Abstract
This study was carried out to assess individuals’ willingness to pay (WTP) for UZIMAX, a novel plant-based biopesticide developed for malaria vector control. The biopesticide is estimated to kill up to 100% of Anopheles larvae within 48 h of application and poses no risks to human health and the environment. However, scaling-up of its adoption requires clear evidence of its acceptance by individuals in malaria-prone areas. We conducted Becker-DeGroot-Marschak (BDM) revealed preference auctions with 204 participants to determine their willingness to pay (WTP) for community-based application of the biopesticide to control malaria vectors. Nearly all participants were willing to pay at the lowest bid price of the biopesticide, and the majority of them expressed great interest in pooling resources to facilitate biopesticide application. Household per capita income and building capacity of households through training significantly increased WTP. These findings imply high adoption potential of the technology and the need to devise inclusive policy tools, especially those that enhance collective action, resource mobilization and capacity building to empower both men and women and stimulate investment in eco-friendly technologies for malaria prevention. Financial and labor resource mechanisms managed by the community could potentially spur adoption of the biopesticides, and in turn, generate health, environmental and economic benefits to households in malaria-prone communities.
1. Introduction
Malaria still poses significant public health and socio-economic burdens on many Sub-Saharan African (SSA) countries in-spite of government efforts that have reduced morbidity and mortality due to the disease by more than 50% between 2000 and 2017 [1,2,3]. Direct control of malaria vectors (Anopheles mosquitoes) is one of the key tools for effective malaria control [4,5,6]. Insecticide-based interventions such as the use of long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) are the most common methods used to control malaria vectors in the region [7,8,9]. While current malaria vector control interventions involving the use of synthetic chemical insecticides have led to significant malaria reduction in the region, challenges associated with exclusive reliance on these approaches hamper the goal of malaria elimination [10,11,12]. First, increased vector resistance against the commonly used insecticides [7,8,9]. Second, the current interventions are expensive for Governments and individuals in many malaria-affected countries to be sustained without support from external donor-funded programs [13]. In addition, while LLINs and IRS may reduce malaria transmission arising from mosquitoes biting people indoors at night, these technologies fail to prevent mosquito bites outdoors [11,12].
The World Health Organization (WHO) advocates for the adoption of integrated vector management (IVM) strategies, targeting different stages of mosquitoes both indoors and outdoors for effective, environment-friendly and sustainable malaria control and elimination [12,14,15,16]. One of the key principals of IVM is that the interventions should be ecologically, environmentally, socially, economically and politically acceptable [14]. This suggests that the IVM practices should be safe for both humans and the environment, have a higher opportunity cost as opposed to the alternative methods and be acceptable among the target communities. IVM manages the vector population in such a way as to reduce or interrupt transmission of disease [6]. Within the IVM context, larval source management (LSM) is believed to be among the most likely interventions to achieve and sustain malaria vector control [17]. Towards this end, microbial larvicides such as Bacillus thuringiensis var. israelensis (Bti) have been piloted in many countries in Africa including Kenya, and evidence shows that the strategy can be sustainably feasible and effective in malaria vector control (e.g., [18,19] in Kenya; [20] in Botswana and Zimbabwe; [21,22] in Burkina Faso; [23,24,25] in Tanzania). However, although this type of intervention presents a great opportunity for sustainable malaria control and elimination, it has not been widely adopted in many SSA countries mainly due to limited availability and awareness. Furthermore, the commercial microbial larvicides (such as Bti) used in SSA are mainly imported, thereby making them costly, unaffordable, and inaccessible to rural communities most affected by malaria. Thus, the development and promotion of cheaper, effective, and ecologically friendly alternatives to Bti are desirable as a means of stimulating the adoption of larviciding at scale in SSA and thus contributing to the development of environmentally friendly and sustainable approaches to malaria management.
To address the challenges highlighted above, scientists have developed an alternative novel plant-derived mosquito bio-larvicide called UZIMAX [26]. Several advantages of the plant-based bio-larvicide have been cited. Unlike conventional synthetic insecticides with a single active ingredient, plant-derived products comprise blends of compounds that act additively and synergistically to inactivate the behavioral and/or physiological processes of mosquitos. Also, natural phytochemical blends such as those in the UZIMAX formulation show resistance-mitigating effects even when used over long periods [27]. They are also effective and eco-friendly for mosquito control, as they contain certain chemicals with exceptional larvicidal activities [28,29]. UZIMAX has been tested for safety and efficacy by the Pesticide Control Products Board (PCPB) of Kenya and is currently in the process of official registration for use in the country. Entomological assessments show that UZIMAX is highly effective against mosquitoes and that it can kill up to 100% of Anopheles larvae under field conditions within 48 h of application [26]. Besides, the product is environmentally safe to both humans and non-target organisms, such as fish, tadpoles, beetles and bees [26], thus guaranteeing environmental integrity and stability.
Like other larvicides, UZIMAX would be most accessible and beneficial to residents in a given area if applied in mosquito-breeding habitats at the community level [30]. This would potentially make it possible for households to cost-effectively control malaria by pooling labor, technical and financial resources needed to purchase and apply bio-larvicides. Preliminary efficacy assessments have estimated that on average, a village in malaria-prone areas in Kenya would require at least ten liters of UZIMAX bio-larvicide each rainy season (about twenty liters per year) [26]. Although the microbial larvicides exhibit potential health benefits to households in the SSA region, research on the acceptance of the technology is still limited. Community participation in the management of mosquitoes and malaria has in the past been shown to be successful, especially where the interventions require substantial labor and financial investments from the community members [31,32].
The objective of this study was to assess an individual’s valuation of UZIMAX, as a biopesticide for controlling the breeding of mosquito larvae and, subsequently, malaria transmission. We used an auction experimental approach to assess whether individuals were willing to pay for the biopesticides to control malaria vectors at the community level. To our knowledge, no study has evaluated the potential demand for community-level larviciding (chemicals and microbial larvicides) in the region. We also compared the individual WTP to an estimated annual cost if the interventions were provided at a community level. Findings from this study will be essential to provide evidence and feedback to the IVM research scientists and development partners as well as policymakers, especially in the health sector, on the potential pathways for sustaining the IVM interventions for malaria elimination in developing countries. Understanding people’s preferences and their willingness to pay and contribute to larviciding at the community level will guide the formulation of effective, sustainable and inclusive policy mechanisms for integrating the new larvicide in the national malaria control program.
2. Materials and Methods
2.1. Study Area
The study was conducted in Malindi in Kilifi County of Kenya (Figure 1). Subsistence agriculture fishing and local trading are the major economic activities in this area. Malaria prevalence currently averages 5% in the population. Inadequate access to health and sanitation services, combined with poverty, contribute to conditions that influence the risk of malaria and other diseases. Malaria is one of the critical causes of morbidity and mortality within the sub-county. A study by [16] reported that Malindi had one of the most successful programs implementing IVM interventions for malaria control in the country. The IVM interventions including the use of LLINs and environmental management have been implemented through a multi-stakeholder collaboration involving government departments, national and international research institutes, nongovernmental organizations (NGOs) and communities. A comprehensive description of the Malindi county is provided by [33].
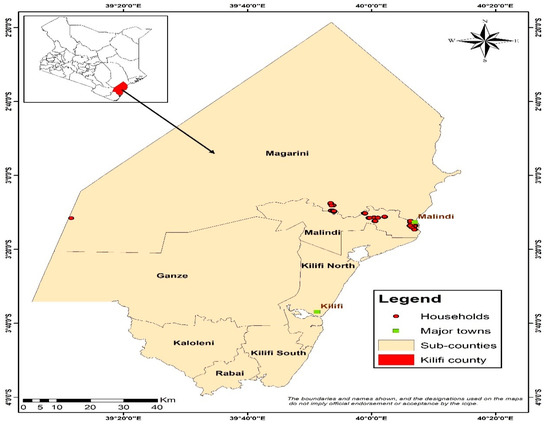
Figure 1.
Map of Kilifi County.
2.2. Auction Design and Mechanism
We use the Becker-DeGroot-Marschak (BDM) auction method to elicit people’s WTP for UZIMAX [34]. The BDM approach is a simulated auction in which the bids of individual participants are not compared to one another but to a randomly generated price. The method involves presenting individual participants with a product or products and then asking them to offer a bid (bid price) for the product. The participant is required to purchase the product at the lower random price if their bid price exceeds a randomly generated price; otherwise, no transaction takes place. Several field studies (e.g., [35,36,37,38]) have demonstrated the usefulness of the BDM mechanism. One attractive feature of the BDM mechanisms is the possibility to allow one or several participants in the experiment at a time. Unlike auction mechanisms such as the second price auctions, the BDM approach permits the elicitation of incentive-compatible valuations without convening participants in groups in a laboratory setting [36]. Participants can be met individually in their homes, and they do not compete for the same product. This keeps the costs of the BDM approach lower than the laboratory-based market research experiments. The method has been used for enlisting households WTP for food products in both the rural and urban areas in Kenya [37,39]. Ref. [37] also notes the BDM procedure can be much faster and more efficient in enlisting WTP data from rural- consumers at their home.
2.3. Experimental/ Auction Procedure
This study used the individual BDM experimental auction with an endowment to elicit the individuals’ WTP for UZIMAX biopesticides for mosquito larviciding. In our experiment, each participant was met at their home by an enumerator. To be eligible for the study, participants were required to be at least 18 years of age and be a decision-maker in the household. Respondent participation was voluntary and oral informed consent was obtained for those who were eligible and agreed to participate. The enumerator first explained the procedure in detail to the participant, emphasizing that the transaction had to be executed if the participant’s bid was higher than the random number. Each participant was then presented with a 250 mL bottle of UZIMAX and informed about the attributes of the biopesticide (safety and effectiveness of the biopesticide, application procedure, the labor, financial requirements for its application, and the benefits associated with it). The participants were also given the chance to see, touch, and read about the content from the label on the bottle. Each participant was then provided with KShs440 (US$4.4) as an endowment to purchase the 250 mL of UZIMAX. All the study participants received the endowment to enable them to evaluate the biopesticide more positively. Each participant was then asked to give their WTP price for each of the ten trials provided on a recording sheet that listed price intervals, as presented in Table 1. The price intervals ranged from Kenya shillings (Kshs) 395–440 (US$3.95 to US$44.0), in increments of Kshs4 (US$0.04). After collecting the recording sheets from the participant, the enumerator asked the participant to randomly select one price (bid price) from the list and revealed it to the participant. The bid price was then compared to a number, randomly drawn from a normal distribution and when the bid was higher, the participant bought the 250 mL bottle of UZIMAX at the lower randomly generated price offered. After the outcome of the bidding was determined, the participant was then compensated for participation and received compensation upon completion of a short questionnaire to avoid biasing the respondent. The field experiment was conducted in November 2018 and covered 216 participants, 123 from 10 rural villages, and 93 from eight urban villages; however, after cleaning the data for missing information and enumerators’ errors, a sample of 204 participants (117 and 87 from rural and urban villages, respectively) was utilized for analysis. Out of the sample, about 74% of the participant’s bids were successful (including 84% in rural areas and 62% in urban areas) (Table 2). The data were collected using tablets (using CSPro 7.1 software). The survey questionnaire captured variables including knowledge about malaria, the use of larvicides, utilization of the personal protective measures and willingness to pay for UZIMAX bio-pesticide, as well as several socio-economic and demographic characteristics of individual participants and their households.

Table 1.
Proportion of participants with successful bid prices for UZIMAX.

Table 2.
Socio-economic and demographic characteristics of the sample.
2.4. Estimating WTP
We estimate both the unconditional and conditional WTP of the biopesticides. We use simple means to measure WTP since the observed bid prices collected using the BDM auctions represent the revealed preference of the participant. We then fit a standard ordinary least squares (OLS) regression model for WTP and predict the conditional WTP values. The regression model also helps to demonstrate the relationship between socioeconomic variables (household and individual) and WTP to inform how pricing policies can target types of households and individuals. The WTP model is specified in Equation (1).
where is a vector of characteristics of interest for participant and their household, and is an error term. We include individual characteristics such as gender, age, education, individual perceptions of larviciding, and trust in the community’s financial contribution to collective action. Household-level variables included income, household size, and participation in malaria training. Many of these variables have been shown to affect consumer acceptance of other malaria control practices in households (e.g., [40,41]). We also include a dummy variable indicating whether the participant’s household has attended any malaria-related training in the past two years. We control for the effect of trust using an index constructed from three indicator variables: whether the participant believes that they can trust other people, whether they believe that other people have good intentions toward them; and whether they believe ‘that one can rely on other people even if one does not know them well’. The index takes values of 0 and 1, corresponding to no information and higher information access, respectively. The economics literature suggests that trust may influence decision making under uncertainty and has been associated with a greater willingness to pay for improved healthcare [42,43]. We thus hypothesize that increased trust can influence one’s preference and valuation of new technology in malaria vector control, thus increasing their likelihood to invest in the technology. We also control for household per capita income. We hypothesize that, unlike their poor counterparts, participants from households with higher income are more likely to invest in purchased malaria prevention technologies such as new biopesticides because they may have the disposable income to purchase them.
3. Results and Discussion
3.1. Descriptive Statistics: Socio-Economic Attributes
3.1.1. Characteristics of Participants and Their Households
Previous work on factors that influence willingness to pay for malaria prevention interventions has considered education, wealth status, household, marital status, household income, gender and level of expenditure on malaria treatment as highly influential factors in the SSA region [44]. These socioeconomic variables are also considered for this study because they accurately represent factors that influence the demand for a good. Table 2 presents the descriptive results of selected socio-economic variables of the participants. A large majority of participants in the sample were females (59% in rural areas and 83% in urban). The average age was 39 years in rural areas and 41 years in urban areas. The high participation rate of women, especially in the urban areas, was a result of their availability during the survey. About 46% of the participants in rural areas were heads of households compared to 31% in urban areas. Male heads dominate the sample; only 15% and 14% of the households are headed by females in rural and urban areas, respectively. For those selected, the average years of formal education are six years in rural areas and seven years in the town. The average household size of participants is three persons in rural and four persons in the town. Wage employment is the main occupation for town dwellers (30%), whereas farming forms the predominant primary source of livelihood in the rural areas. The results of other important socioeconomic variables are presented below in Table 2. Unlike previous studies on WTP for malaria control measures (e.g., [30,45]), this study also provides an elaborate comparison between genders and across study sites (rural versus urban). These comparisons are important for understanding the differences in the effect of gender and urbanization status on willingness to participate in communal malaria control interventions.
3.1.2. Knowledge of Malaria Transmission, and Adoption of Malaria Control Practices
A substantial number of both male and female participants generally appear to have adequate knowledge of mosquitoes and malaria transmission, and the trend is similar for participants residing in both rural and urban villages (Table 3). This high awareness is a confirmation of high priority and investment that SSA countries have had towards controlling malaria. About 94% (in rural) and 80% (in urban) of the participants correctly stated that mosquitoes bite both humans and animals. Regarding the seasonality and mosquito abundance, about 79% correctly stated that mosquitoes are mainly abundant during the rainy season. Similarly, about 74% of the rural-based participants and 76% of the urban participants mentioned stagnant water pools as the main breeding sites for mosquitoes. Regarding mosquito biting time, 63% of the participants were aware that nighttime is the biting time of mosquito. A good number of the surveyed households (52%) identified indoor dark areas as the resting places of mosquitoes during the day.

Table 3.
Participant’s knowledge of malaria and its transmission, by gender and residence (%).
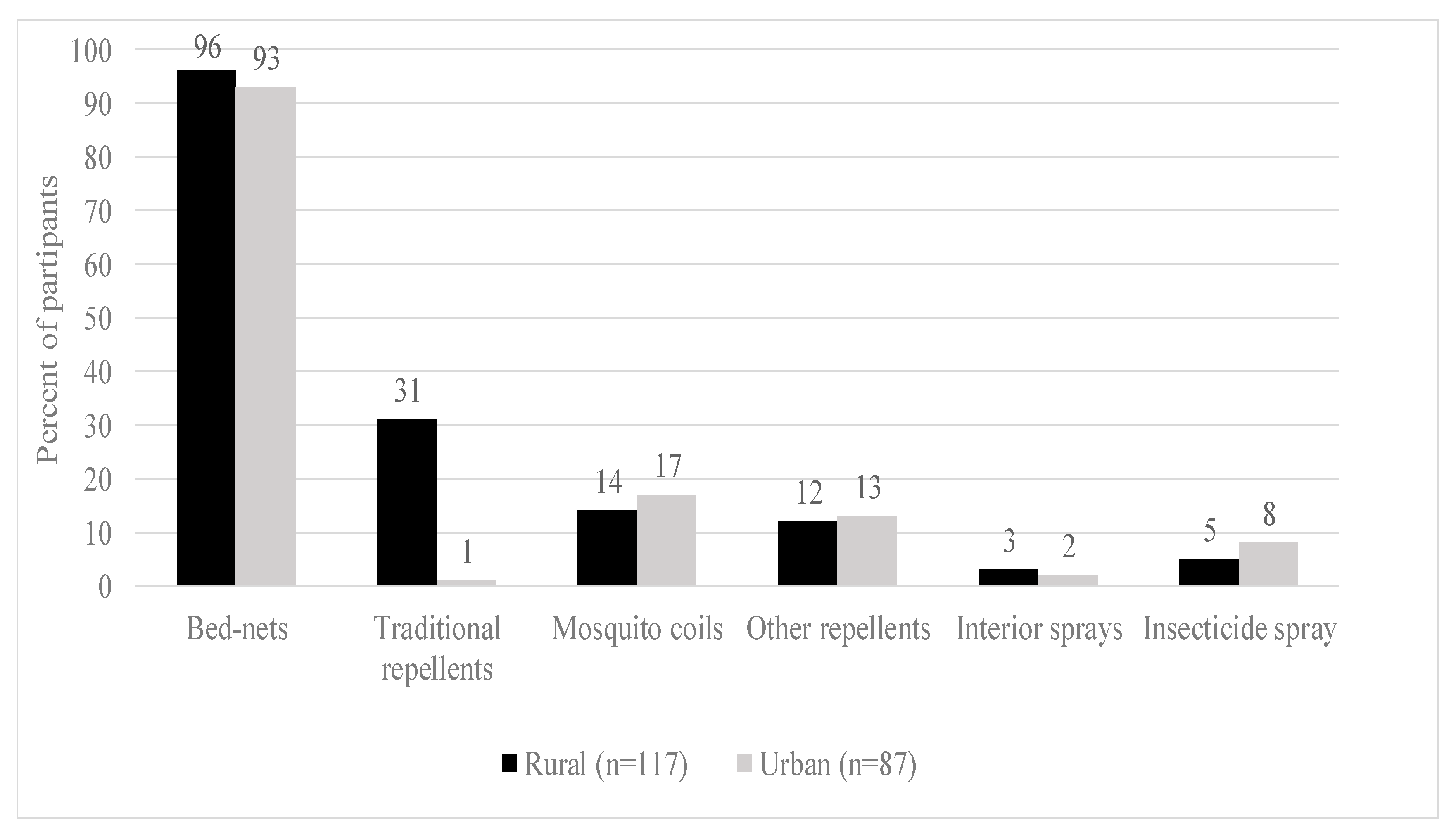
Figure 2 below shows that there is a marginal distribution of the prevention technologies used by the surveyed households. Insecticide-treated nets (ITNs) constitute the dominant technology used by households to control malaria in most households surveyed (more than 90%), for both rural and urban areas. A few households use mosquito coils and insecticide sprays (less than 20%). Traditional repellents such as neem trees and cow dung are also used in rural areas (32%).
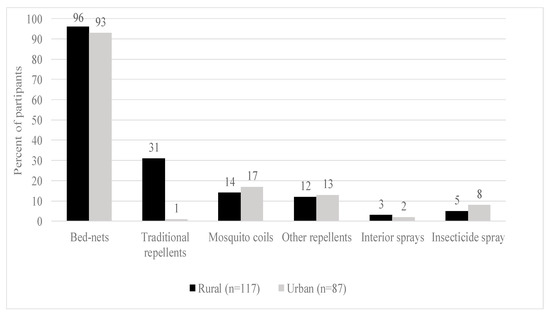
Figure 2.
Malaria prevention practices.
3.1.3. Participant’s Perceptions of Larviciding
The majority of the surveyed participants were not familiar with the use of larvicides to reduce mosquito populations: For instance, only 23% of the participants mentioned that they were aware of the use of Bti for malaria prevention; more male participants (30%) than females (20%) being aware of the intervention. Awareness of the technology was also found to be more prevalent among rural residents (29%) than the urban (14%). Many of those who were aware of larviciding (about 80%) also reported that Bti had been applied in water pools to control mosquitoes in their villages; further noting that Bti is a very effective strategy for controlling malaria vectors (78%) (Table 4). The relatively low overall numbers of survey participants being aware of Bti use for malaria treatment is consistent with the novelty of the control method and its low penetration in communities.

Table 4.
Knowledge of larviciding for malaria prevention by gender.
3.2. Willingness to Pay for UZIMAX Biopesticides to Control Mosquitoes
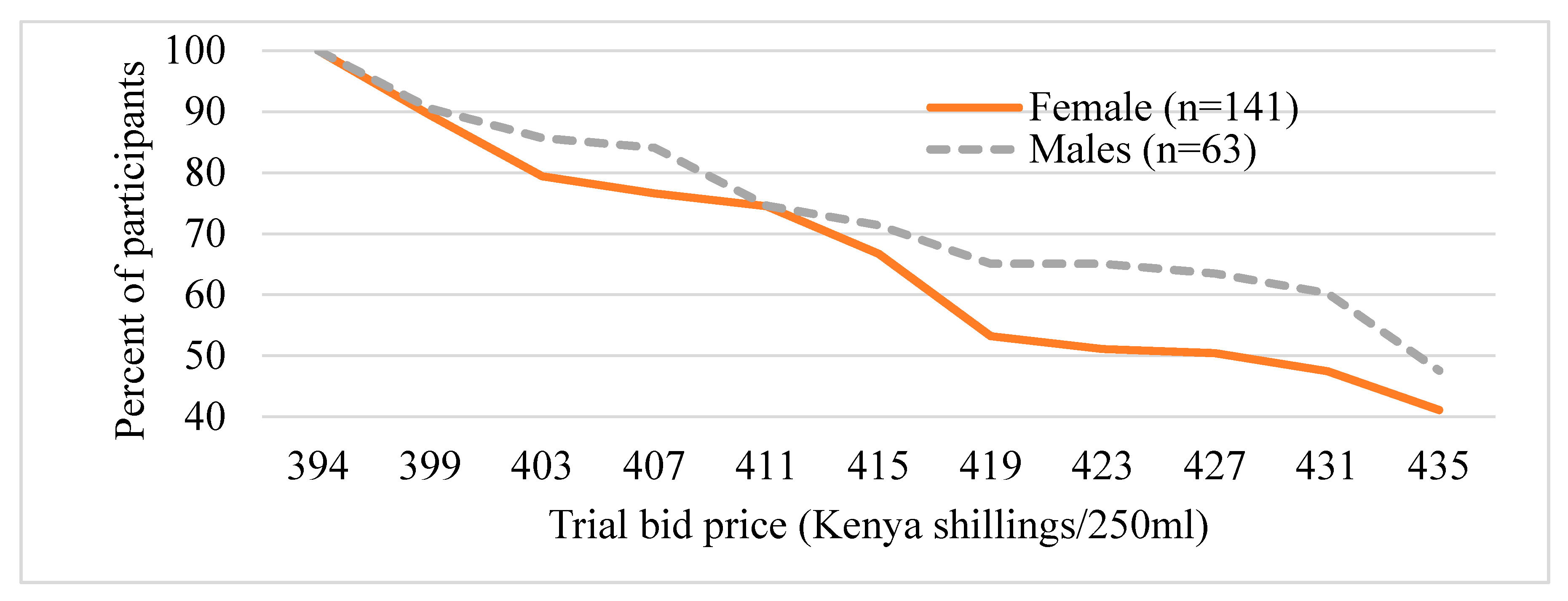
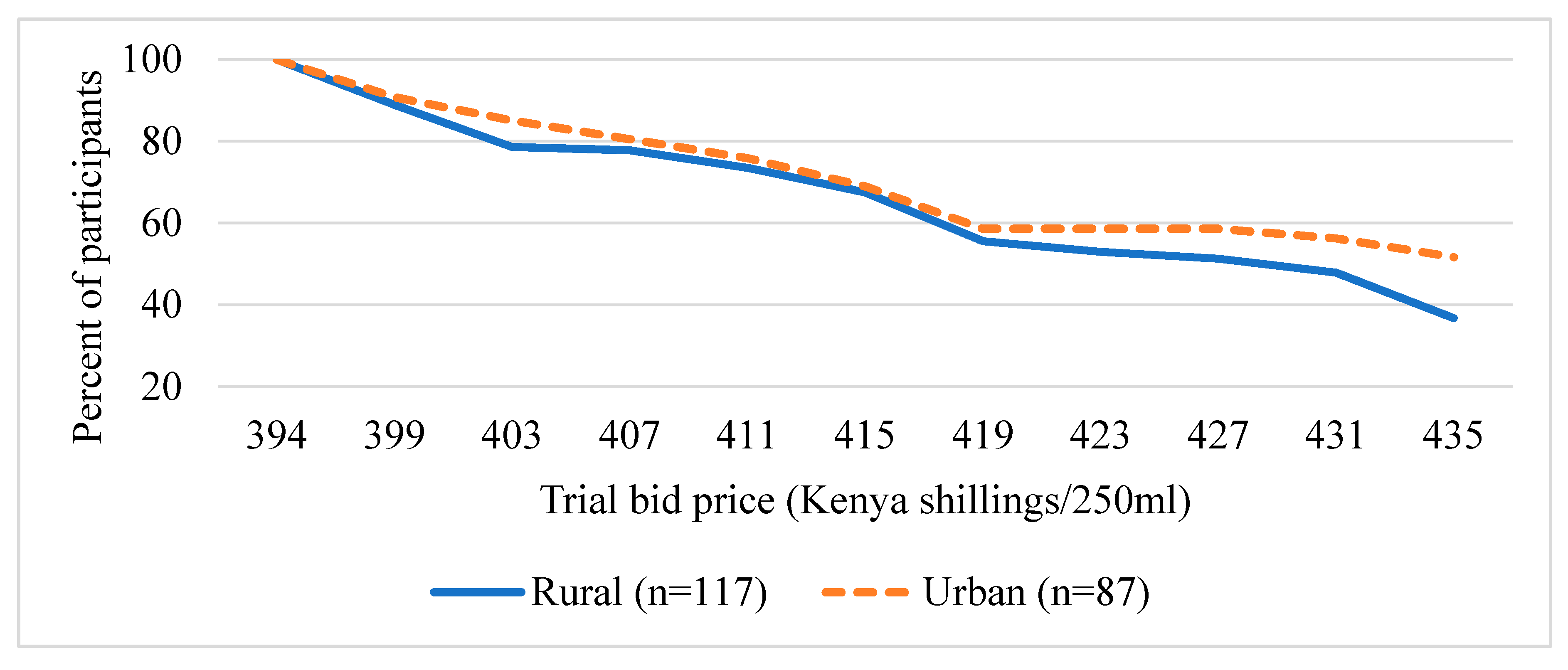
Figure 3 and Figure 4 present the distribution of the trail bid prices (presented during the survey) the participants are willing to pay for UZIMAX. The results show a high potential demand for bio-pesticide when supplied to the market. As can be seen in the figure, all participants were willing to pay at the lowest bid price of Kshs395 (about US$3.95) for 250 mL of the biopesticide. Their WTP for the biopesticides would be offset by the economic and health gains, the reduction in expenditure on insecticide and the environmental and human preservation accruing from the technology. As expected, results are consistent with economic theory. The willingness to pay declined with increase in the bid price for the bio-pesticide. The downward sloping WTP curves resemble demand curves where there tend to be more unwilling participants as the bid prices increase [45,46,47]. The results also show that more males were willing to pay higher bid prices relative to their female counterparts (Figure 3). Similarly, more urban-based participants were willing to pay higher prices than those in rural areas (Figure 4). These results, especially for gender, have been consistent across several studies. A systematic review and meta-analysis of willingness to pay values on malaria control interventions by [44] revealed that gender of the participants was a consistent and significant positively related factor, revealing a higher willingness to pay among male survey participants. Most literature points to differences in access to financial resources between males and females and between rural and urban residents as well as differences in access to medical/health information as the main causes of the differences.
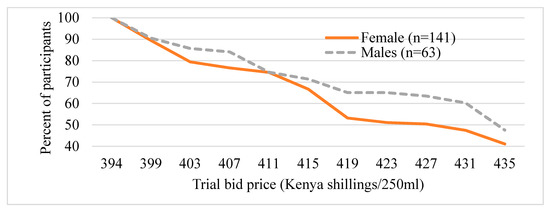
Figure 3.
Distribution of trial bids (exchange rate, 1US$ equal to 100Kenya shillings), by gender of the participant.
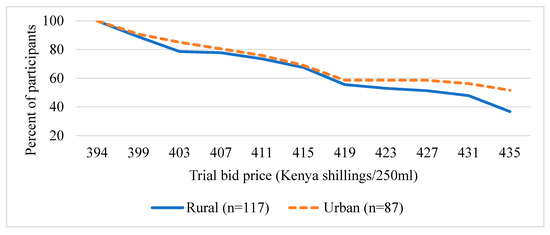
Figure 4.
Distribution of trial bid prices of UZIMAX (exchange rate, 1US$ equal to 100Kenya shillings), by residence.
Estimated WTP for UZIMAX
Table 5 shows summary statistics (mean and median values) of both the unconditional and conditional prices participants are willing to pay for a 250 mL bottle of UZIMAX biopesticide, comparing rural and urban residents as well as female and male participants. We note that the conditional WTP prices are generally comparable to the unconditional WTP prices, although the latter values are slightly higher. The average WTP for the sample was Kshs420 ($4.2) per 250 mL bottle of UZIMAX. Mean WTP values were higher among male participants (Kshs420) than female participants (Kshs414). We also note that urban residents were willing to pay a slightly higher price (Kshs421.8) compared to the rural residents (Kshs419). The trend was similar for median prices. We compared median differences between urban and rural residents and between female and male participants within each residence category and for the whole sample using Wilcoxon rank-sum tests. We found significant differences in median WTP between bid price by women and men and in rural and urban residences. Considering that the average cost price is Kshs394 (US$3.94) per a 250-mL bottle of UZIMAX, during the time of the experiment, more than 80% of the participants were generally willing to pay a 7 % premium (Kshs26) for the same package of UZIMAX.

Table 5.
Mean WTP for UZIMAX, by gender and residence.
During the rainy season (lasting about 2 months) about 10 liters of UZIMAX would be needed to apply in each village, which costs about Kshs15,127 (about US$151.3) per season per village (for two months). Assuming 100 households per village, each household would contribute Kshs320 per year (approximately Kshs160 per month for a two-month rainy season) towards the purchase of UZIMAX for mosquito larvae control in the village of residence. Thus, the mean valuation (WTP) of UZIMAX by participants (Kshs420/250 mL) is in line with the expected annual contribution towards larviciding. Moreover, these projections are consistent with Mboera et al. [47] who found that 73.4% of the survey participants expressed WTP to make a nominal household contribution of US$1.76 per month towards communal larviciding interventions in Tanzania. Besides, Trapero-Betran et al. [44] in their meta-analysis of willingness to pay for malaria control interventions reported that the mean WTP for malaria preventive goods/services was about US$2.6. Larviciding interventions for mosquito control all over the world have generally received positive responses from the population given their ease of application and safety to non-target organisms [21,47]. This result may, however, reflect a linear projection of costs for the UZIMAX application, which may not necessarily hold if the number of willing participants in a village is varied.
To understand the individual interest in resource pooling as a strategy for effective and sustainable use of UZIMAX to control malaria vector, participants were further asked if they would be willing to contribute money to purchase the biopesticides at the community level. About 97% of the participants were interested in contributing money every quarter towards larviciding in their villages, and about 86% of these indicated that they would be happy to start contributions immediately (Table 6). The results also show interest by many participants to volunteer their time (labor) during the application of the bio-pesticide in their village. Nonetheless, despite the high willingness to contribute to malaria control initiatives reported among the population, rural dwellers have expressed little ability to contribute due to widespread poverty [48,49]. Ref. [50] also noted that proper application of the larvicides requires the existence of good organizational structures and locally trained personnel in the proper application of the larvicides. Ultimately, these challenges might limit the widespread use of the larvicide at a community scale.

Table 6.
Interest in collective biopesticide purchase and application in the village.
3.3. Factors Associated with Participants’ WTP
Table 7 presents the results of OLS regression models for the factors that contribute to the participants’ WTP for biopesticide in the study area. We estimate a pooled model as well as separate models for male and female participants. The results show that the gender of the participant is significantly associated with the amount they are WTP for the biopesticides. The coefficient on gender is negative suggesting that male individuals in this sample are WTP higher price for the biopesticides compared to their female counterparts. The other important variables in the regression include the size of the household, household per capita income, participation in malaria training and public health activities implemented by the Government and local and international institutions in their communities. Participants’ WTP for UZIMAX increased with the size of the household. The results also show that a participant residing in a household where at least one member received training related to malaria control is WTP about Kshs6 more for UZIMAX relative to those from a household without a trainee. Participation in such training activities increases public health awareness and may induce behavioral change among participants. We also note that participants from HHs with higher per capita income are WTP higher prices relative to those from HH with low per capita income. Higher per capita income is likely to stimulate increased investment in disease prevention.

Table 7.
Factors influencing WTP for UZIMAX.
Our findings further show some differences between the determinants of WTP by male and female participants. For example, the coefficients on household size and per capita income, are significant in the model for male participants but not for their female counterparts. In particular, higher per capita income is associated with increased WTP for the biopesticides. As earlier noted in the descriptive statics, female participants were associated with lower income per capita households, mainly derived from farming. The proportion of female participants who participated in nonfarm income-generating activities is about 44% relative to 52% for their male counterparts. This result points to the promotion of gender-inclusive income diversification, such as promoting non-farm- income generation, to improve the purchasing power of women and their households, which is likely to increase investment in malaria vector control technologies such as the UZIMAX biopesticides. The direction and significance of factors associated with participant’s WTP for UZIMAX are similar to the results of a meta-analysis of studies aimed at establishing the willingness to pay for various malaria control interventions by [44]. The study also reported that socioeconomic status, gender, marital status, age, household size, recent exposure to malaria sickness, income and cost of treating malaria were important and significant factors affecting the use of the various control interventions.
4. Conclusions and Policy Implications
The results of this study indicate that nearly all participants surveyed were interested in community-based larviciding using UZIMAX biopesticide and were willing to pay for the biopesticide due to the health and environmental benefits of this technology. Participants are willing to pay a premium of Kshs420 ($4.2) per 250 mL of UZIMAX. We note that willingness to pay is higher in urban and reduces with an increase in the bid process. Moreover, there are apparent gender differences; male participants are willing to pay a higher price for the biopesticides. We find that participants are willing to work together by pooling labor and finances in their villages to ensure the sustainable and effective application of the biopesticide during the rainy season. Thus, designing and implementing strong community-based financial and labor resources mechanisms could be a key pathway for promoting widespread use of the biopesticides, generating health, environmental and economic benefits in malaria-prone communities. Designs of efficient poor and rural incentive UZIMAX based interventions should be considered by embedding mechanisms that increase rural-based low-income earners to contribute resources for sustained malaria vector control.
We also find that WTP is dependent on household participation in capacity building and public health programs implemented by the Government and local and international institutions. The results support a popular view that facilitating the development of an integrated health innovation system with strong multi-stakeholder collaboration is essential for creating sustainable health care systems in Africa [51]. Because UZIMAX is still a new product, appropriate capacity building, communication and awareness creation are needed to demonstrate the effectiveness of the biopesticide and its potential health, environmental and economic impacts. Training and awareness campaigns need to target key stakeholders, including carefully developed community-based health groups, public health workers in the county government, the private sector such as private change agents in the health sector and local leaders. The creation of more awareness and training is needed for female residents and in rural areas. Besides, proper marketing structures need to be developed and facilitated to ensure increased and sustainable access and availability of the biopesticide.
Author Contributions
G.M.D.: Conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft; M.K. (Michael Kidoido): visualization, writing—review and editing; B.W.M.: Visualization, writing—review and editing; N.G.G.: data collection, Literature review, writing—review and editing; M.K. (Menale Kassie): Investigation, supervision, Visualization, writing—review and editing; R.M.: Project administration, investigation; J.B.O.: Project administration, investigation; C.M.M.: Supervision, writing—review and editing, Project Administration, Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Biovision Foundation Switzerland (Grant number BV HH-07/2016-18). The article processing charge (APC) was funded by ICIPE core funding from the UK’s Foreign, Commonwealth & Development Office (FCDO), the Swiss Agency for Development and Cooperation (SDC), the Federal Democratic Republic of Ethiopia and the Kenyan Government. The funding bodies have no role in the design of the study, collection, analysis, and interpretation of data or in writing the manuscript. The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of ICIPE or the donors.
Acknowledgments
The authors are grateful for the useful comments received from the anonymous reviewers of the paper.
Conflicts of Interest
The authors do declare that we do not have any conflict of interest with this work.
References
- Sachs, J.; Malaney, P. The Economic and Social Burden of Malaria. Nature 2002, 415, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.M.; Kinyoki, D.K.; Mundia, C.W.; Kabaria, C.W.; Mutua, J.W.; Alegana, V.A.; Fall, I.S.; Snow, R.W. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000–10: A spatial and temporal analysis of transmission intensity. Lancet 2014, 383, 1739–1747. [Google Scholar] [CrossRef]
- Macharia, P.M.; Giorgi, E.; Noor, A.M.; Waqo, E.; Kiptui, R.; A Okiro, E.; Snow, R.W. Spatio-temporal analysis of Plasmodium falciparum prevalence to understand the past and chart the future of malaria control in Kenya. Malar. J. 2018, 17, 340. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region); South African Institute for Medical Research: Johannesburg, South Africa, 1968; pp. 1–343. [Google Scholar]
- Barat, L.M.; Mills, A.; Basu, S.; Palmer, N.; Hanson, K.; Worrall, E. Do Malaria Control Interventions Reach the Poor? A View through the Equity Lens. Am. J. Trop. Med. Hyg. 2004, 71, 174–178. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Strategic Framework for Integrated Vector Management; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Ranson, H.; N’Guessan, R.; Lines, J.; Moiroux, N.; Nkuni, Z.; Corbel, V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011, 27, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J. The role of vector control in stopping the transmission of malaria: Threats and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130431. [Google Scholar] [CrossRef] [PubMed]
- Butler, D. Malaria Vaccine Results Face Scrutiny. Nature 2011, 478, 439–440. [Google Scholar] [CrossRef]
- Bhattarai, A.; Ali, A.S.; Kachur, S.P.; Mårtensson, A.; Abbas, A.K.; Khatib, R.; Al-Mafazy, A.-W.; Ramsan, M.; Rotllant, G.; Gerstenmaier, J.F.; et al. Impact of Artemisinin-Based Combination Therapy and Insecticide-Treated Nets on Malaria Burden in Zanzibar. PLoS Med. 2007, 4, e309. [Google Scholar] [CrossRef]
- Sharp, B.L.; Ridl, F.C.; Govender, D.; Kuklinski, J.; Kleinschmidt, I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar. J. 2007, 6, 52–56. [Google Scholar] [CrossRef]
- Govella, N.J.; Ferguson, H. Why Use of Interventions Targeting Outdoor Biting Mosquitoes will be Necessary to Achieve Malaria Elimination. Front. Physiol. 2012, 3, 199. [Google Scholar] [CrossRef]
- Leach-Kemon, K.; Chou, D.P.; Schneider, M.T.; Tardif, A.; Dieleman, J.L.; Brooks, B.P.; Hanlon, M.; Murray, C.J. The Global Financial Crisis Has Led to a Slowdown in Growth of Funding to Improve Health in Many Developing Countries. Health Aff. 2012, 31, 228–235. [Google Scholar] [CrossRef]
- Beier, J.C.; Keating, J.; Githure, J.I.; Macdonald, M.B.; Impoinvil, D.E.; Novak, R.J. Integrated vector management for malaria control. Malar. J. 2008, 7, S4. [Google Scholar] [CrossRef]
- Mutero, C.M.; Schlodder, D.; Kabatereine, N.; Kramer, R. Integrated Vector Management for Malaria Control in Uganda: Knowledge, Perceptions and Policy Development. Malar. J. 2012, 11, 1–10. [Google Scholar] [CrossRef]
- Mutero, C.M.; Mbogo, C.; Mwangangi, J.; Imbahale, S.; Kibe, L.; Orindi, B.O.; Girma, M.; Njui, A.; Lwande, W.; Affognon, H.; et al. An Assessment of Participatory Integrated Vector Management for Malaria Control in Kenya. Environ. Health Perspect. 2015, 123, 1145–1151. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Larval Source Management: A Supplementary Malaria Vector Control Measure: An Operational Manual; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Zhou, G.; Wiseman, V.; Atieli, H.E.; Lee, M.-C.; Githeko, A.K.; Yan, G. The impact of long-lasting microbial larvicides in reducing malaria transmission and clinical malaria incidence: Study protocol for a cluster randomized controlled trial. Trials 2016, 17, 423. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Mweresa, N.G.; Wanjala, C.L.; Gilbreath, T.M., III; Zhou, G.; Lee, M.-C.; Githeko, A.K.; Yan, G. Evaluation of Long-Lasting Microbial Larvicide for Malaria Vector Control in Kenya. Malar. J. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, M.; Becker, P.; Mudambo, K.; De Jager, C. Field effectiveness of microbial larvicides on mosquito larvae in malaria areas of Botswana and Zimbabwe. Malar. J. 2016, 15, 586. [Google Scholar] [CrossRef]
- Dambach, P.; Schleicher, M.; Korir, P.; Ouedraogo, S.; Dambach, J.; Sie, A.; Dambach, M.; Becker, N. Nightly Biting Cycles of Anopheles Species in Rural Northwestern Burkina Faso. J. Med. Entomol. 2018, 55, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Dambach, P.; Schleicher, M.; Stahl, H.-C.; Traoré, I.; Becker, N.; Kaiser, A.; Sie, A.; Sauerborn, R. Routine implementation costs of larviciding with Bacillus thuringiensis israelensis against malaria vectors in a district in rural Burkina Faso. Malar. J. 2016, 15, 380. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, U.; Lindsay, S.W. Larval Source Management for Malaria Control in Africa: Myths and Reality. Malar. J. 2011, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maheu-Giroux, M.; Castro, M.C. Impact of Community-Based Larviciding on the Prevalence of Malaria Infection in Dar es Salaam, Tanzania. PLoS ONE 2013, 8, e71638. [Google Scholar] [CrossRef]
- Rahman, R.; Lesser, A.; Mboera, L.; Kramer, R. Cost of microbial larviciding for malaria control in rural Tanzania. Trop. Med. Int. Health 2016, 21, 1468–1475. [Google Scholar] [CrossRef]
- International Centre of Insect Physiology and Ecology (icipe): Annual Report 2018; Icipe Press: Nairobi, Kenya, 2019; Available online: http://www.icipe.org/publications/annual-reports (accessed on 14 October 2020).
- Bouzid, M.; Colón-González, F.J.; Lung, T.; Lake, I.R.; Hunter, P.R. Climate Change and the Emergence of Vector-Borne Diseases in Europe: Case Study of Dengue Fever. BMC Public Health 2014, 14, 781. [Google Scholar] [CrossRef]
- Watanabe, K.; Shono, Y.; Kakimizu, A.; Okada, A.; Matsuo, N.; Satoh, A.; Nishimura, H. New mosquito repellent from Eucalyptus camaldulensis. J. Agric. Food Chem. 1993, 41, 2164–2166. [Google Scholar] [CrossRef]
- Kocher, D.K.; Riat, A.K. Larvicidal Potential of Eucalyptus Globulus Oil against Anopheles Stephensi. Int. J. Mosq. Res. 2019, 6, 24–26. [Google Scholar]
- Onwujekwe, O.; Uguru, N.; Etiaba, E.; Chikezie, I.; Uzochukwu, B.; Adjagba, A. The Economic Burden of Malaria on Households and the Health System in Enugu State Southeast Nigeria. PLoS ONE 2013, 8, e78362. [Google Scholar] [CrossRef]
- Ghebreyesus, A.T.; Witten, K.H.; Getachew, A.; O’Neill, K.; Bosman, A.; Teklehaimanot, A. Community-based malaria control in Tigray, northern Ethiopia. Parassitologia 1999, 41, 367–371. [Google Scholar]
- Yasuoka, J.; Levins, R.; Mangione, T.W.; Spielman, A. Community-based rice ecosystem management for suppressing vector anophelines in Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 995–1006. [Google Scholar] [CrossRef]
- Government of Kenya (Gok). Kilifi County Integrated Development Plan 2013–2017. Available online: https://www.cog.go.ke/phocadownload/FirstCIDPs/KIlifi%20.pdf (accessed on 14 October 2020).
- Becker, G.M.; DeGroot, M.H.; Marschak, J. Measuring Utility by a Single-response Sequential Method. Behav. Sci. 1964, 9, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bohm, P.; Lindén, J.; Sonnegård, J. Eliciting Reservation Prices: Becker—DeGroot—Marschak Mechanisms vs. Markets. Econ. J. 1997, 107, 1079–1089. [Google Scholar] [CrossRef]
- Wertenbroch, K.; Skiera, B. Measuring Consumers’ Willingness to Pay at the Point of Purchase. J. Mark. Res. 2002, 39, 228–241. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C.; Morawetz, U.B. Estimating consumer willingness to pay for food quality with experimental auctions: The case of yellow versus fortified maize meal in Kenya. Agric. Econ. 2010, 42, 1–16. [Google Scholar] [CrossRef]
- Berry, J.; Fischer, G.; Guiteras, R. Eliciting and Utilizing Willingness to Pay: Evidence from Field Trials in Northern Ghana. J. Political Econ. 2020, 128, 1436–1473. [Google Scholar] [CrossRef]
- Kimenju, S.C.; De Groote, H. Consumer willingness to pay for genetically modified food in Kenya. Agric. Econ. 2008, 38, 35–46. [Google Scholar] [CrossRef]
- Tassew, A.; Hopkins, R.J.; Deressa, W. Factors influencing the ownership and utilization of long-lasting insecticidal nets for malaria prevention in Ethiopia. Malar. J. 2017, 16, 262. [Google Scholar] [CrossRef] [PubMed]
- Pell, C.; Straus, L.; Andrew, E.V.W.; Meñaca, A.; Pool, R. Social and Cultural Factors Affecting Uptake of Interventions for Malaria in Pregnancy in Africa: A Systematic Review of the Qualitative Research. PLoS ONE 2011, 6, e22452. [Google Scholar] [CrossRef] [PubMed]
- Habibov, N.; Cheung, A.; Auchynnikava, A. Does social trust increase willingness to pay taxes to improve public healthcare? Cross-sectional cross-country instrumental variable analysis. Soc. Sci. Med. 2017, 189, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Roosen, J.; Bieberstein, A.; Blanchemanche, S.; Goddard, E.; Marette, S.; Vandermoere, F. Trust and willingness to pay for nanotechnology food. Food Policy 2015, 52, 75–83. [Google Scholar] [CrossRef]
- Trapero-Bertran, M.; Mistry, H.; Shen, J.; Fox-Rushby, J. A Systematic Review and Meta-analysis of Willingness-to-pay Values: The Case of Malaria Control Interventions. Health Econ. 2013, 22, 428–450. [Google Scholar] [CrossRef]
- Yukich, J.; Briët, O.J.T.; Ahorlu, C.K.; Nardini, P.; Keating, J. Willingness to pay for small solar powered bed net fans: Results of a Becker—DeGroot—Marschak auction in Ghana. Malar. J. 2017, 16, 1–5. [Google Scholar] [CrossRef][Green Version]
- Dickinson, K.; Paskewitz, S. Willingness to Pay for Mosquito Control: How Important Is West Nile Virus Risk Compared to the Nuisance of Mosquitoes? Vector-Borne Zoonotic Dis. 2012, 12, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Mboera, L.E.; Kramer, R.; Miranda, M.L.; Kilima, S.P.; Shayo, E.H.; Lesser, A. Community Knowledge and Acceptance of Larviciding for Malaria Control in a Rural District of East-Central Tanzania. Int. J. Environ. Res. Public Health 2014, 11, 5137–5154. [Google Scholar] [CrossRef] [PubMed]
- Opiyo, A.P.; Mukabana, W.R.; Kiche, I.; Mathenge, E.; Killeen, G.F.; Fillinger, U. An Exploratory Study of Community Factors Relevant for Participatory Malaria Control on Rusinga Island, Western Kenya. Malar. J. 2007, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Ingabire, C.M.; Alaii, J.; Hakizimana, E.; Kateera, F.; Muhimuzi, D.; Nieuwold, I.; Bezooijen, K.; Rulisa, S.; Kaligirwa, N.; Muvunyi, C.M.; et al. Community mobilization for malaria elimination: Application of an open space methodology in Ruhuha sector, Rwanda. Malar. J. 2014, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Derua, Y.A.; Kweka, E.J.; Kisinza, W.N.; Githeko, A.K.; Mosha, F.W. Bacterial larvicides used for malaria vector control in sub-Saharan Africa: Review of their effectiveness and operational feasibility. Parasites Vectors 2019, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chataway, J.; Chaturvedi, K.; Hanlin, R.; Mugwagwa, J.; Smith, J.; Wield, D. In Science, Technology and Innovation for Public Health in Africa. Available online: http://asiandrivers.open.ac.uk/NEPAD_health_final.pdf (accessed on 14 October 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).