A Snapshot on Food Allergies: A Case Study on Edible Flowers
Abstract
:1. Food Allergies
1.1. Mechanisms of Food Allergy
1.2. Sensitization and Cross-Reactivity
- the individual becomes “tolerant” to the allergen, and subsequent exposures will not result in the production of antibodies;
- the individual becomes “sensitized” to the allergen, and subsequent exposure develops an IgE-mediated response with unwanted symptoms; or
- the individual develops an immune response, involving the production of other types of immunoglobulins (i.e., IgG) that may reappear on consequent exposure, but that does not necessarily give symptoms.
1.3. Adverse Reactions to Foods: Symptoms and Syndromes
2. Food Allergens
2.1. Classification of Food Allergens
2.2. Database of Allergens
3. Food Processing and Allergenicity
Influence of Processing on Food Allergenicity
4. Labeling and Novel Foods: European Regulations
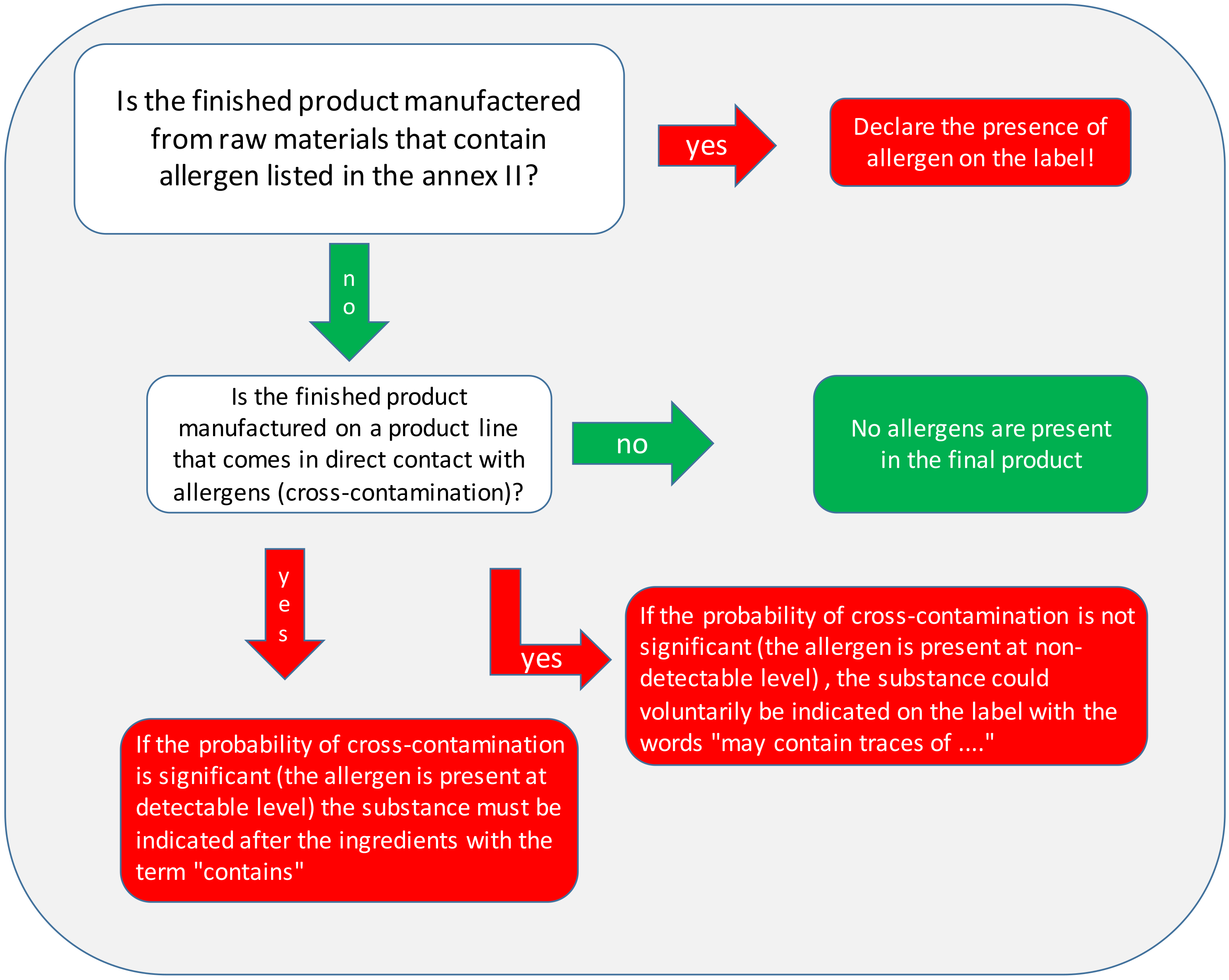
4.1. Labeling
- (1)
- Directive 2003/89/EC [36] (and further amendments) introduced Annex IIIa, which is the list of fourteen allergenic foods that, if present in a product, must always be shown on the package label, as follows:
- Cereals containing gluten (i.e., wheat, rye, barley, oats, spelt, kamut, or their hybridized strains) and products thereof
- Crustaceans and products thereof
- Eggs and products thereof
- Fish and products thereof
- Peanuts and products thereof
- Soybeans and products thereof
- Milk and products thereof (including lactose)
- Nuts i.e., almonds, hazelnuts, walnuts, cashews, pecan nuts, Brazil nuts, pistachio nuts, macadamia nuts, and Queensland nuts, and products thereof
- Celery and products thereof
- Mustard and products thereof
- Sesame seeds and products thereof
- Sulfur dioxide and sulfites at concentrations of more than 10 mg/kg or 10 mg/liter expressed as SO2.
- Lupin and products thereof
- Mollusks and products thereof
- (2)
- Directive 2007/68/EC [91] is the most recent amendment of Annex IIIa. It contains the list of allergens that must be labeled and the products deriving from these allergens for which it is not necessary to add the allergen on the label.
4.2. Novel Food Regulation
5. Focus on the Edible Flowers
5.1. Edible Flowers: A Source of Bioactive Compounds
5.2. Safety Issues of Edible Flowers
- -
- the presence of toxic compounds and
- -
- the possibility to develop allergic reactions.
5.2.1. Potential Toxic Compounds in Edible Flowers
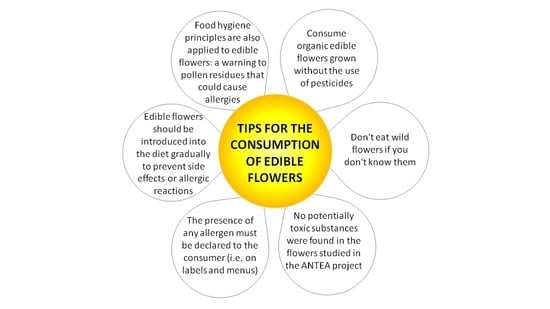
5.2.2. Risks Associated with the Consumption of Edible Flowers: Consumer Safety Precautions
- -
- the presence of any allergens must be declared to the consumer on the food label or menus, if the meal is eaten away from home;
- -
- to avoid problems related to cross-contamination, especially for allergic people, the hygiene rules in use for all foods must be respected—pollen residues or allergens could give rise to allergic reactions;
- -
- sensitive individuals must gradually introduce new varieties of flowers into their diet to check for any allergic reactions; and
- -
- flowers that are not found to be consumed in Europe before 15 May 1997 must be registered as a novel food.
5.3. A Case Study: The ANTEA Project
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andreas, L. Allergenicity of food and impact of processing. In Novel Food Processing; CRC Press: Boca Raton, FL, USA, 2009; pp. 459–478. [Google Scholar]
- Sicherer, S.H.; Sampson, H.A. Peanut allergy: Emerging concepts and approaches for an apparent epidemic. J. All. Clin. Immunol. 2007, 120, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food Allergies: The Basics. Gastroenterology 2015, 148, 1120–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathe, S.K.; Liu, C.; Zaffran, V.D. Food Allergy. Annu. Rev. Food Sci. Technol. 2016, 7, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Nuray Bayar Muluk, M.D.; Cemal Cingi, M.D. Oral allergy syndrome. Am. J. Rhinol. Allergy 2018, 32, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Iweala, O.I.; Choudhary, S.K.; Commins, S.P. Food Allergy. Curr. Gastroenterol. Rep. 2018, 20, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Rodriguez, T.W.; Garcia-Neuer, M.; Alenazy, L.A.; Castells, M. Anaphylaxis in the 21st century: Phenotypes, endotypes, and biomarkers. J. Asthma Allergy 2018, 11, 121–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Waserman, S.; Bégin, P.; Watson, W. IgE-mediated food allergy. Allergy Asthma Clin. Immunol. 2018, 14, 55. [Google Scholar] [CrossRef] [Green Version]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clinic. Rev. Allergy Immunol. 2019, 57, 244. [Google Scholar] [CrossRef] [Green Version]
- Nowak-Węgrzyn, A.; Katz, Y.; Mehr, S.S.; Koletzko, S. Non-IgE-mediated gastrointestinal food allergy. Allergy Asthma Clin. Immunol. 2018, 14, 55. [Google Scholar] [CrossRef]
- Burton, O.T.; Oettgen, H.C. Beyond immediate hypersensitivity: Evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol Rev. 2011, 242, 128–143. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307, quiz 8. Epub 2014/01/07. [Google Scholar] [CrossRef]
- Hogan, S.P.; Wang, Y.H.; Strait, R.; Finkelman, F.D. Food-induced anaphylaxis: Mast cells as modulators of anaphylactic severity. Semin. Immunopathol. 2012, 34, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, F.; Hawranek, T.; Gruber, P.; Wopfner, N.; Mari, A. Allergic cross-reactivity: From gene to the clinic. Allergy 2004, 59, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Ansotegui, I.J.; Pawankar, R.; Schmid-Grendelmeier, P.; van Hage, M.; Baena-Cagnani, C.E.; Melioli, G.; Nunes, C.; Passalacqua, G.; Rosenwasser, L.; et al. A WAO—ARIA—GALEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: A practice parameter update-2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025. [Google Scholar] [CrossRef]
- Popescu, F.D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef]
- Egger, M.; Mutschlechner, S.; Wopfner, N.; Gadermaier, G.; Briza, P.; Ferreira, F. Pollen-food syndromes associated with weed pollinosis: An update from the molecular point of view. Allergy 2006, 61, 461–476. [Google Scholar] [CrossRef]
- Han, Y.; Kim, J.; Ahn, K. Food allergy. Korean J. Pediatr. 2012, 55, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.D. Molecular biomarkers for grass pollen immunotherapy. World J. Methodol. 2014, 4, 26–45. [Google Scholar] [CrossRef]
- Werfel, T.; Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Enrique, E.; Knulst, A.C.; Mari, A.; Muraro, A.; Ollert, M.; Poulsen, L.K.; et al. Position paper of the EAACI: Food allergy due to immunological cross-reactions with common inhalant allergens. Allergy 2015, 70, 1079–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.P.; Bansil, S.; Uygungil, B. Signs and symptoms of food allergy and food-induced anaphylaxis. Ped. Clin. N. Am. 2015, 62, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Oriel, R.C.; Wang, J.J. Diagnosis and management of food allergy. Ped. Clin. N. Am. 2019, 66, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Sommergrube, K.; Mills, E.N. Food allergen protein families and their structural characteristics and application in component-resolved diagnosis: New data from the EuroPrevall project. Anal. Bioanal. Chem. 2009, 395, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121, 847–852. [Google Scholar] [CrossRef]
- Jenkins, J.A.; Griffiths-Jones, S.; Shewry, P.R.; Breiteneder, H.; Mills, E.N. Structural relatedness of plant food allergens with specific reference to cross-reactive allergens: An in silico analysis. J. Allergy Clin. Immunol. 2005, 115, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, J.A.; Breiteneder, H.; Mills, E.N. Evolutionary distance from human homologs reflects allergenicity of animal food proteins. J. Allergy Clin. Immunol. 2007, 120, 1399–1405. [Google Scholar] [CrossRef]
- Radauer, C.; Breiteneder, H. Pollen allergens are restricted to few protein families and show distinct patterns of species distribution. J. All. Clin. Immunol. 2006, 1, 141–147. [Google Scholar]
- Breiteneder, H.; Mills, E.N. Molecular properties of food allergens. J. Allergy Clin. Immunol. 2005, 115, 14–23. [Google Scholar] [CrossRef]
- Dall’Antonia, F.; Pavkov-Keller, T.; Zangger, K.; Keller, W. Structure of allergens and structure based epitope predictions. Methods 2014, 66, 3–21. [Google Scholar] [CrossRef]
- FDA. The Threshold Working Group. Report. Approaches to Establish Thresholds for Major Food Allergens and for Gluten in Food; FDA: Silver Spring, MD, USA, 2006. [Google Scholar]
- Blom, W.M.; Vlieg-Boerstra, B.J.; Kruizinga, A.G.; van der Heide, S.; Houben, G.F.; Dubois, A.E. Threshold dose distributions for 5 major allergenic foods in children. J. Allergy Clin. Immunol. 2013, 131, 172–179. [Google Scholar] [CrossRef]
- Council of the European Union. Directive 2003/89/EC (and further amendments) introduced Annex IIIa. Off. J. Eur. Union 2003. Available online: https://www.fsai.ie/uploadedFiles/Dir2003.89.pdf (accessed on 18 September 2020).
- Allergen Nomenclature. Available online: http://www.allergen.org/ (accessed on 12 July 2020).
- COMprehensive Protein Allergen Resource. COMPARE 2020 DB Release Date: 01/29/2020. Available online: https://comparedatabase.org/ (accessed on 12 July 2020).
- Tong, J.C.; Lim, S.J.; Muh, H.C.; Chew, F.T.; Tammi, M.T. Allergen Atlas: A comprehensive knowledge center and analysis resource for allergen information. Bioinformatics 2009, 25, 979–980. [Google Scholar] [CrossRef] [Green Version]
- Radauer, C. Navigating through the Jungle of Allergens: Features and Applications of Allergen Databases. Int. Arch. Allergy Immunol. 2017, 173, 1–11. [Google Scholar] [CrossRef]
- Allergome. Available online: http://www.allergome.org/script/about.php (accessed on 12 July 2020).
- AllergenOnline. Available online: http://www.allergenonline.org/ (accessed on 12 July 2020).
- Structural Database of Allergenic Proteins (SDAP). Available online: https://fermi.utmb.edu/ (accessed on 12 July 2020).
- Immune Epitope Database (IEDB). Available online: https://www.iedb.org/ (accessed on 12 July 2020).
- Lorenz, A.R.; Scheurer, S.; Vieths, S. Food allergens: Molecular and immunological aspects, allergen databases and cross-reactivity. Chem. Immunol. Allergy 2015, 101, 18–29. [Google Scholar] [CrossRef]
- Pekar, J.; Ret, D.; Untersmayr, E. Stability of allergens. Mol. Immunol. 2018, 100, 14–20. [Google Scholar] [CrossRef]
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53, S151–S183. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Thomas, K.; Herouet-Guicheney, C.; Ladics, G.; Bannon, G.; Cockburn, A.; Crevel, R.; Fitzpatrick, J.; Mills, C.; Privalle, L.; Vieths, S. Evaluating the effect of food processing on the potential human allergenicity of novel proteins: International workshop report. Food Chem. Toxicol. 2007, 45, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.N.C.; Mackie, A.R. The impact of processing on allergenicity of food. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Saiz, R.S.; Benede, E.; Lopez-Exposito, M.I. Effect of processing technologies on the allergenicity of food products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1902–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepski, S.; Brockmeyer, J. Impact of dietary factors and food processing on food allergy. Mol. Nutr. Food Res. 2013, 57, 145–152. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the evaluation of allergenic foods and food ingredients for labeling purposes. EFSA J. 2014, 12, 3894. [Google Scholar]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Vanga, S.K.; Singh, A.; Raghavan, V. Review of conventional and novel food processing methods on food allergens. Crit. Rev. Food Sci. Nutr. 2017, 57, 2077–2094. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Effects of daily food processing on allergenicity. Crit. Rev. Food Sci. Nutr. 2017, 59, 31–42. [Google Scholar] [CrossRef]
- Mills, E.N.C.; Sancho, A.; Kostyra, H. The effect of food processing on allergens. In Managing Allergens in Food; Hoffmann-Somergruber, K., Mills, C., Wichers, H.J., Eds.; Woodhead Publishing: Cambridge, UK, 2006. [Google Scholar]
- Cabanillas, B.; Jappe, U.; Novak, N. Allergy to peanut, soybean, and other legumes: Recent advances in allergen characterization, stability to processing and IgE cross-reactivity. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Sathe, S.K.; Teuber, S.S.; Roux, K.H. Effects of food processing on the stability of food allergens. Biotechnol. Adv. 2005, 23, 423–429. [Google Scholar] [CrossRef]
- Andreas, L.; Sathe, S.K.; Sharma, G.M. Effects of food processing on food allergens. Mol. Nutr. Food Res. 2009, 53, 970–978. [Google Scholar]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Ahrazem, O.; Jimeno, L.; Lopez-Torrejon, G.; Herrero, M.; Espada, J.L.; Sanchez-Monge, R.; Duffort, O.; Barber, D.; Salcedo, G. Assessing allergen levels in peach and nectarine cultivars. Ann. All. Asthma Immunol. 2007, 99, 42–47. [Google Scholar] [CrossRef]
- Boyano-Martınez, T.; Pedrosa, M.; Belver, T.; Quirce, S.; Garcıa-Ara, C. Peach allergy in Spanish children: Tolerance to the pulp and molecular sensitization profile. Pediat. All. Immunol. 2013, 24, 168–172. [Google Scholar] [CrossRef]
- Pravettoni, V.; Primavesi, L.; Farioli, L.; Brenna, O.V.; Pompei, C.; Conti, A.; Scibilia, J.; Piantanida, M.; Mascheri, A.; Pastorello, E.A. Tomato allergy: Detection of IgE-binding lipid transfer proteins in tomato derivatives and in fresh tomato peel, pulp, and seeds. J. Agric. Food Chem. 2009, 57, 10749–10754. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.K.; Raghavan, V. Effect of pre-harvest and post-harvest conditions on the fruit allergenicity: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1027–1043. [Google Scholar] [CrossRef]
- Tulipani, S.; Marzban, G.; Herndl, A.; Laimer, M.; Mezzetti, B.; Battino, M. Influence of environmental and genetic factors on healthrelated compounds in strawberry. Food Chem. 2011, 124, 906–913. [Google Scholar] [CrossRef]
- Lopez-Matas, M.A.; Larramendi, C.H.; Ferrer, A.; Huertas, A.J.; Pagan, J.A.; Garcıa-Abujeta, J.L.; Dolle, S.; Lehmann, K.; Schwarz, D.; Weckwert, W.; et al. Allergenic activity of different tomato cultivars in tomato allergic subjects. Clin. Exp. Allergy 2011, 41, 1643–1652. [Google Scholar]
- Schmitz-Eiberger, M.; Matthes, A. Effect of harvest maturity, duration of storage and shelf life of apples on the allergen Mal d 1, polyphenoloxidase activity and polyphenol content. Food Chem. 2011, 127, 1459–1464. [Google Scholar] [CrossRef]
- Wang, J.; Liang, S.; Ma, H.; Zhang, P.; Shi, W. Effects of ethephon on fresh In-Husk walnut preservation and its possible relationship with phenol metabolism. J. Food. Sci. 2016, 81, C1921–C1927. [Google Scholar] [CrossRef]
- Teodorowicz, M.; van Neerven, J.; Savelkoul, H. Food processing: The influence of the maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Maillard reaction in food allergy: Pros and cons. Crit. Rev. Food Sci. Nutr. 2018, 58, 208–226. [Google Scholar] [CrossRef]
- Toda, M.; Hellwig, M.; Henle, T.; Vieths, S. Influence of the maillard reaction on the allergenicity of food proteins and the development of allergic inflammation. Curr. Allergy Asthma Rep. 2019, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, M.; Wellner, A.; Gadermaier, G.; Ilchmann, A.; Briza, P.; Krause, M.; Henle, T. Ovalbumin modified with pyrraline, a Maillard reaction product, shows enhanced T-cell immunogenicity. J. Biol. Chem. 2014, 289, 7919–7928. [Google Scholar] [CrossRef] [Green Version]
- Gruber, P.; Becker, W.M.; Hofmann, T. Influence of the Maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. J. Agric. Food Chem. 2005, 53, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; L’Hocine, L.; Karboune, S. Allergenicity of potato proteins and of their conjugates with galactose, galactooligosaccharides, and galactan in native, heated, and digested forms. J. Agric. Food Chem. 2014, 62, 3591–3598. [Google Scholar] [CrossRef]
- De Jongh, H.H.; Robles, C.L.; Timmerman, E.; Nordlee, J.A.; Lee, P.W.; Baumert, J.L.; Hamilton, R.G.; Taylor, S.L.; Koppelman, S.J. Digestibility and IgE-binding of glycosylated codfish parvalbumin. Biomed. Res. Int. 2013, 2013, 756789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupa, P.; Nakamura, S.; Katayama, S.; Min, Y. Effects of ovalbumin glycoconjugates on alleviation of orally induced egg allergy in mice via dendritic-cell maturation and T-cell activation. Mol. Nutr. Food Res. 2014, 58, 405–417. [Google Scholar] [CrossRef]
- Rupa, P.; Nakamura, S.; Katayama, S.; Mine, Y. Attenuation of allergic immune response phenotype by mannosylated egg white in orally induced allergy in BALB/c mice. J. Agric. Food Chem. 2014, 62, 9479–9487. [Google Scholar] [CrossRef]
- Kroghsbo, S.; Rigby, N.M.; Johnson, P.E.; Adel-Patient, K.; Bøgh, K.L.; Salt, L.J.; Mills, E.C.; Madsen, C.B. Assessment of the sensitizing potential of processed peanut proteins in Brown Norway rats: Roasting does not enhance allergenicity. PLoS ONE 2014, 7, e96475. [Google Scholar] [CrossRef]
- Iwan, M.; Vissers, Y.M.; Fiedorowicz, E.; Kostyra, H.; Kostyra, E.; Savelkoul, H.F.; Wichers, H.J. Impact of Maillard reaction on immunoreactivity and allergenicity of the hazelnut allergen Cor a 11. J. Agric. Food Chem. 2011, 59, 7163–7171. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, S.; Das, M.; Dwivedi, P.D. Impact of thermal processing on legume allergens. Plant Foods Hum. Nutr. 2012, 67, 430–441. [Google Scholar] [CrossRef]
- Cuadrado, C.; Cabanillas, B.; Pedrosa, M.M.; Varela, A.; Guillamon, E.; Muzquiz, M.; Crespo, J.F.; Rodriguez, J.; Burbano, C. Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Mol. Nutr. Food Res. 2009, 53, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.A.; Pravettoni, V.; Farioli, L.; Primavesi, L.; Scibilia, J.; Piantanida, M.; Mascheri, A.; Conti, A. Green bean (Phaseolus vulgaris): A new source of IgE-binding lipid transfer protein. J. Agric. Food Chem. 2009, 58, 4513–4516. [Google Scholar] [CrossRef] [PubMed]
- Kasera, R.; Singh, A.B.; Lavasa, S.; Nagendra, K.; Arora, N. Purification and immunobiochemical characterization of a 31 kDa crossreactive allergen from Phaseolus vulgaris (kidney bean). PLoS ONE 2013, 8, e63063. [Google Scholar] [CrossRef] [PubMed]
- Vitaliti, G.; Morselli, I.; Di Stefano, V.; Lanzafame, A.; La Rosa, M.; Leonardi, S. Urticaria and anaphylaxis in a child after inhalation of lentil vapours: A case report and literature review. Ital. J. Pediatr. 2012, 38, 71. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Lahti, T.; Tanaka, T.; Nickerson, M.T. Egg proteins: Fractionation, bioactive peptides and allergenicity. J. Sci. Food Agric. 2018, 98, 5547–5558. [Google Scholar] [CrossRef]
- Esteghlal, S.; Gahruie, H.H.; Niakousari, M.; Barba, F.J.; Bekhit, A.E.; Mallikarjunan, K.; Roohinejad, S. Bridging the knowledge gap for the impact of non-thermal processing on proteins and amino acids. Foods 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Potential utility of high-pressure processing to address the risk of food allergen concerns. Compr. Rev. Food Sci. Technol. 2014, 13, 78–90. [Google Scholar] [CrossRef]
- Yang, A.; Zuo, L.; Cheng, Y.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Council of the European Union. Directive 2000/13/EC of the European Parliament and of the Council of 20 March 2000 on the approximation of the laws of the Member States relating to the labelling, presentation and advertising of foodstuffs. Off. J. Eur. Union 2000. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32000L0013&from=EN (accessed on 18 September 2020).
- Council of the European Union. COMMISSION DIRECTIVE 2007/68/EC of 27 November 2007 amending Annex IIIa to Directive 2000/13/EC of the European Parliament and of the Council as regards certain food ingredients. Off. J. Eur. Union 2007. Available online: http://extwprlegs1.fao.org/docs/pdf/eur75647.pdf (accessed on 18 September 2020).
- Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1169&from=EN (accessed on 18 September 2020).
- Council of the European Union. Regulation EC No. 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients. Off. J. Eur. Comm. 1997, 40, L43. [Google Scholar]
- European Commission. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods. Available online: https://ec.europa.eu/food/safety/novel_food/legislation_en (accessed on 20 October 2020).
- European Commission. List containing all authorised novel foods (Commission Implementing Regulation (EU) 2017/2470). Available online: https://ec.europa.eu/food/safety/novel_food/authorisations/union-list-novel-foods_en (accessed on 20 September 2020).
- European Commission. Novel Food Catalogues. Available online: https://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm (accessed on 20 September 2020).
- Cunningham, E. What nutritional contribution do edible flowers make? J. Acad. Nutr. Diet 2015, 115, 856. [Google Scholar] [CrossRef]
- Murphy, H. Foods indigenous to the Western hemisphere. In American Indian Health Diet Project; Available online: http://www.aihd.ku.edu/foods/squash.html (accessed on 18 September 2020).
- Kirker, C.L.; Newman, M. Edible Flowers: A Global History, 1st ed.; Reaktion Books Ltd.: London, UK, 2016; pp. 19–21. [Google Scholar]
- Stradley, L. Edible flowers are the new rage in haute cuisine. In What’s Cooking America Website; Available online: http://whatscookingamerica.net/EdibleFlowers/EdibleFlowersMain.htm (accessed on 27 February 2015).
- Falconnier, D. Incredible Edible Flowers; University of Illinois Extension: Urbana, IL, USA, 2006; pp. 1–6. [Google Scholar]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. An Overview on the market of edible flowers. Food Rev. Int. 2019, 36, 258–275. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible flowers—A new promising source of mineral elements in human nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Chensom, S.; Okumura, H.; Mishima, T. Primary screening of antioxidant activity, total polyphenol content, carotenoid content, and nutritional composition of 13 edible flowers from Japan. Prev. Nutr. Food Sci. 2019, 24, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.J.; Choung, M.G. Anthocyanin compositions and biological activities from the red petals of Korean edible rose (Rosa hybrida cv. Noblered). Food Chem. 2011, 129, 272–278. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Zhang, Y.; Wang, L.; Zhang, R. In vitro antioxidant properties of different parts of pomegranate flowers. Food Bioprod. Process. 2011, 89, 234–240. [Google Scholar] [CrossRef]
- Arya, V.; Kumar, D.; Gautam, M. Phytopharmacological review on flowers: Source of inspiration for drug discovery. Biomed. Prev. Nutr. 2014, 4, 45–51. [Google Scholar] [CrossRef]
- Zeng, Y.; Deng, M.; Lv, Z.; Peng, Y. Evaluation of antioxidant activities of extracts from 19 Chinese edible flowers. SpringerPlus 2014, 3, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, T.C.; Dias, M.I.; Barros, L.; Ferreira, I.C. Nutritional and chemical characterization of edible petals and corresponding infusions: Valorization as new food ingredients. Food Chem. 2017, 220, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, P.; Wojdyło, A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef]
- US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Available online: http://www.ars.usda.gov/Services/docs..htm?docid¼8964 (accessed on 8 March 2015).
- Plumb, J.; Pigat, S.; Bompola, F.; Cushen, M.; Pinchen, H.; Nørby, E.; Astley, S.; Lyons, J.; Kiely, M.; Finglas, P. eBASIS (Bioactive Substances in Food Information Systems) and bioactive intakes: Major updates of the bioactive compound composition and beneficial bio effects database and the development of a probabilistic model to assess intakes in Europe. Nutrients 2017, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- Lara-Cortes, E.; Osorio-Dıaz, P.; Jiménez-Aparicio, A.; Bautista-Bañios, S. Nutritional content, functional properties and conservation of edible flowers. Review. Arch. Latinoam. Nutr. 2013, 63, 197–208. [Google Scholar]
- Matyjaszczyka, E.; Śmiechowsk, M. Edible flowers. Benefits and risks pertaining to their consumption. Trends Food Sci. Tech. 2019, 91, 670–674. [Google Scholar] [CrossRef]
- Kristanc, L.; Kreft, S. European medicinal and edible plants associated with subacute and chronic toxicity part I: Plants with carcinogenic, teratogenic and endocrine- disrupting effects. Food Chem. Toxicol. 2016, 92, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C.F.R. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. 2017, 110, 165–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egebjerg, M.M.; Olesen, P.T.; Eriksen, F.D.; Ravn-Haren, G.; Bredsdorff, L.; Pilegaard, K. Are wild and cultivated flowers served in restaurants or sold by local producers in Denmark safe for the consumer? Food Chem Toxicol. 2018, 120, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klintschar, M.; Beham-Schimdt, C.; Radner, H.; Henning, G.; Roll, P. Colchicine poisoning by accidental ingestion of meadow saffron (Colchicum autumnale): Pathological and medicolegal aspects. Forensic Sci. Int. 1999, 106, 191–200. [Google Scholar] [CrossRef]
- Korkmaz, M.F.; Bostancı, M.; Onur, H.; Çağan, E. Datura stramonium poisoning: A case report and review of the literature. Eur. Res. J. 2019, 5, 186–188. [Google Scholar] [CrossRef] [Green Version]
- Farkhondeh, T.; Kianmehr, M.; Kazemi, T.; Samarghandian, S.; Khazdair, M.R. Toxicity effects of Nerium oleander, basic and clinical evidence: A comprehensive review. Hum. Exp. Toxicol. 2020, 39, 773–784. [Google Scholar] [CrossRef]
- Adler, L.S. The ecological significance of toxic nectar. Oikos 2001, 91, 409–420. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Martena, M.J.; Boersma, M.G.; Spiegelenberg, W.; Alink, G.M. Molecular mechanisms of toxicity of important food-borne phytotoxins. Mol. Nutr. Food Res. 2005, 49, 131–158. [Google Scholar] [CrossRef]
- Smith, R.L.; Adams, T.B.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Rogers, A.E.; et al. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances—Methyl eugenol and estragole. Food Chem. Toxic. 2002, 40, 851–870. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Meller, E.; Wysocka, G. Mineral composition of some edible flowers. J. Elem. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Drava, G.; Iobbi, V.; Govaerts, R.; Minganti, V.; Copetta, A.; Ruffoni, B.; Bisio, A. Trace elements in edible flowers from Italy: Further insights into health benefits and risks to consumers. Molecules 2020, 25, 2891. [Google Scholar] [CrossRef]
- Lu, B.; Li, M.; Yin, R. Phytochemical Content, Health Benefits, and Toxicology of Common Edible Flowers: A Review (2000–2015). Crit. Rev. Food Sci. Nutr. 2016, 56, S130–S148. [Google Scholar] [CrossRef] [PubMed]
- Akindahunsi, A.A.; Olaleye, M.T. Toxicological investigation of aqueous-methanolic extract of the calyces of Hibiscus sabdariffa L. J. Ethnopharmacol. 2003, 89, 161–164. [Google Scholar] [CrossRef]
- Fakeye, T.O.; Pal, A.; Bawankule, D.U.; Yadav, N.P.; Khanuja, S.P.S. Toxic effects of oral administration of extracts of dried calyx of Hibiscus sabdariffa Linn. (Malvaceae). Phytother. Res. 2009, 23, 412–416. [Google Scholar] [CrossRef]
- Mahmoud, Y.I. Effect of extract of Hibiscus on the ultrastructure of the testis in adult mice. Acta Histochem. 2012, 114, 342–348. [Google Scholar] [CrossRef] [PubMed]
- De Arruda, A.; Cardoso, C.A.L.; Vieira, M.D.C.; Arena, A.C. Safety assessment of Hibiscus sabdariffa after maternal exposure on male reproductive parameters in rats. Drug Chem. Toxicol. 2015, 39, 1003938. [Google Scholar]
- Mazzocchi, A.; Venter, C.; Maslin, K.; Agostoni, C. The role of nutritional aspects in food allergy: Prevention and management. Nutrients. 2017, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Coimbra, A.; Vítor, A.; Aguiar, R.; Ferreira, A.L.; Todo-Bom, A. Food allergy-From food avoidance to active treatment. Scand. J. Immunol. 2020, 91, e12824. [Google Scholar] [CrossRef]
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea plant; identification, applications and use as an emerging food source—Review. Food Rev. Int. 2020, 1–16. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A.; Chapuis, C.; Rubin, M.; Starkenmann, C. Volatile composition of oyster leaf (Mertensia maritima (L.) Gray). J. Agric. Food Chem. 2012, 60, 11681–11690. [Google Scholar] [CrossRef]
- Najar, B.; Marchioni, I.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Volatilomic analysis of four edible flowers from Agastache genus. Molecules 2019, 24, 4480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli La Pistelli, L.U. Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, I.; Pistelli, L.; Ferri, B.; Cioni, P.; Pistelli, L.; Ruffoni, B. Preliminary studies on edible saffron bio-residues during different post-harvest storages. Bulg. Chem. Commun. 2019, 51, 131–136. [Google Scholar]
- Bazzurro, V.; Milanese, M.; Gatta, E.; Bonifacino, T.; Diaspro, A.; Bonanno, G. Analisi in-vitro delle potenziali attivita’ citotossiche su campioni rappresentativi di 40 estratti totali di fiori eduli. Available online: http://interregantea.eu/Poster.aspx (accessed on 18 September 2020).
- Feo, F.; Martinez, J.; Martinez, A.; Galindo, P.A.; Cruz, A.; Garcia, R.; Guerra, F.; Palacios, R. Occupational allergy in saffron workers. Allergy 1997, 52, 633–641. [Google Scholar] [CrossRef]



| Environmental Allergen | Fruits | Vegetables | Nuts | Spices | Legumes and Other Foods |
|---|---|---|---|---|---|
| Tree pollen | Apple, apricot, cherry, kiwi, nectarine, peach, plum, prune, strawberry, lychee, jackfruit, Sharon fruit, tomato | Carrot, celeriac, celery, green pepper, potato, parsnip | Walnut, almond, hazelnut | Basil, caraway, anise, dill, thyme, pepper, tarragon, paprika, fennel, marjoram, oregano, parsley, cumin, coriander, chicory | Bean, pea, soybean, peanut, lentil, sunflower seed |
| Grass and grain pollen | Kiwi, orange tomato, melon, watermelon, date | Potato | Peas, peanut, sunflower seed, flour, bran, legume | ||
| Mugwort pollen | Mango, grapes, pineapple, avocado, banana, tomato, lychee, peach, watermelon, melon, apple, orange | Celeriac, parsnip, carrot, celery, onion, green pepper, potato | Chestnut | Basil, caraway, anise, dill, thyme, pepper, tarragon, paprika, fennel, marjoram, oregano, parsley, mustard, coriander | Sunflower seed, chamomile |
| Ragweed | Melon, banana, watermelon | Zucchini, cucumber |
| Symptoms Which May Result from Food Allergy | IgE Mediated (Immediate Reaction) | Non-IgE Mediated (Delayed Reaction) |
|---|---|---|
| Systemic | ||
| Anaphylaxis | ✓ | |
| Gastrointestinal | ||
| Swelling and itching of lips and mouth | ✓ | |
| Nausea | ✓ | |
| Vomiting | ✓ | ✓ |
| Diarrhea | ✓ | ✓ |
| Crampy pain | ✓ | ✓ |
| Respiratory | ||
| Rhinitis | ✓ | |
| Asthma | ✓ | |
| Swelling of larynx | ✓ | |
| Cough | ✓ | |
| Chest tightness | ✓ | |
| Bronchospasm | ✓ | |
| Skin | ||
| Pruritus | ✓ | ✓ |
| Urticaria | ✓ | |
| Erythema | ✓ | ✓ |
| Eczema | ✓ | ✓ |
| Conjunctivitis | ✓ | |
| Cardiovascular | ||
| Presyncope/syncope | ✓ | |
| Hypotension | ✓ | |
| Tachycardia | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, M.; Copetta, A.; Durazzo, A.; Gabrielli, P.; Lombardi-Boccia, G.; Lupotto, E.; Santini, A.; Ruffoni, B. A Snapshot on Food Allergies: A Case Study on Edible Flowers. Sustainability 2020, 12, 8709. https://doi.org/10.3390/su12208709
Lucarini M, Copetta A, Durazzo A, Gabrielli P, Lombardi-Boccia G, Lupotto E, Santini A, Ruffoni B. A Snapshot on Food Allergies: A Case Study on Edible Flowers. Sustainability. 2020; 12(20):8709. https://doi.org/10.3390/su12208709
Chicago/Turabian StyleLucarini, Massimo, Andrea Copetta, Alessandra Durazzo, Paolo Gabrielli, Ginevra Lombardi-Boccia, Elisabetta Lupotto, Antonello Santini, and Barbara Ruffoni. 2020. "A Snapshot on Food Allergies: A Case Study on Edible Flowers" Sustainability 12, no. 20: 8709. https://doi.org/10.3390/su12208709
APA StyleLucarini, M., Copetta, A., Durazzo, A., Gabrielli, P., Lombardi-Boccia, G., Lupotto, E., Santini, A., & Ruffoni, B. (2020). A Snapshot on Food Allergies: A Case Study on Edible Flowers. Sustainability, 12(20), 8709. https://doi.org/10.3390/su12208709