An Investigation into Performance of Cement-Stabilized Kaolinite Clay with Recycled Seashells Exposed to Sulphate
Abstract
1. Introduction
2. Used Materials
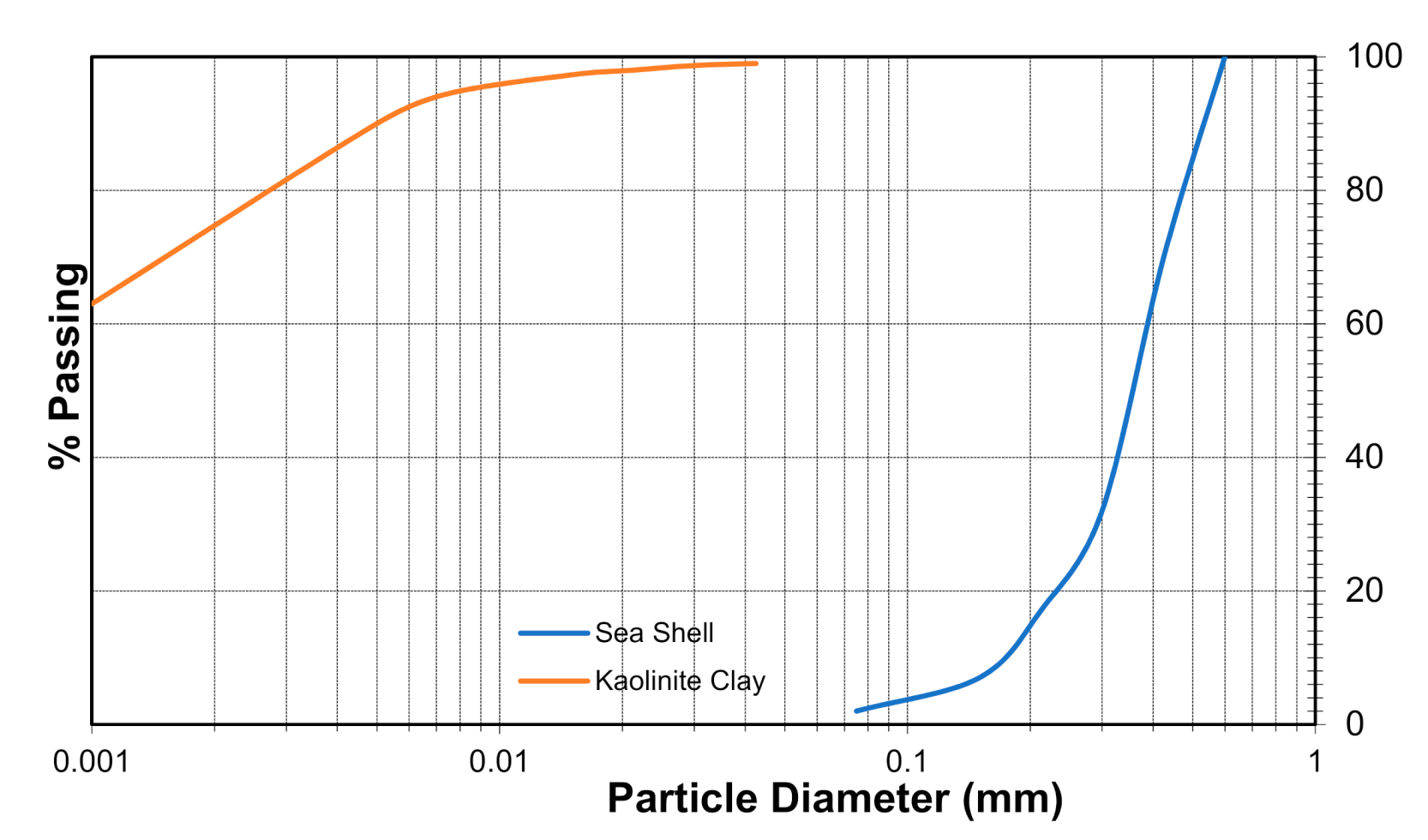
2.1. Kaolinite Clay
2.2. Portland Cement (PC)
2.3. Seashell
3. Methodology and Specimens Preparation
3.1. Standard Proctor Test
3.2. Preparation of UCS Tests
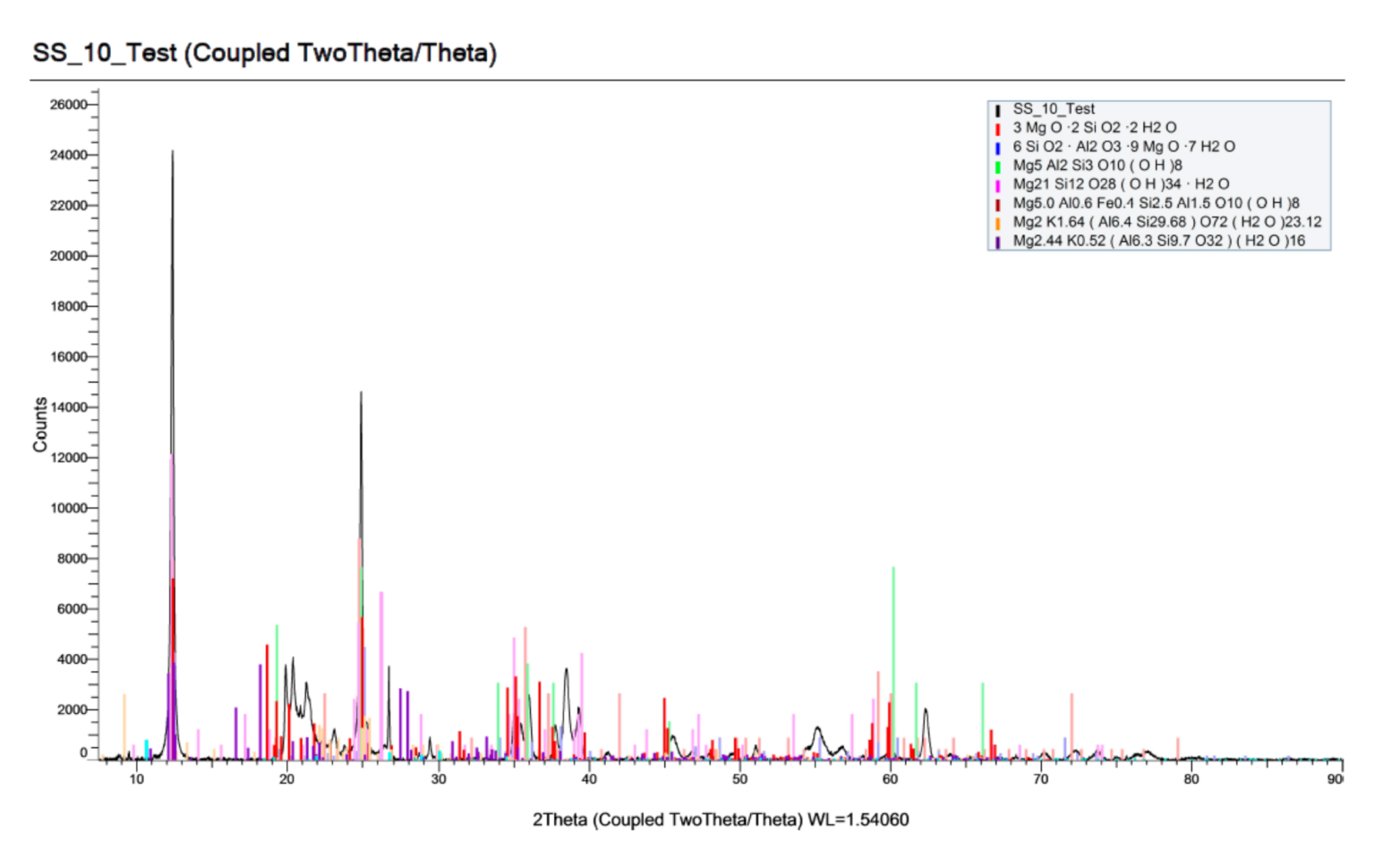
3.3. X-ray Diffraction
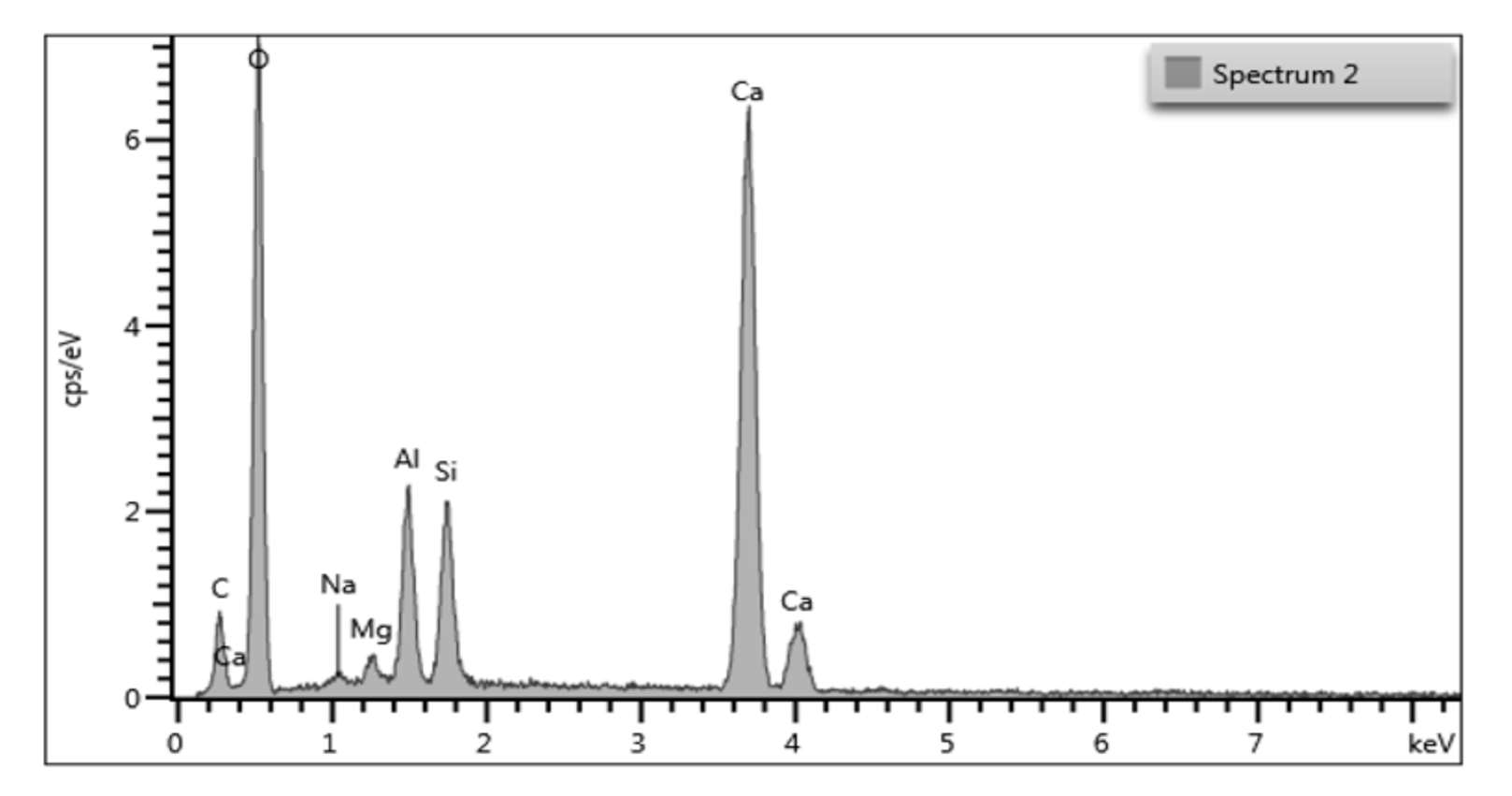
3.4. Scanning Electron Microscope
4. Results and Discussion
4.1. Compaction Test
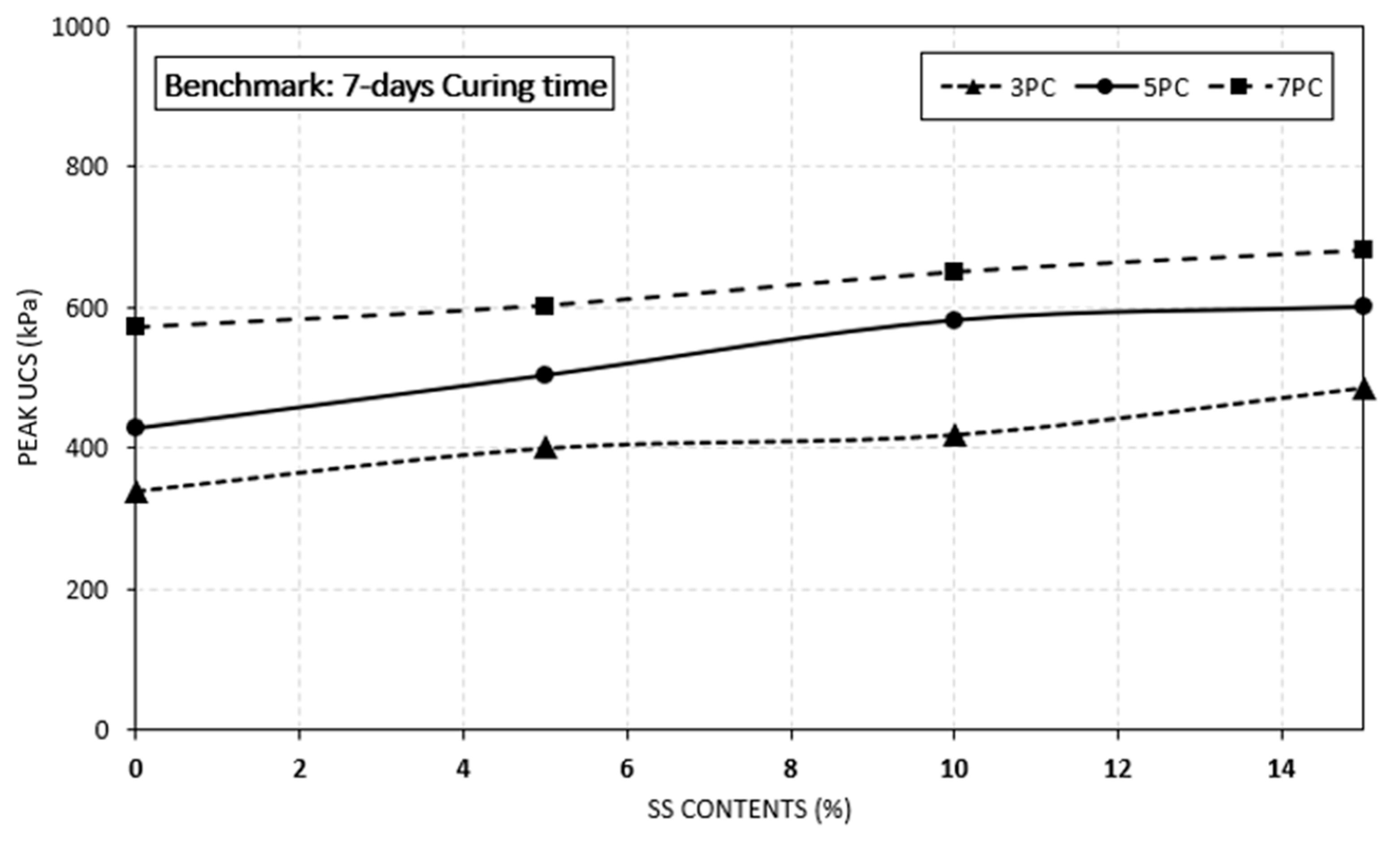
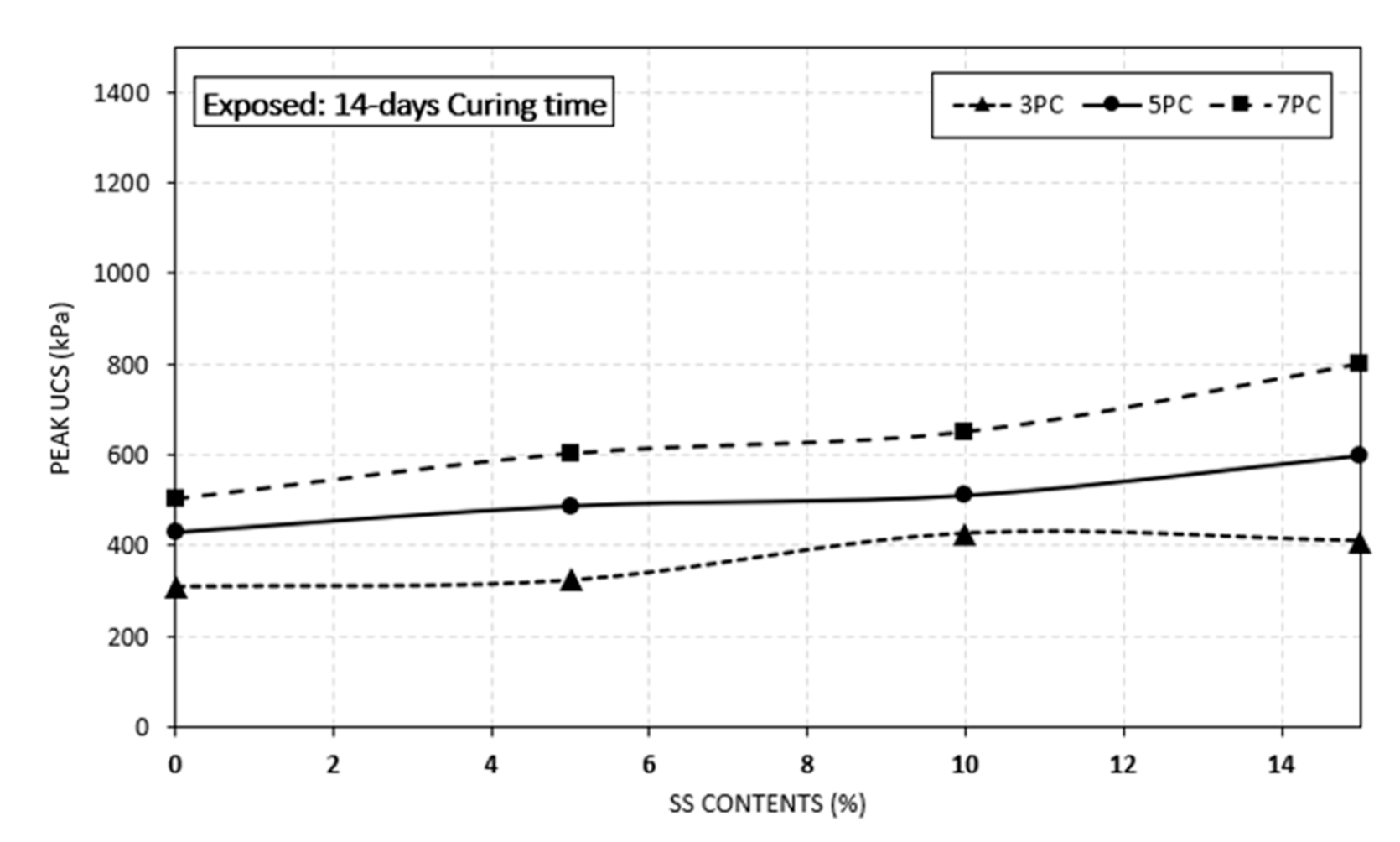
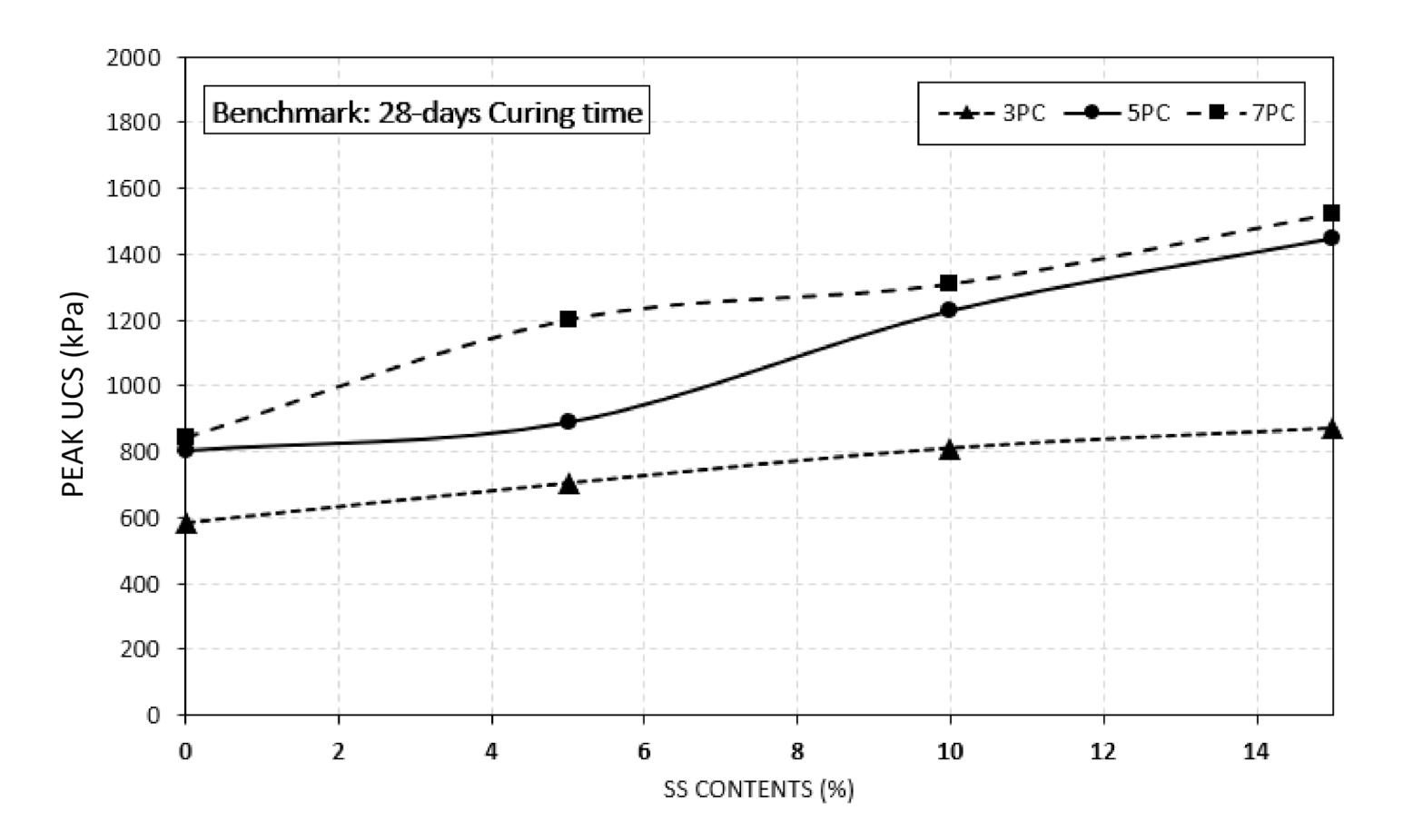
4.2. Effect of Crushed Seashell on Sulphate Resistance
4.3. Microstructural and Crystallography Analysis
4.3.1. Scanning Electron Microscopic Characterization
4.3.2. X-ray Diffraction (XRD) Analysis
5. Conclusions
- The study has shown that the addition of seashell (SS) is effective to improve peak UCS value of the specimens in both benchmark and exposed specimens. However, the rate of increase in exposed specimens was lower than the benchmark specimens.
- Increasing the curing time along with increasing the seashell contents enhanced the peak UCS values. The highest UCS values were recorded at 28 days of curing period for both benchmark and exposed specimens.
- SEM analysis had shown that the seashells are able to bind with the kaolinite and cement power which supports the replacement of cement by seashell replacement.
- XRD analysis had shown that the presence of magnesium sulphate is able to reduce the formation of CSH with a smaller amorphous hump in sulphate-attacked specimens in comparison to benchmark specimens. This provided evidence that specimens under sulphate attacks had reduced formation of CSH, which had led to the deterioration of CSH due to formation of the magnesium silicate hydrate (MSH) after exposure of the specimens to the magnesium sulphate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snedker, E.A.; Temporal, J. M40 motorway banbury IV contract-lime stabilisation. Highw. Transp. 1990, 37, 7–8. [Google Scholar]
- Rollings, R.S.; Burkes, J.P.; Rollings, M.P. Sulfate Attack on Cement-Stabilized Sand. J. Geotech. Geoenviron. Eng. 1999, 125, 364–372. [Google Scholar] [CrossRef]
- Chegenizadeh, A.; Keramatikerman, M.; Panizza, S.; Nikraz, H. Effect of Powdered Recycled Tire on Sulfate Resistance of Cemented Clay. J. Mater. Civ. Eng. 2017, 29, 04017160. [Google Scholar] [CrossRef]
- Chegenizadeh, A.; Keramatikerman, M.; Miceli, S.; Nikraz, H.; Sabbar, A.S. Investigation on Recycled Sawdust in Controlling Sulphate Attack in Cemented Clay. Appl. Sci. 2020, 10, 1441. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S.; Jin, F. Magnesium sulfate attack onclays stabilised by carbide slag-and magnesia-ground granulated blastfurnace slag. Géotech. Lett. 2015, 5, 306–312. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Sulfate attackresearch—Whither now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Xie, S. Effect of magnesium sulfate on theunconfined compressive strength of cement-treated soils. J. Test. Eval. 2012, 40, 1–8. [Google Scholar] [CrossRef]
- Little, D.; Nair, S.; Herbert, B. Addressing sulfate-induced heave in lime treated soils. J. Geotech. Geoenviron. Eng. 2010, 136, 110–118. [Google Scholar] [CrossRef]
- Lawrence, C.D. Sulphate attack on concrete. Mag. Concr. Res. 1990, 42, 249–264. [Google Scholar] [CrossRef]
- Collepardi, M. A state-of-the-art review on delayed ettringite attack on concrete. Cem. Concr. Compos. 2003, 25, 401–407. [Google Scholar] [CrossRef]
- Damidot, D.; Atkins, M.; Kindness, A.; Glasser, F. Sulphate attack on concrete: Limits of the AFt stability domain. Cem. Concr. Res. 1992, 22, 229–234. [Google Scholar] [CrossRef]
- Khatri, R.; Sirivivatnanon, V.; Yang, J. Role of permeability in sulphate attack. Cem. Concr. Res. 1997, 27, 1179–1189. [Google Scholar] [CrossRef]
- Tasong, W.A.; Wild, S.; Tilley, R.J. Mechanisms by which ground granulated blast furnace slag prevents sulphate attack of lime-stabilised kaolinite. Cem. Concr. Res. 1999, 29, 975–982. [Google Scholar] [CrossRef]
- Al-Rkaby, A.H.J.; Chegenizadeh, A.; Nikraz, H. Anisotropic strength of large scale geogrid-reinforced sand: Experimental study. Soils Found. 2017, 57, 557–574. [Google Scholar] [CrossRef]
- Sabbar, A.S.; Chegenizadeh, A.; Nikraz, H. Static liquefaction of very loose sand–slag–bentonite mixtures. Soils Found. 2017, 57, 341–356. [Google Scholar] [CrossRef]
- Hasan, U.; Chegenizadeh, A.; Budihardjo, M.A.; Nikraz, H. Shear strength evaluation of bentonite stabilised with recycled materials. J. GeoEng. 2016, 11, 59–73. [Google Scholar]
- Patel, A.; Mishra, C.B. Performance of Seashell Powder on Sub-grade Soil Stabilization. Civ. Eng. 2017, 1, 150–156. [Google Scholar]
- Ab Manan, W.N.; Ab Aziz, N.A. Optimization of Soil pH by Using Calcium Carbonate (CaCO3) Obtained from Seashell Waste. Gading J. Sci. Technol. 2018, 1, 81–86. [Google Scholar]
- Suteu, D.; Bilba, D.; Aflori, M.; Doroftei, F.; Lisa, G.; Badeanu, M.; Malutan, T. The Seashell Wastes as Biosorbent for Reactive Dye Removal from Textile Effluents. Clean Soil Air Water 2011, 40, 198–205. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Boutouil, M.; Sebaibi, N.; Leleyter, L.; Baraud, F. Valorization of seashell by-products in pervious concrete pavers. Constr. Build. Mater. 2013, 49, 151–160. [Google Scholar] [CrossRef]
- Rainbow, A.; Wilson, F.N. Composting for soil improvement in the United Kingdom. In Proceedings of the 12th International Soil Conservation Organization Conference, Beijing, China, 26–31 May 2002; Tsinghua University Press: Beijing, China, 2002; Volume 67. [Google Scholar]
- Lam, K.P.; Wu, S.; Chu, J. Field trial of a membrane less vacuum preloading system for soft soil improvement. Proc. Inst. Civ. Eng.-Ground Improv. 2020, 173, 40–50. [Google Scholar] [CrossRef]
- Moustafa, H.; Duquesne, S.; Darwish, N.A.; Youssef, A.M. Highly flame retardant green composites using seashells. Soc. Plast. Eng. 2016. [Google Scholar] [CrossRef]
- Kudoh, T. Soil Improvement Material Derived from Marine Resources and Process for Producing the Same. U.S. Patent Application 10/550,403, 10 May 2007. [Google Scholar]
- Thurner, R.; Reinisch, J.; Tschuchnigg, F. Influence of the Carbonate Content on CPT-Results and Practical Relevance for the Soil Improvement Works on Major Land Reclamation Projects. In Proceedings of the International Congress and Exhibition “Sustainable Civil Infrastructures: Innovative Infrastructure Geotechnology”, Cairo, Egypt, 24–28 November 2018. [Google Scholar] [CrossRef]
- Remonsellez, F.; Zarrias, N.; Bol, R.; Fuentes, B. Characterization and low-cost treatment of an industrial arid soil polluted with lead sulfide in northern Chile. Environ. Earth Sci. 2017, 76, 1–11. [Google Scholar] [CrossRef]
- Australian Standard. Methods of Testing Soils for Engineering Purposes; AS 1289.5. 1.1; Australian Standard: Sydney, Australia, 2004. [Google Scholar]
- ASTM D2166. Standard Test Method for Unconfined Compressive Strength of Cohesive Soil; Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2003. [Google Scholar]
- ASTM C1012/C1012M-18b. Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Keramatikerman, M.; Chegenizadeh, A.; Nikraz, H.; Yilmaz, Y. Mechanical Behaviour of Atrazine-Contaminated Clay. Appl. Sci. 2020, 10, 2457. [Google Scholar] [CrossRef]
- Kolias, S.; Kasselouri-Rigopoulou, V.; Karahalios, A. Stabilisation of clayey soils with high calcium fly ash and cement. Cem. Concr. Compos. 2005, 27, 301–313. [Google Scholar] [CrossRef]
- Keramatikerman, M.; Chegenizadeh, A.; Nikraz, H. Experimental study on effect of fly ash on liquefaction resistance of sand. Soil Dyn. Earthq. Eng. 2017, 93, 1–6. [Google Scholar] [CrossRef]
- Mousavi, S.; Wong, L.S. Performance of compacted and stabilized clay with cement, peat ash and silica sand. Jordan J. Civ. Eng. 2015, 9, 20–32. [Google Scholar]
- Hasan, U.; Chegenizadeh, A.; Budihardjo, M.A.; Nikraz, H. Experimental Evaluation of Construction Waste and Ground Granulated Blast Furnace Slag as Alternative Soil Stabilisers. Geotech. Geol. Eng. 2016, 34, 1707–1722. [Google Scholar] [CrossRef]
- Osinubi, K.J. Stabilisation of Tropical Black Clay with Cement and Pulverised Coal Bottom Ash Admixture. In Proceedings of the Geo-Denver 2000, Denver, CO, USA, 5–8 August 2000; pp. 289–302. [Google Scholar] [CrossRef]
- Keramatikerman, M.; Chegenizadeh, A. Effect of Particle Shape on Monotonic Liquefaction: Natural and Crushed Sand. Exp. Mech. 2017, 57, 1341–1348. [Google Scholar] [CrossRef]
- Eskisar, T.; Eskisar, T. Influence of Cement Treatment on Unconfined Compressive Strength and Compressibility of Lean Clay with Medium Plasticity. Arab. J. Sci. Eng. 2015, 40, 763–772. [Google Scholar] [CrossRef]
- Chen, M.; Shen, S.-L.; Arulrajah, A.; Wu, H.-N.; Hou, D.-W.; Xu, Y.-S. Laboratory evaluation on the effectiveness of polypropylene fibers on the strength of fiber-reinforced and cement-stabilized Shanghai soft clay. Geotext. Geomembr. 2015, 43, 515–523. [Google Scholar] [CrossRef]
- Peethamparan, S.; Olek, J. Study of the Effectiveness of Cement Kiln Dusts in Stabilizing Na-Montmorillonite Clay. J. Mater. Civ. Eng. 2008, 20, 137–146. [Google Scholar] [CrossRef]
- Yadav, J.S.; Tiwari, S. A study on the potential utilization of crumb rubber in cement treated soft clay. J. Build. Eng. 2017, 9, 177–191. [Google Scholar] [CrossRef]
- Yoobanpot, N.; Jamsawang, P.; Horpibulsuk, S. Strength behavior and microstructural characteristics of soft clay stabilized with cement kiln dust and fly ash residue. Appl. Clay Sci. 2017, 141, 146–156. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.; Trezza, M.A.; González, M. Thaumasite formation in limestone filler cements exposed to sodium sulphate solution at 20 °C. Cem. Concr. Compos. 2005, 27, 77–84. [Google Scholar] [CrossRef]
- Kurdowski, W. The protective layer and decalcification of C-S-H in the mechanism of chloride corrosion of cement paste. Cem. Concr. Res. 2004, 34, 1555–1559. [Google Scholar] [CrossRef]
- El-Hachem, R.; Rozière, E.; Grondin, F.; Loukili, A. Multi-criteria analysis of the mechanism of degradation of Portland cement based mortars exposed to external sulphate attack. Cem. Concr. Res. 2012, 42, 1327–1335. [Google Scholar] [CrossRef]
- Le Bescop, P.; Solet, C. External sulphate attack by ground water Experimental study on CEM I cement pastes. Revue Européenne de Génie Civ. 2006, 10, 1127–1145. [Google Scholar] [CrossRef]
- Wild, S.; Khatib, J.; O’Farrell, M. Sulphate resistance of mortar, containing ground brick clay calcined at different temperatures. Cem. Concr. Res. 1997, 27, 697–709. [Google Scholar] [CrossRef]
- Consoli, N.C.; Lopes, L.D.S.; Foppa, D.; Heineck, K.S. Key parameters dictating strength of lime/cement-treated soils. Proc. Inst. Civ. Eng. Geotech. Eng. 2009, 162, 111–118. [Google Scholar] [CrossRef]
- Latifi, N.; Vahedifard, F.; Ghazanfari, E.; Rashid, A.S.A. Sustainable Usage of Calcium Carbide Residue for Stabilization of Clays. J. Mater. Civ. Eng. 2018, 30, 04018099. [Google Scholar] [CrossRef]






| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | Na2O | |
|---|---|---|---|---|---|---|---|
| GP Portland Cement | 63.9 | 21.1 | 4.8 | 2.7 | 2.6 | 2.0 | 0.5 |
| No. | Sample ID | PC (%) | SS (%) | Curing Time (Days) | Testing Type: Benchmark (B)/Exposed (E) |
|---|---|---|---|---|---|
| 1 | K | 0 | 0 | 0 | - |
| 2 | 3PC | 3 | 0 | 7, 14, 28 | B/E |
| 3 | 5PC | 5 | 0 | 7, 14, 28 | B/E |
| 4 | 7PC | 7 | 0 | 7, 14, 28 | B/E |
| 5 | 3PC-10SS | 3 | 10 | 7, 14, 28 | B/E |
| 6 | 3PC-10SS | 5 | 10 | 7, 14, 28 | B/E |
| 7 | 3PC-10SS | 7 | 10 | 7, 14, 28 | B/E |
| 8 | 5PC-20SS | 3 | 20 | 7, 14, 28 | B/E |
| 9 | 5PC-20SS | 5 | 20 | 7, 14, 28 | B/E |
| 10 | 5PC-20SS | 7 | 20 | 7, 14, 28 | B/E |
| 11 | 7PC-30SS | 3 | 30 | 7, 14, 28 | B/E |
| 12 | 7PC-30SS | 5 | 30 | 7, 14, 28 | B/E |
| 13 | 7PC-30SS | 7 | 30 | 7, 14, 28 | B/E |
| No. | Sample ID | PC (%) | SS (%) | OMC (%) | MDD (t/m3) |
|---|---|---|---|---|---|
| 1 | K | 0 | 0 | 23.0 | 1.42 |
| 2 | 3PC | 3 | 0 | 23.6 | 1.41 |
| 3 | 5PC | 5 | 0 | 24.1 | 1.39 |
| 4 | 7PC | 7 | 0 | 25.2 | 1.36 |
| 5 | 3PC-10SS | 3 | 10 | 22.0 | 1.39 |
| 6 | 3PC-20SS | 3 | 20 | 21.2 | 1.37 |
| 7 | 3PC-30SS | 3 | 30 | 21.0 | 1.36 |
| 8 | 5PC-10SS | 5 | 10 | 23.0 | 1.38 |
| 9 | 5PC-20SS | 5 | 20 | 22.4 | 1.37 |
| 10 | 5PC-30SS | 5 | 30 | 22.1 | 1.35 |
| 11 | 7PC-10SS | 7 | 10 | 24.0 | 1.33 |
| 12 | 7PC-20SS | 7 | 20 | 22.4 | 1.32 |
| 13 | 7PC-30SS | 7 | 30 | 21.0 | 1.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chegenizadeh, A.; Keramatikerman, M.; Afzal, F.; Nikraz, H.; Keong Lau, C. An Investigation into Performance of Cement-Stabilized Kaolinite Clay with Recycled Seashells Exposed to Sulphate. Sustainability 2020, 12, 8367. https://doi.org/10.3390/su12208367
Chegenizadeh A, Keramatikerman M, Afzal F, Nikraz H, Keong Lau C. An Investigation into Performance of Cement-Stabilized Kaolinite Clay with Recycled Seashells Exposed to Sulphate. Sustainability. 2020; 12(20):8367. https://doi.org/10.3390/su12208367
Chicago/Turabian StyleChegenizadeh, Amin, Mahdi Keramatikerman, Faizan Afzal, Hamid Nikraz, and Chee Keong Lau. 2020. "An Investigation into Performance of Cement-Stabilized Kaolinite Clay with Recycled Seashells Exposed to Sulphate" Sustainability 12, no. 20: 8367. https://doi.org/10.3390/su12208367
APA StyleChegenizadeh, A., Keramatikerman, M., Afzal, F., Nikraz, H., & Keong Lau, C. (2020). An Investigation into Performance of Cement-Stabilized Kaolinite Clay with Recycled Seashells Exposed to Sulphate. Sustainability, 12(20), 8367. https://doi.org/10.3390/su12208367






