Geochemical Distribution Characteristics of Rare Earth Elements in Different Soil Profiles in Mun River Basin, Northeast Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analysis
2.3. Data Treatment
3. Results and Discussion
3.1. Contents of Rare Earth Elements in Soil
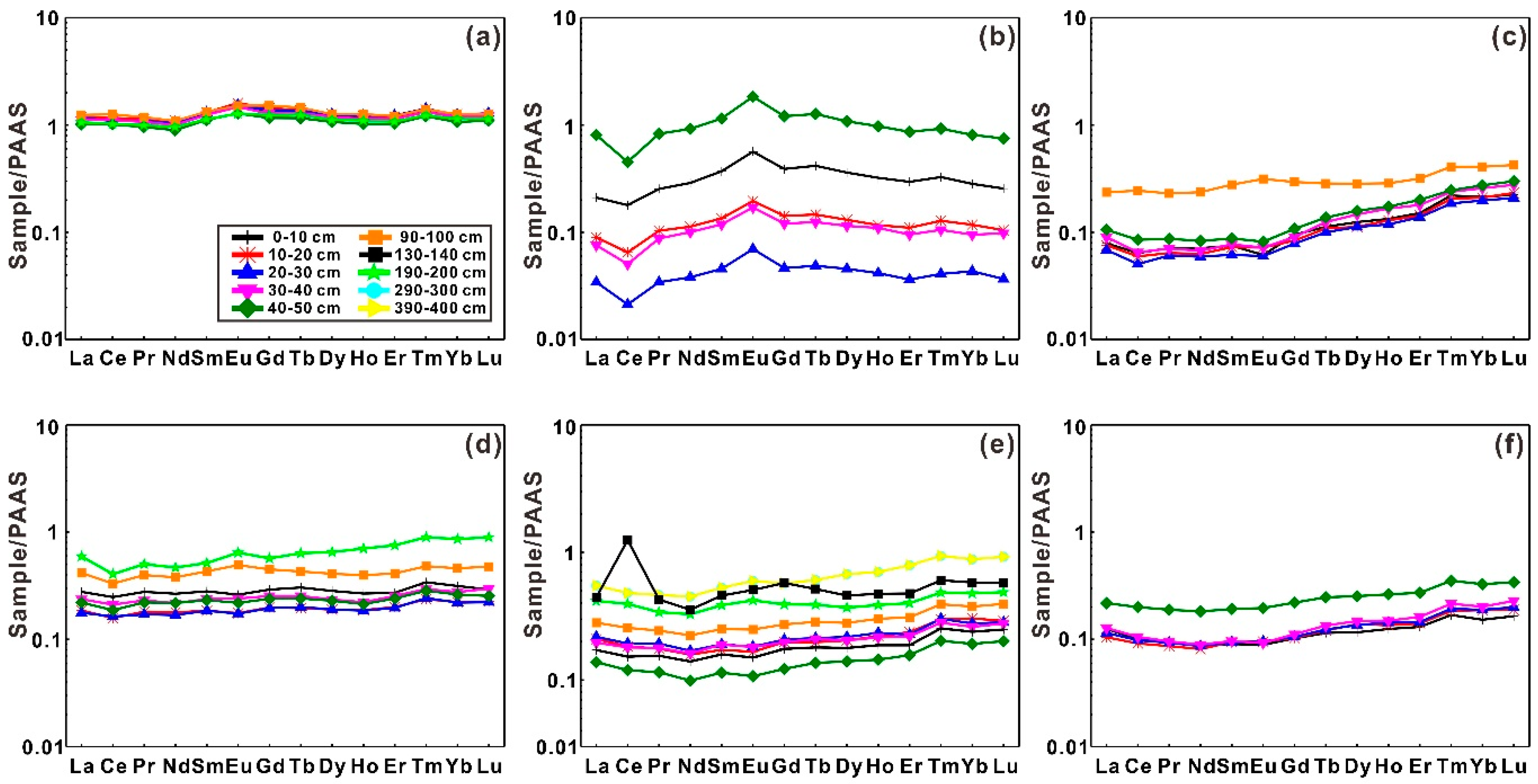
3.2. PAAS-Normalized REE Patterns
3.2.1. Fractionation between LREEs and HREEs
3.2.2. Ce and Eu Anomalies
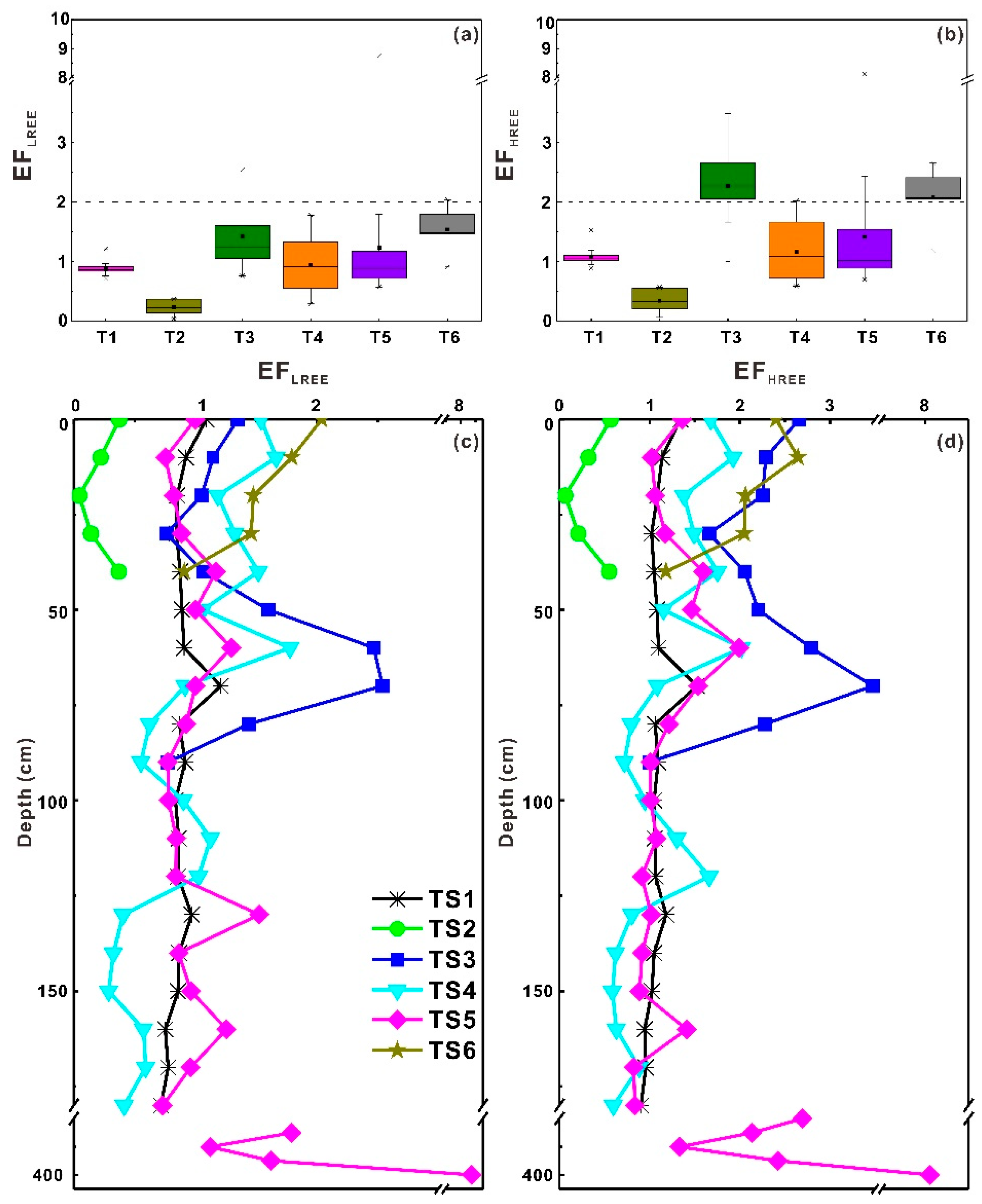
3.3. Enrichment Factors (EFs) of REEs
3.3.1. Distribution Patterns of EFs
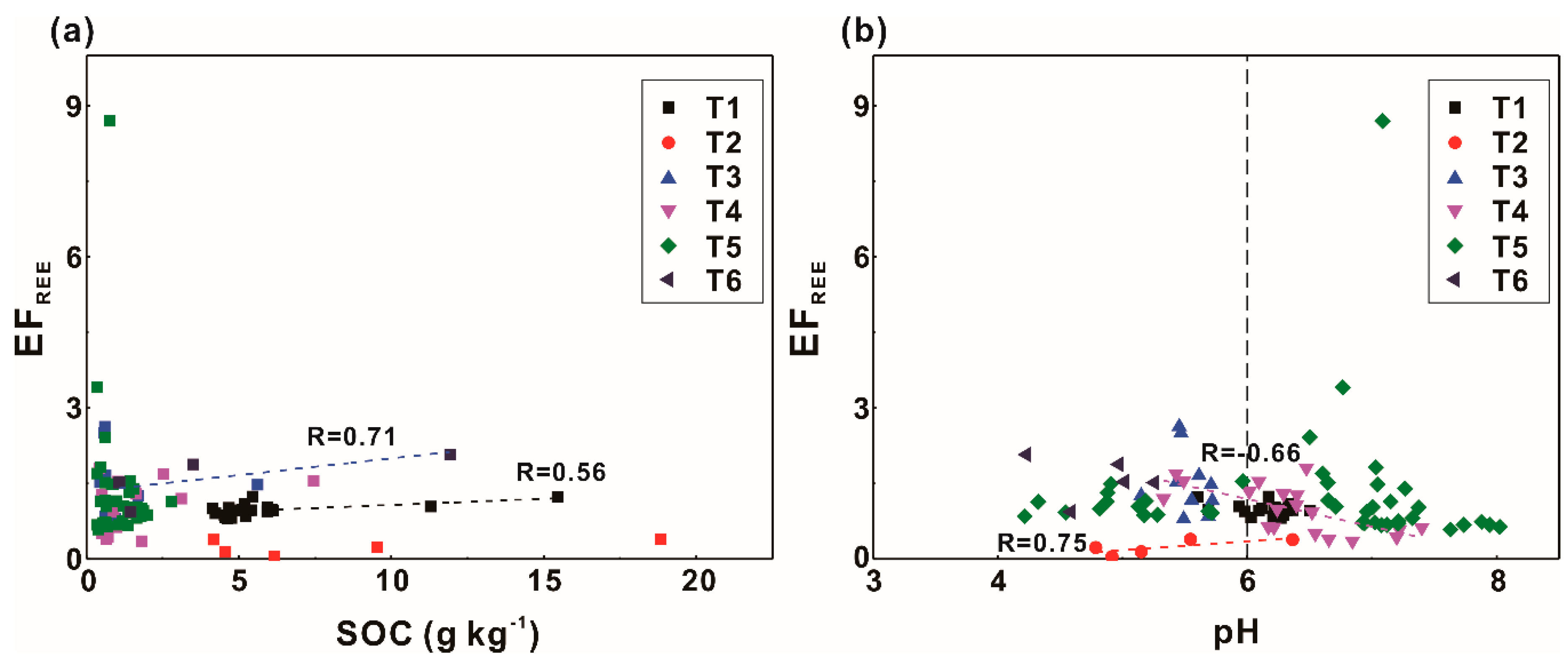
3.3.2. Factors Influencing EFs of Rare Earth Elements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, H.; Hao, F.; Ouyang, W.; Liu, S.; Chunye, L.I.N.; Wenjing, Y. Vertical distribution of rare earth elements in a wetland soil core from the Sanjiang Plain in China. J. Rare Earths 2012, 30, 731–738. [Google Scholar] [CrossRef]
- Dołęgowska, S.; Migaszewski, Z.M. Anomalous concentrations of rare earth elements in the moss–soil system from south-central Poland. Environ. Pollut. 2013, 178, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Han, G.L.; Song, Z.L.; Tang, Y. Geochemistry of rare earth elements in soils under different land uses in a typical karst area, Guizhou Province, Southwest China. Can. J. Soil Sci. 2017, 97, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Welch, S.A.; Christy, A.G.; Isaacson, L.; Kirste, D. Mineralogical control of rare earth elements in acid sulfate soils. Geochim. Cosmochim. Acta 2009, 73, 44–64. [Google Scholar] [CrossRef]
- Tyler, G. Vertical distribution of major, minor, and rare elements in a Haplic Podzol. Geoderma 2004, 119, 277–290. [Google Scholar] [CrossRef]
- Ohlander, B.; Land, M.; Ingri, J.; Widerlund, A. Mobility of rare earth elements during weathering of till in northern Sweden. Appl. Geochem. 1996, 11, 93–99. [Google Scholar] [CrossRef]
- Patino, L.C.; Velbel, M.A.; Price, J.R.; Wade, J.A. Trace element mobility during spheroidal weathering of basalts and andesites in Hawaii and Guatemala. Chem. Geol. 2003, 202, 343–364. [Google Scholar] [CrossRef]
- Davranche, M.; Pourret, O.; Gruau, G.; Dia, A.; Le Coz-Bouhnik, M. Adsorption of REE (III)-humate complexes onto MnO2: Experimental evidence for cerium anomaly and lanthanide tetrad effect suppression. Geochim. Cosmochim. Acta 2005, 69, 4825–4835. [Google Scholar] [CrossRef]
- Cao, X.D.; Chen, Y.; Wang, X.R.; Deng, X.H. Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 2001, 44, 655–661. [Google Scholar] [CrossRef]
- Nesbitt, H.W. Mobility and fractionation of rare-earth elements during weathering of a granodiorite. Nature 1979, 279, 206–210. [Google Scholar] [CrossRef]
- Davranche, M.; Grybos, M.; Gruau, G.; Pedrot, M.; Dia, A.; Marsac, R. Rare earth element patterns: A tool for identifying trace metal sources during wetland soil reduction. Chem. Geol. 2011, 284, 127–137. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Peng, A. Application of rare-earth elements in the agriculture of china and its environmental behavior in soil. Environ. Sci. Pollut. Res. Int. 2002, 9, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Prabnakorn, S.; Maskey, S.; Suryadi, F.X.; de Fraiture, C. Rice yield in response to climate trends and drought index in the Mun River Basin, Thailand. Sci. Total Environ. 2018, 621, 108–119. [Google Scholar] [CrossRef]
- Pagano, G.; Guida, M.; Tommasi, F.; Oral, R. Health effects and toxicity mechanisms of rare earth elements-Knowledge gaps and research prospects. Ecotox. Environ. Safe. 2015, 115, 40–48. [Google Scholar] [CrossRef]
- Wijnhoud, J.D.; Konboon, Y.; Lefroy, R.D.B. Nutrient budgets: Sustainability assessment of rainfed lowland rice-based systems in northeast Thailand. Agric. Ecosyst. Environ. 2003, 100, 119–127. [Google Scholar] [CrossRef]
- Zhou, W.X.; Han, G.L.; Liu, M.; Li, X.Q. Effects of soil pH and texture on soil carbon and nitrogen in soil profiles under different land uses in Mun River Basin, Northeast Thailand. Peer J. 2019, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Qu, R.; Han, G.; Liu, M.; Li, X. The mercury behavior and contamination in soil profiles in Mun River Basin, Northeast Thailand. Int. J. Environ. Res. Public Health 2019, 16, 4131. [Google Scholar] [CrossRef] [Green Version]
- Khunthasuvon, S.; Rajastasereekul, S.; Hanviriyapant, P.; Romyen, P.; Fukai, S.; Basnayake, J.; Skulkhu, E. Lowland rice improvement in northern and northeast Thailand 1. Effects of fertiliser application and irrigation. Field Crop. Res. 1998, 59, 99–108. [Google Scholar] [CrossRef]
- Akter, A.; Babel, M.S. Hydrological modeling of the Mun River basin in Thailand. J. Hydrol. 2012, 452–453, 232–246. [Google Scholar] [CrossRef]
- Nimnate, P.; Thitimakorn, T.; Choowong, M.; Hisada, K. Imaging and locating paleo-channels using geophysical data from meandering system of the Mun River, Khorat Plateau, Northeastern Thailand. Open Geosci. 2017, 9, 675–688. [Google Scholar] [CrossRef] [Green Version]
- Dheeradilok, P. Mineral resources and landuse planning for industrial development in Nakhon Ratchasrima, Northeastern Thailand. J. Southeast Asian Earth Sci. 1993, 8, 567–571. [Google Scholar] [CrossRef]
- Löffler, E.; Thompson, W.P.; Liengsakul, M. Quaternary geomorphological development of the lower Mun river basin, North East Thailand. CATENA 1984, 11, 321–330. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.L.; Zhang, Q.; Song, Z.L. Variations and indications of 13CSOC and 15NSON in soil profiles in karst critical zone observatory (CZO), Southwest China. Sustainability 2019, 11, 16. [Google Scholar]
- Liu, M.; Han, G.; Zhang, Q. Effects of agricultural abandonment on soil aggregation, soil organic carbon storage and stabilization: Results from observation in a small karst catchment, Southwest China. Agric. Ecosyst. Environ. 2020, 288, 106719. [Google Scholar] [CrossRef]
- Han, G.; Li, F.; Tang, Y. Organic matter impact on distribution of rare earth elements in soil under different land uses. Clean-Soil Air Water 2017, 45. [Google Scholar] [CrossRef]
- Alfaro, M.R.; do Nascimento, C.W.A.; Biondi, C.M.; da Silva, Y.; da Silva, Y.; Accioly, A.; Montero, A.; Ugarte, O.M.; Estevez, J. Rare-earth-element geochemistry geochemistry in soils developed in different geological settings of Cuba. Catena 2018, 162, 317–324. [Google Scholar] [CrossRef]
- Sadeghi, M.; Morris, G.A.; Carranza, E.J.M.; Ladenberger, A.; Andersson, M. Rare earth element distribution and mineralization in Sweden: An application of principal component analysis to foregs soil geochemistry. J. Geochem. Explor. 2013, 133, 160–175. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A review on the potentiality of Rare Earth Elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: London, UK, 1985. [Google Scholar]
- Compton, J.S.; White, R.A.; Smith, M. Rare earth element behavior in soils and salt pan sediments of a semi-arid granitic terrain in the Western Cape, South Africa. Chem. Geol. 2003, 201, 239–255. [Google Scholar] [CrossRef]
- Reimann, C.; De Caritat, P. Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ. Sci. Technol. 2000, 34, 5084–5091. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental-crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Huang, H.; Lin, C.; Yu, R.; Yan, Y.; Hu, G.; Wang, Q. Spatial distribution and source appointment of rare earth elements in paddy soils of Jiulong River Basin, Southeast China. J. Geochem. Explor. 2019, 200, 213–220. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Haneklaus, S.; Sparovek, G.; Schnug, E. Rare earth elements in soils. Commun. Soil Sci. Plant Anal. 2006, 37, 1381–1420. [Google Scholar] [CrossRef]
- Chapela Lara, M.; Buss, H.L.; Pett-Ridge, J.C. The effects of lithology on trace element and REE behavior during tropical weathering. Chem. Geol. 2018, 500, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.C.B.D.; Le Pera, E.; Oliveira, C.S.D.; Souza Júnior, V.S.D.; Pedron, F.D.A.; Corrêa, M.M.; Azevedo, A.C.D. Impact of weathering on REE distribution in soil-saprolite profiles developed on orthogneisses in Borborema Province, NE Brazil. Geoderma 2019, 347, 103–117. [Google Scholar] [CrossRef]
- Zeng, J.; Yue, F.-J.; Wang, Z.-J.; Wu, Q.; Qin, C.-Q.; Li, S.-L. Quantifying depression trapping effect on rainwater chemical composition during the rainy season in karst agricultural area, southwestern China. Atmos. Environ. 2019, 218, 116998. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Landing, W.M.; Lewis, B.L. Ocean particle chemistry: The fractionation of rare earth elements between suspended particles and seawater. Geochim. Cosmochim. Acta 1994, 58, 1567–1579. [Google Scholar] [CrossRef]
- Mihajlovic, J.; Bauriegel, A.; Stärk, H.-J.; Roßkopf, N.; Zeitz, J.; Milbert, G.; Rinklebe, J. Rare earth elements in soil profiles of various ecosystems across Germany. Appl. Geochem. 2019, 102, 197–217. [Google Scholar] [CrossRef]
- Han, G.L.; Xu, Z.F.; Tang, Y.; Zhang, G.P. Rare earth element patterns in the karst terrains of Guizhou Province, China: Implication for water/particle interaction. Aquat. Geochem. 2009, 15, 457–484. [Google Scholar] [CrossRef]
- Cao, X.; Wu, P.; Cao, Z. Element geochemical characteristics of a soil profile developed on dolostone in central Guizhou, southern China: Implications for parent materials. Acta Geochim. 2016, 35, 445–462. [Google Scholar] [CrossRef]
- Akagi, T.; Fu, F.F.; Yabuki, S. Absence of Ce anomaly in the REE patterns of peat moss and peat grass in the Ozegahara peatland. Geochem. J. 2002, 36, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Laveuf, C.; Cornu, S.; Juillot, F. Rare earth elements as tracers of pedogenetic processes. C. R. Geosci. 2008, 340, 523–532. [Google Scholar] [CrossRef]
- Han, G.; Song, Z.; Tang, Y.; Wu, Q.; Wang, Z. Ca and Sr isotope compositions of rainwater from Guiyang city, Southwest China: Implication for the sources of atmospheric aerosols and their seasonal variations. Atmos. Environ. 2019, 214, 116854. [Google Scholar] [CrossRef]
- Semhi, K.; Chaudhuri, S.; Clauer, N.; Boeglin, J.L. Impact of termite activity on soil environment: A perspective from their soluble chemical components. Int. J. Environ. Sci. Technol. 2008, 5, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Alderton, D.H.M.; Pearce, J.A.; Potts, P.J. Rare-earth Element mobility during granite alteration—Evidence from southwest England. Earth Planet. Sci. Lett. 1980, 49, 149–165. [Google Scholar] [CrossRef]
- Carling, P.A. The geology of the lower Mekong River. In The Mekong; Elsevier: Amsterdam, The Netherlands, 2009; pp. 13–28. [Google Scholar]
- Sanematsu, K.; Kon, Y.; Imai, A.; Watanabe, K.; Watanabe, Y. Geochemical and mineralogical characteristics of ion-adsorption type REE mineralization in Phuket, Thailand. Miner. Depos. 2013, 48, 437–451. [Google Scholar] [CrossRef]
- Bau, M.; Koschinsky, A. Oxidative scavenging of cerium on hydrous Fe oxide: Evidence from the distribution of rare earth elements and yttrium between Fe oxides and Mn oxides in hydrogenetic ferromanganese crusts. Geochem. J. 2009, 43, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Shimizu, H.; Usui, A.; Kagi, H.; Nomura, M. Direct observation of tetravalent cerium in ferromanganese nodules and crusts by X-ray-absorption near-edge structure (XANES). Geochim. Cosmochim. Acta 2000, 64, 2929–2935. [Google Scholar] [CrossRef]
- Elias, M.S.; Ibrahim, S.; Samuding, K.; Kantasamy, N.; Daung, J.A.D.; Rahman, S.A.; Hashim, A. Dataset on concentration and enrichment factor of rare earth elements (REEs) in sediments of Linggi River, Malaysia. Data Brief 2019, 25, 103983. [Google Scholar] [CrossRef]
- Condie, K.C.; Dengate, J.; Cullers, R.L. Behavior of rare-earth elements in a paleoweathering profile on granodiorite in the front range, Colorado, USA. Geochim. Cosmochim. Acta 1995, 59, 279–294. [Google Scholar] [CrossRef]
- Tang, Y.; Han, G.L. Characteristics of major elements and heavy metals in atmospheric dust in Beijing, China. J. Geochem. Explor. 2017, 176, 114–119. [Google Scholar] [CrossRef]
- Yong, R.; Zheng, L. The distribution of exogenous REEs in Chinese major types of soil. Rare Earth Elem. 1992, 2, 31–35. [Google Scholar]
- Wu, Z.H.; Luo, J.; Guo, H.Y.; Wang, X.R.; Yang, C.S. Adsorption isotherms of lanthanum to soil constituents and effects of pH, EDTA and fulvic acid on adsorption of lanthanum onto goethite and humic acid. Chem. Speciat. Bioavail. 2001, 13, 75–81. [Google Scholar]
- Davranche, M.; Pourret, O.; Gruau, G.; Dia, A.; Jin, D.; Gaertner, D. Competitive binding of REE to humic acid and manganese oxide: Impact of reaction kinetics on development of cerium anomaly and REE adsorption. Chem. Geol. 2008, 247, 154–170. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Wu, Q.; Tang, Y. Effects of agricultural alkaline substances on reducing the rainwater acidification: Insight from chemical compositions and calcium isotopes in a karst forests area. Agric. Ecosyst. Environ. 2020, 290. [Google Scholar] [CrossRef]
- Hendershot, W.; Lalande, H.; Duquette, M. Soil reaction and exchangeable acidity. In Soil Sampling and Methods of Analysis; CRC Press Company: Boca Raton, FL, USA, 1993; Volume 2. [Google Scholar]


| Sampling Point | Land Cover | Location | Important Field Records |
|---|---|---|---|
| T1 | Rice | 14°56′32.59″ N 101°58′34.10″ E | Relatively uniform soil at different depths |
| T2 | Forest | 14°33′5.03″ N 104°56′14.76″ E | Brown, red, and compact soil |
| T3 | Rice | 15°09′0.77″ N 105°10′56.44″ E | 0–12 cm: Gray-black root layer |
| 12–100 cm: Fine silt | |||
| >100 cm: Iron–manganese nodules | |||
| T4 | Wild grass | 15°03′55.86″ N 104°55′7.50″ E | 0–20 cm: Plant root layer |
| 20–112 cm: Fine silt | |||
| 112–180 cm: Red weathering crust | |||
| 180–193 cm: Gray-green sand | |||
| >193 cm: Bedrock | |||
| T5 | Artificial building | 14°58′33.11″ N 104°23′44.07″ E | 0–105 cm: Fine silt |
| 105–205 cm: Iron–manganese nodules | |||
| 205–405 cm: Clay layer | |||
| T6 | Forest | 15°11′59.35″ N 104°30′28.23″ E | |
| Fine silt |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Han, G.; Liu, M.; Song, C.; Li, X. Geochemical Distribution Characteristics of Rare Earth Elements in Different Soil Profiles in Mun River Basin, Northeast Thailand. Sustainability 2020, 12, 457. https://doi.org/10.3390/su12020457
Zhou W, Han G, Liu M, Song C, Li X. Geochemical Distribution Characteristics of Rare Earth Elements in Different Soil Profiles in Mun River Basin, Northeast Thailand. Sustainability. 2020; 12(2):457. https://doi.org/10.3390/su12020457
Chicago/Turabian StyleZhou, Wenxiang, Guilin Han, Man Liu, Chao Song, and Xiaoqiang Li. 2020. "Geochemical Distribution Characteristics of Rare Earth Elements in Different Soil Profiles in Mun River Basin, Northeast Thailand" Sustainability 12, no. 2: 457. https://doi.org/10.3390/su12020457





