles, psychrotrophs, and coliforms in raw and pasteurized milk. J. Food Prot. 1977, 40, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Baron, F.; Cochet, M.-F.; Ablain, W.; Grosset, N.; Madec, M.-N.; Gonnet, F.; Jan, S.; Gautier, M. Rapid and cost-effective method for micro-organism enumeration based on miniaturization of the conventional plate-counting technique. Le Lait 2006, 86, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, S. Evaluating most probable number method to count and isolate viable methylotrophs. Braz. J. Microbiol. 2011, 42, 46–48. [Google Scholar] [CrossRef] [Green Version]
- Kurm, V.; Van Der Putten, W.H.; Hol, W.H.G. Cultivation-success of rare soil bacteria is not influenced by incubation time and growth medium. PLoS ONE 2019, 14, e0210073. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Nace, G.W.; Irwin, P.L. A 6×6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods 2003, 55, 475–479. [Google Scholar] [CrossRef]
- Sieuwerts, S.; De Bok, F.; Mols, E.; De Vos, W.; Vlieg, J.E.T.V.H. A simple and fast method for determining colony forming units. Lett. Appl. Microbiol. 2008, 47, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-T.; Maxcy, R.B.; Stroup, W.W. Colony-forming unit enumeration by a plate-MPN method. J. Food Prot. 1983, 46, 836–841. [Google Scholar] [CrossRef] [PubMed]
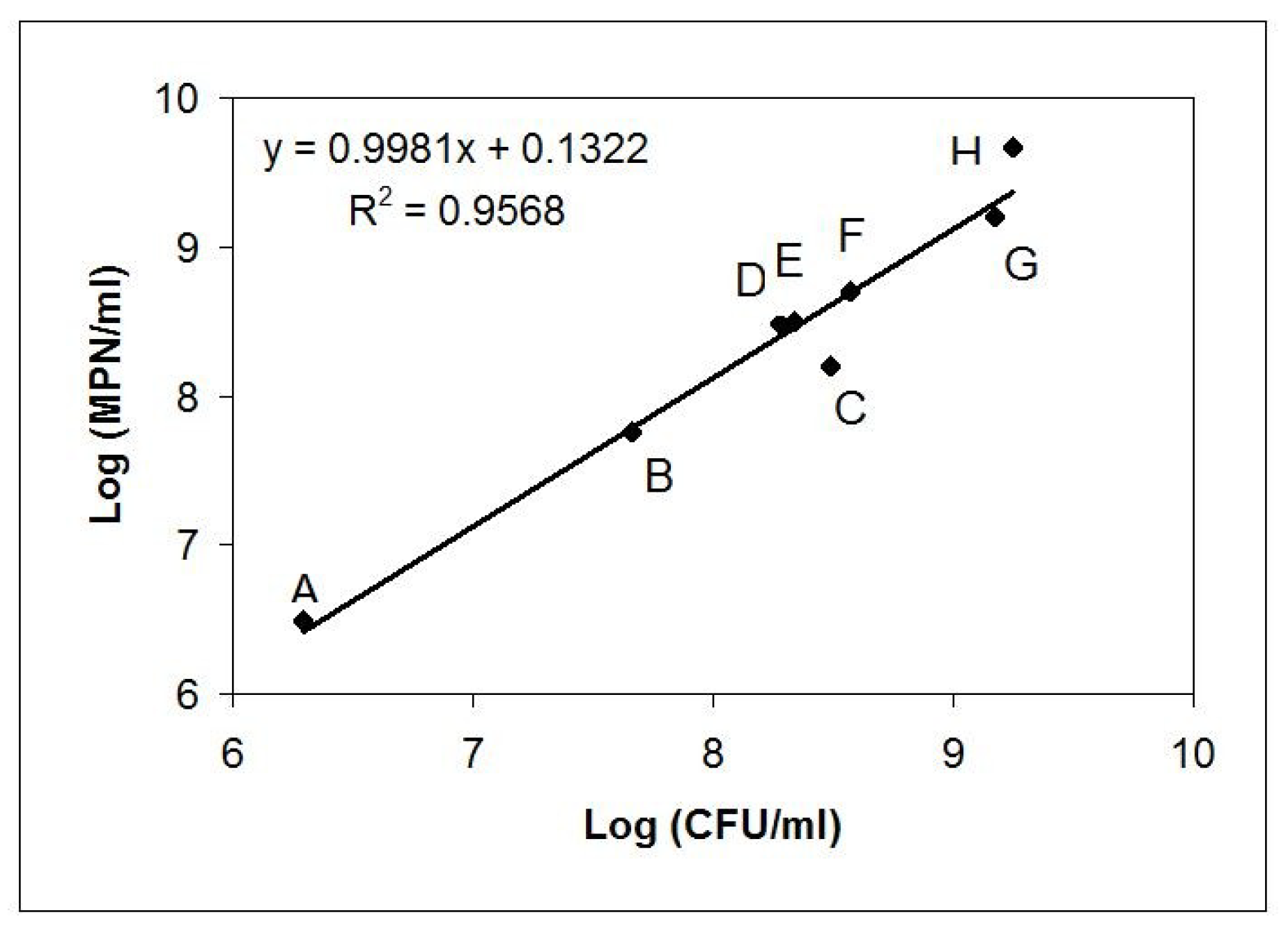
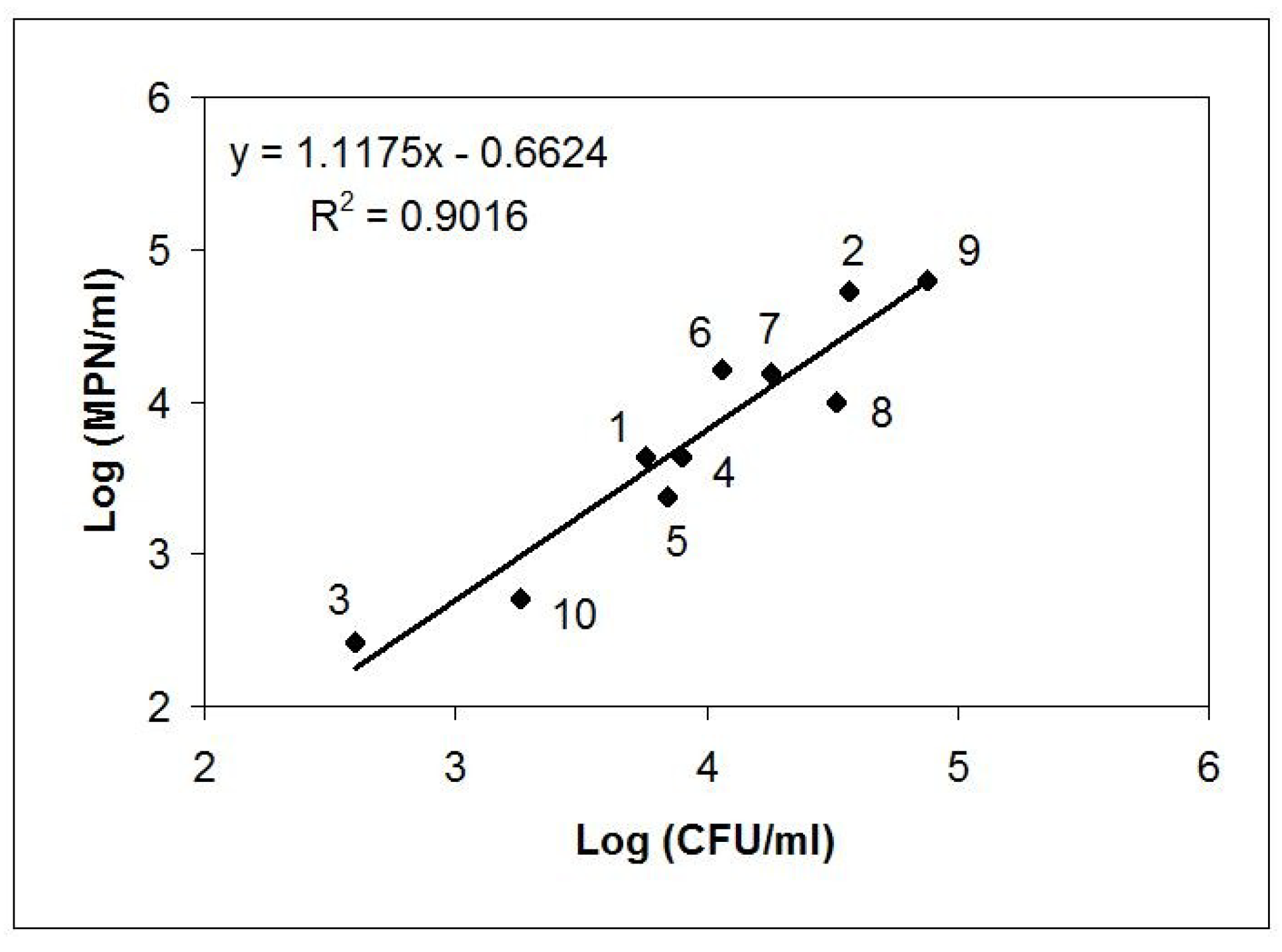
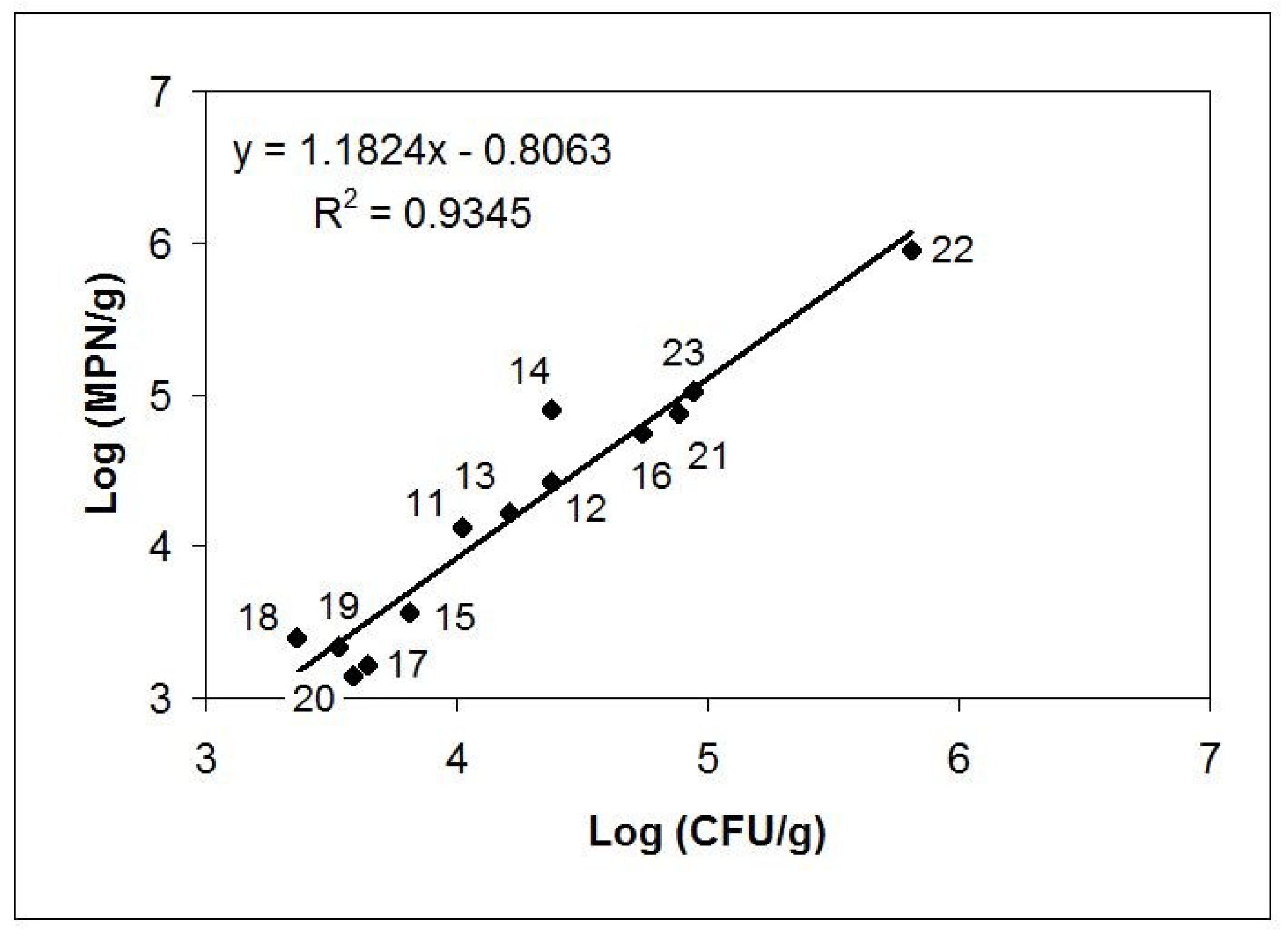
| Sample | Matrix | Description; GPS Position (Czech Republic Territory) |
|---|---|---|
| 1 | Surface water | Public swimming pool; N 49°56.38670′, E 14°44.05240′ |
| 2 | Surface water | Creek bellow output of biological wastewater treatment unit; N 50°12.96942′, E 14°8.43233′ |
| 3 | Groundwater | Drinking water well; N 49°56.28245′, E 14°41.84592′ |
| 4 | Groundwater | Drinking water well; N 49°28.75600′, E 15°44.22397′ |
| 5 | Mine water | Kutná Hora; N 49°58.46962′, E 15°16.96935′ |
| 6 | Groundwater (contaminated) | Pump-and-Treat system, Crystal Bohemia factory, Poděbrady; N 50°8.71315′, E 15°7.65625′ |
| 7 | Groundwater (contaminated) | Pump-and-Treat system, Crystal Bohemia factory, Poděbrady; N 50°8.71542′, E 15°7.62857′ |
| 8 | Groundwater (contaminated) | Pump-and-Treat system, Crystal Bohemia factory, Poděbrady; N 50°8.72520′, E 15°7.61672′ |
| 9 | Groundwater (contaminated) | Pump-and-Treat system, Spolana, Neratovice; N 50°16.23263′, E 14°30.93505′ |
| 10 | Groundwater (contaminated) | Pump-and-Treat system, Karlovy Vary; N 50°13.73613′, E 12°50.73683′ |
| 11 | Soil (2 m depth) | Sampling borehole, Praha; N 50°2.92988′, E 14°19.43730′ |
| 12 | Soil (4 m depth) | Sampling borehole, Praha; N 50°2.92988′, E 14°19.43730′ |
| 13 | Soil (6 m depth) | Sampling borehole, Praha; N 50°2.92988′, E 14°19.43730′ |
| 14 | Soil (2 m depth) | Sampling borehole, Praha; N 50°2.91913′, E 14°19.34815′ |
| 15 | Soil (4 m depth) | Sampling borehole, Praha; N 50°2.91913′, E 14°19.34815′ |
| 16 | Soil (2 m depth) | Sampling borehole, Neratovice; N 50°16.23263′, E 14°30.93505′ |
| 17 | Soil (6 m depth) | Sampling borehole, Neratovice; N 50°16.23263′, E 14°30.93505′ |
| 18 | Soil (4 m depth) | Sampling borehole, Neratovice; N 50°16.23963′, E 14°30.93377′ |
| 19 | Soil (3 m depth) | Sampling borehole, Neratovice; N 50°16.23963′, E 14°30.93377′ |
| 20 | Soil (2 m depth) | Sampling borehole, Neratovice; N 50°16.23963′, E 14°30.93377′ |
| 21 | Sediment (0.15 m depth) | Lagoon Dobroutov; N 49°28.43400′, E 15°45.15958′ |
| 22 | Sediment (surface) | Lagoon Dobroutov; N 49°28.43400′, E 15°45.15958′ |
| 23 | Soil (5 m depth) | Sampling borehole, Neratovice; N 50°16.23963′, E 14°30.93377′ |
| Method | MPN Drop Agar Method | Spread Plate Method |
|---|---|---|
| Nutrient agar (mL) | 37.5 a | 150 b |
| Quantity of carriers | 1 tissue plate (5 × 5) | 10 Petri dishes |
| Additional material | 1 tip for pouring medium | 5 spreaders |
| Dimensions of the plate with lid (mm) | 20 × 101 × 101 height × width × depth | 90 × 160 diameter × height (10 plates) |
| Space occupied (cm3) | 202 | 1017 |
| Weight of plates (g) | 46.6 | 126.8 |
| Mean weight of additional material (g) | 5 | 9 |
| Mean handling time c (min) | 5 | 20 |
| Cost (EUR) | ||||
|---|---|---|---|---|
| Method | Carrier | Medium/per Sample/Unit | Aditional Material | Sum |
| Thermo Fisher Scientific Inc. company (https://www.thermofisher.com/cz/en/home.html, 7 September 2020) | ||||
| MPN drop agar method | 2.3 EUR/1 tissue plate | 0.2 EUR/37.5 mL a | 0.3 EUR/1 tip | 2.8 EUR |
| Spread plate method | 2.1 EUR/10 Petri plates | 0.8 EUR/150 mL b | 1 EUR/5 spreaders | 3.9 EUR |
| P-LAB a.s. company (https://www.p-lab.cz/, 7 September 2020) | ||||
| MPN drop agar method | 1.5 EUR/1 tissue plate | 0.2 EUR/37.5 mL a | 0.4 EUR/1 tip | 2.1 EUR |
| Spread plate method | 1.0 EUR/10 Petri plates | 0.8 EUR/150 mL b | 1 EUR/5 spreaders | 2.8 EUR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumchalová, J.; Kubal, M. MPN Drop Agar Method for Determination of Heterotrophic Microorganisms in Soil and Water Samples Using Tissue Plate as a Carrier. Sustainability 2020, 12, 8252. https://doi.org/10.3390/su12198252
Chumchalová J, Kubal M. MPN Drop Agar Method for Determination of Heterotrophic Microorganisms in Soil and Water Samples Using Tissue Plate as a Carrier. Sustainability. 2020; 12(19):8252. https://doi.org/10.3390/su12198252
Chicago/Turabian StyleChumchalová, Jana, and Martin Kubal. 2020. "MPN Drop Agar Method for Determination of Heterotrophic Microorganisms in Soil and Water Samples Using Tissue Plate as a Carrier" Sustainability 12, no. 19: 8252. https://doi.org/10.3390/su12198252
APA StyleChumchalová, J., & Kubal, M. (2020). MPN Drop Agar Method for Determination of Heterotrophic Microorganisms in Soil and Water Samples Using Tissue Plate as a Carrier. Sustainability, 12(19), 8252. https://doi.org/10.3390/su12198252





