Removal of Basic Brown 16 from Aqueous Solution Using Durian Shell Adsorbent, Optimisation and Techno-Economic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Durian Shell as an Adsorbent
2.2. Characterisation of Adsorbent
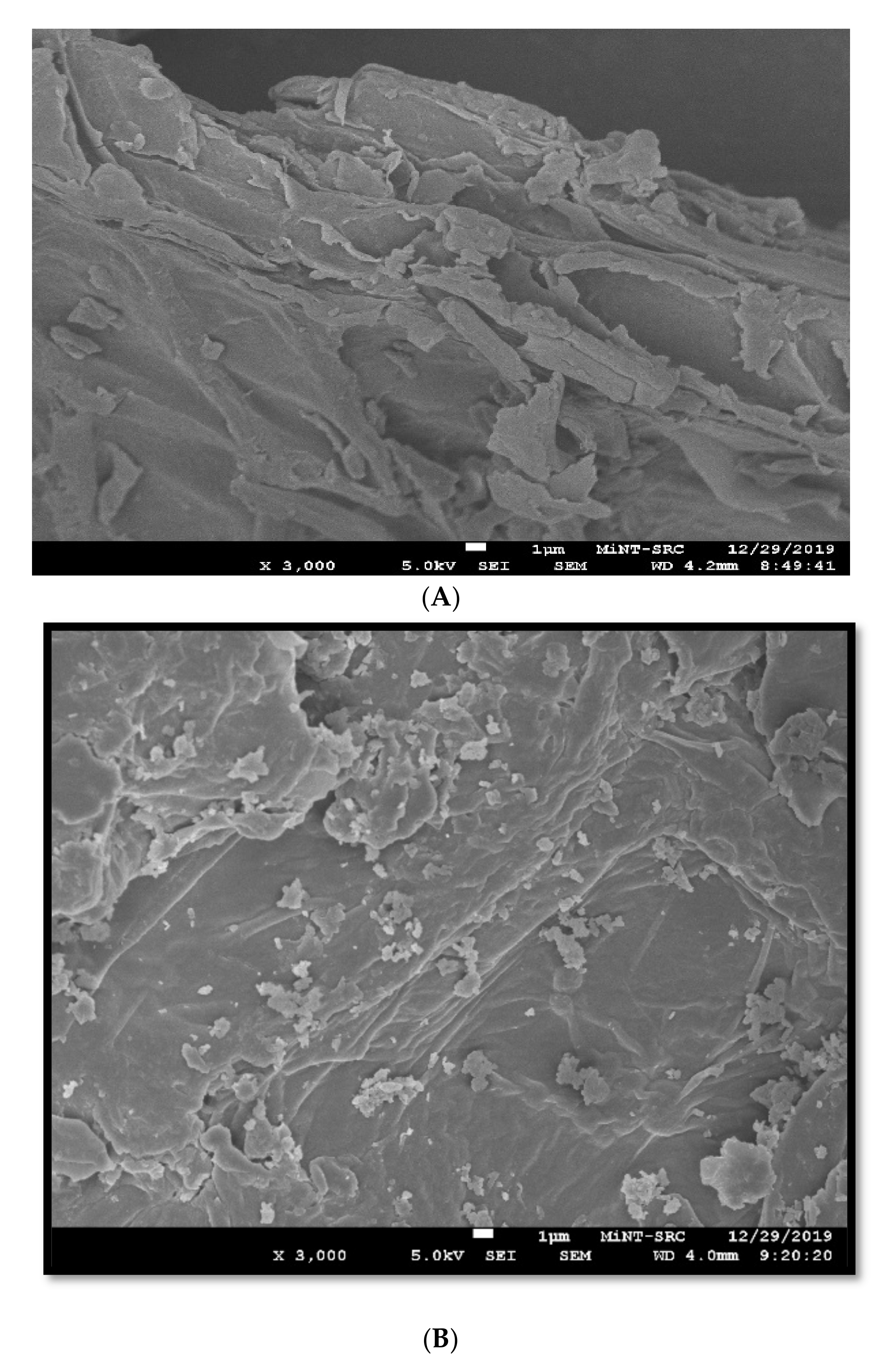
2.2.1. Field Emission Scanning Electron Microscope (FESEM)
2.2.2. Fourier Transform Infrared (FTIR)
2.3. Preparation of C.I BB16 in Aqueous Solution
2.4. Experimental Set-up
2.4.1. Adsorption of C.I BB16
2.4.2. Optimisation of Adsorption of C.I BB16
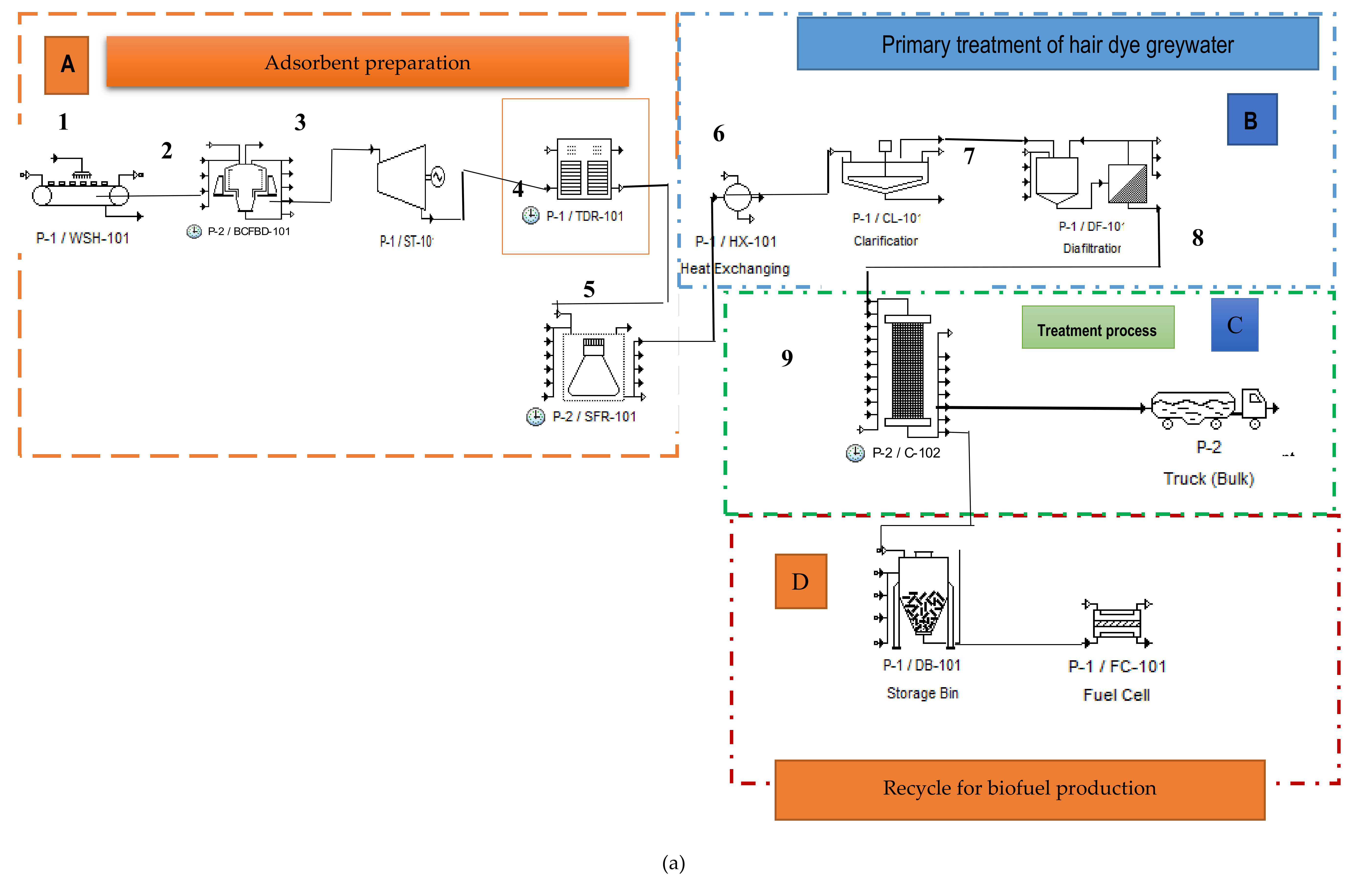
2.5. Techno-Economic Analysis for Hair Dye Greywater Treatment Using Durian Shell Adsorbent
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Durian Adsorbent
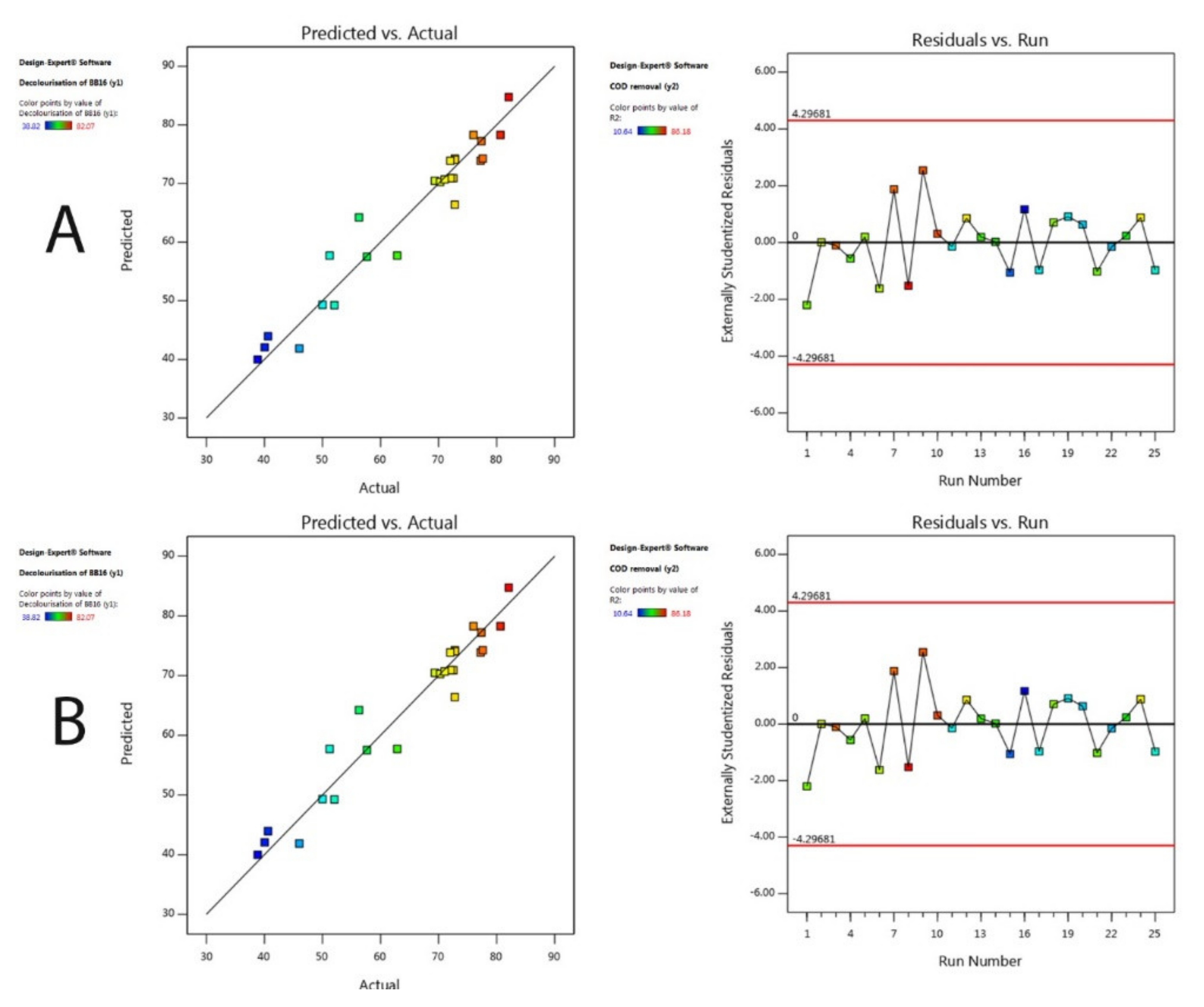
3.2. Optimisation of Adsorption of C.I BB16
Validation of the Optimal Parameters
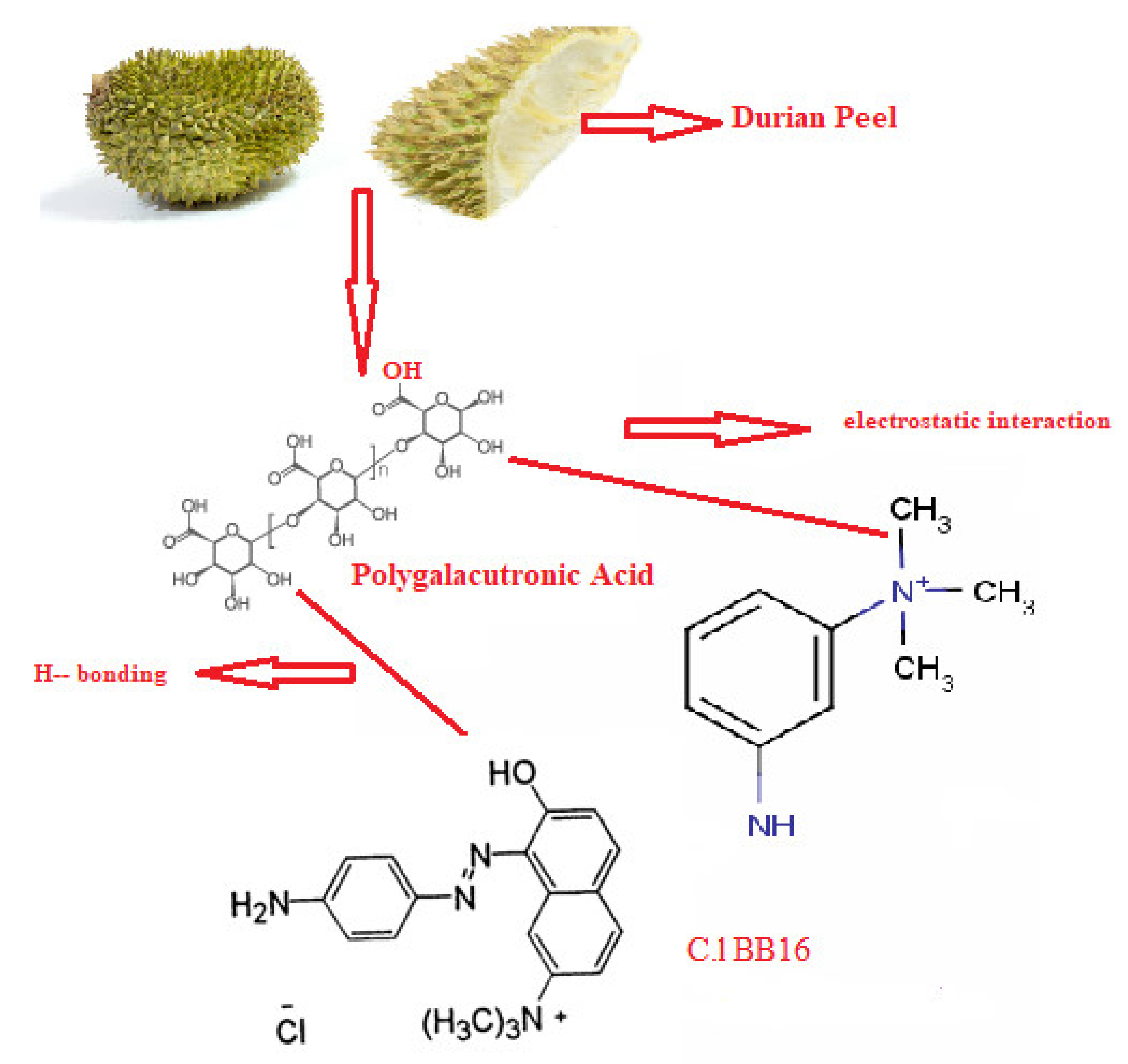
3.3. Mechanism of Adsorption
3.4. Techno-Economic Analysis for Preparation and Application of Durian Shell for Hair Dye Greywater Treatment
3.4.1. Annual Operation Cost
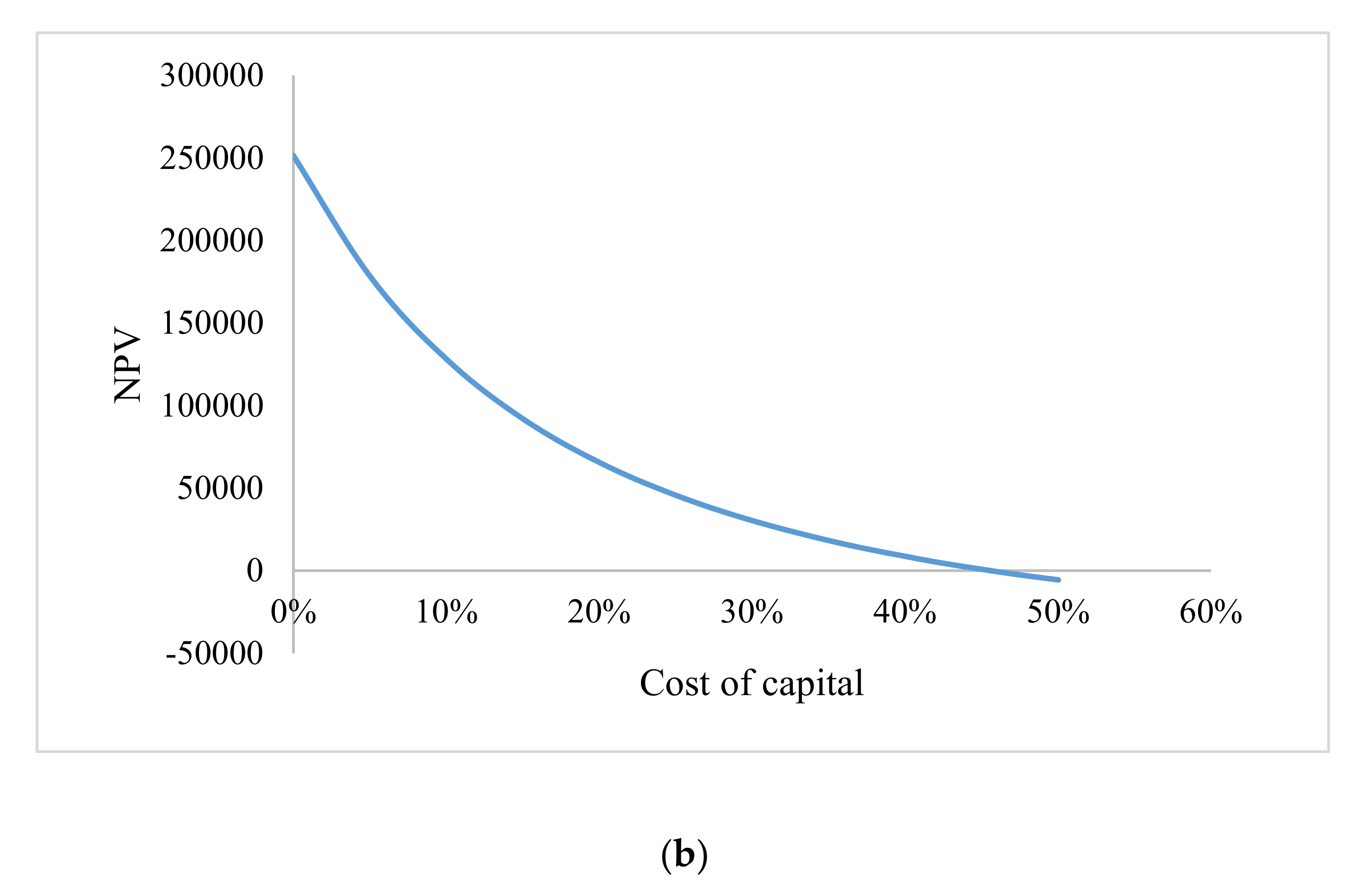
3.4.2. Annual Profitability and Revenue
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandes, N.C.; Brito, L.B.; Costa, G.; Taveira, S.F.; Cunha-Filho, M.; Oliveira, G.A.R.; Marreto, R. Removal of azo dye using Fenton and Fenton-like processes: Evaluation of process factors by Box–Behnken design and ecotoxicity tests. Chem. Interact. 2018, 291, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Radhakrishnan, R.C.; Johnson, J.K.; Joy, S.P.; Thomas, J. Ion-exchange mediated removal of cationic dye-stuffs from water using ammonium phosphomolybdate. Mater. Chem. Phys. 2020, 242, 122488. [Google Scholar] [CrossRef]
- Rosu, C.M.; Vochita, G.; Mihășan, M.; Avădanei, M.; Mihai, C.; Gherghel, D. Performances of Pichia kudriavzevii in decolorization, biodegradation, and detoxification of C.I. Basic Blue 41 under optimized cultural conditions. Environ. Sci. Pollut. Res. 2018, 26, 431–445. [Google Scholar] [CrossRef]
- Aguilar, Z.G.; Brillas, E.; Salazar, M.; Nava, J.L.; Sirés, I.; Sadornil, I.S. Evidence of Fenton-like reaction with active chlorine during the electrocatalytic oxidation of Acid Yellow 36 azo dye with Ir-Sn-Sb oxide anode in the presence of iron ion. Appl. Catal. B Environ. 2017, 206, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Obiora-Okafo, I.A.; Onukwuli, O.D. Optimization of Coagulation-Flocculation for Colour Removal from Azo Dye Using Natural Polyemers: Response Surface Methodological Approach. Niger. J. Technol. 2017, 36, 482–495. [Google Scholar] [CrossRef] [Green Version]
- Isarain-Chávez, E.; Baro, M.D.; Rossinyol, E.; Morales-Ortiz, U.; Sort, J.; Brillas, E.; Pellicer, E. Comparative electrochemical oxidation of methyl orange azo dye using Ti/Ir-Pb, Ti/Ir-Sn, Ti/Ru-Pb, Ti/Pt-Pd and Ti/RuO2 anodes. Electrochim. Acta 2017, 244, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Castro, F.D.; Bassin, J.P.; Dezotti, M. Treatment of a simulated textile wastewater containing the Reactive Orange 16 azo dye by a combination of ozonation and moving-bed biofilm reactor: Evaluating the performance, toxicity, and oxidation by-products. Environ. Sci. Pollut. Res. 2016, 24, 6307–6316. [Google Scholar] [CrossRef]
- Bahia, M.; Passos, F.; Adarme, O.F.H.; Aquino, S.F.; Silva, S.Q. Anaerobic-Aerobic Combined System for the Biological Treatment of Azo Dye Solution Using Residual Yeast. Water Environ. Res. 2018, 90, 729–737. [Google Scholar] [CrossRef]
- Zong, E.; Liu, X.; Jiang, J.; Fu, S.; Chu, F. Preparation and characterization of zirconia-loaded lignocellulosic butanol residue as a biosorbent for phosphate removal from aqueous solution. Appl. Surf. Sci. 2016, 387, 419–430. [Google Scholar] [CrossRef]
- Pathak, P.; Gupta, D.K. Strontium contamination in the environment. In The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Gonçalves, J.A.C.; Schwantes, D.; Campagnolo, M.A.; Dragunski, D.C.; Tarley, C.R.T.; Silva, A.K.D.S. Removal of toxic metals using endocarp of açaí berry as biosorbent. Water Sci. Technol. 2018, 77, 1547–1557. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.-L.; Tan, Y.P.; Abdullah, A.H.; Ong, S.-T. Equilibrium, kinetic and thermodynamic studies of a new potential biosorbent for the removal of Basic Blue 3 and Congo Red dyes: Pineapple (Ananas comosus) plant stem. J. Taiwan Inst. Chem. Eng. 2016, 61, 306–315. [Google Scholar] [CrossRef]
- Jain, S.N.; Gogate, P.R. Acid Blue 113 removal from aqueous solution using novel biosorbent based on NaOH treated and surfactant modified fallen leaves of Prunus Dulcis. J. Environ. Chem. Eng. 2017, 5, 3384–3394. [Google Scholar] [CrossRef]
- Heraldy, E.; Lestari, W.W.; Permatasari, D.; Arimurti, D.D. Biosorbent from tomato waste and apple juice residue for lead removal. J. Environ. Chem. Eng. 2018, 6, 1201–1208. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, C.; Lin, Z.-Y.; Zou, Z. Microwave-Hydrothermal Treated Grape Peel as an Efficient Biosorbent for Methylene Blue Removal. Int. J. Environ. Res. Public Heal. 2018, 15, 239. [Google Scholar] [CrossRef] [Green Version]
- Machrouhi, A.; Farnane, M.; Elhalil, A.; Abdennouri, M.; Tounsadi, H.; Barka, N. Heavy metals adsorption by Thapsia transtagana stems powder: Kinetics, equilibrium and thermodynamics. Moroc. J. Chem. 2019, 7, 98–110. [Google Scholar]
- Yildirim, A.; Acay, H.; Baran, F. Synthesis and characterisation of mushroom-based nanocomposite and its efficiency on dye biosorption via antimicrobial activity. Int. J. Environ. Anal. Chem. 2020, 1–18. [Google Scholar] [CrossRef]
- Saueprasearsit, P. Adsorption of chromium (Cr+6) using durian peel. Int. Conf. Biotechnol. Environ. Manag. 2011, 18, 33–38. [Google Scholar]
- Mohammed, S.A.; Najib, N.W.A.Z.; Muniandi, V. Durian rind as a low cost adsorbent. Int. J. Civ. Environ. Eng. 2012, 12, 51–56. [Google Scholar]
- Foo, K.Y.; Hameed, B. Transformation of durian biomass into a highly valuable end commodity: Trends and opportunities. Biomass Bioenergy 2011, 35, 2470–2478. [Google Scholar] [CrossRef]
- Koay, S.C.; Subramanian, V.; Chan, M.Y.; Pang, M.M.; Tshai, K.Y.; Cheah, K.H. Preparation and Characterization of Wood Plastic Composite Made Up of Durian Husk Fiber and Recycled Polystyrene Foam. MATEC Web Conf. 2018, 152, 02019. [Google Scholar] [CrossRef]
- Lazim, Z.M.; Hadibarata, T.; Othman, M.H.D.; Yusop, Z.; Wirasnita, R.; Nor, N.M. Utilization of durian peel as potential adsorbent for bisphenol a removal in aquoeus solution. J. Teknol. 2015, 74, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Méndez, A.A.; Pena, L.B.; Curto, L.M.; Sciorra, M.D.; Ulloa, R.M.; Aguilar, S.M.G.; Ramos, J.M.V.; Gallego, S.M. Optimization of recombinant maize CDKA;1 and CycD6;1 production in Escherichia coli by response surface methodology. Protein Expr. Purif. 2019, 165, 105483. [Google Scholar] [CrossRef] [PubMed]
- Ciric, A.R.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Hamid, S.R.A.; Yusop, M.F.M.; Aziz, H.B.A. Optimization of microwave-assisted durian seed based activated carbon preparation conditions for methylene blue dye removal. In Proceedings of the International Conference of Global Network for Innovative Technology and Awam International Conference in Civil Engineering (Ignite-Aicce&Rsquo;17): Malaysia Sustainable Technology and Practice for Infrastructure and Community Resilience, Penang, Malaysia, 8–10 August 2017; Volume 1892, p. 040019. [Google Scholar]
- Reshadi, M.A.M.; Bazargan, A.; McKay, G. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci. Total. Environ. 2020, 731, 138863. [Google Scholar] [CrossRef] [PubMed]
- Fahimmunisha, B.A.; Ishwarya, R.; AlSalhi, M.; Devanesan, S.; Govindarajan, M.; Vaseeharan, B. Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: A novel drug delivery approach. J. Drug Deliv. Sci. Technol. 2020, 55, 101465. [Google Scholar] [CrossRef]
- Barrera, H.; Cruz-Olivares, J.; Frontana-Uribe, B.A.; Gómez-Díaz, A.; Reyes-Romero, P.G.; Barrera-Diaz, C.E. Electro-Oxidation–Plasma Treatment for Azo Dye Carmoisine (Acid Red 14) in an Aqueous Solution. Materials 2020, 13, 1463. [Google Scholar] [CrossRef] [Green Version]
- Mondal, N.K.; Samanta, A.; Roy, P.; Das, B. Optimization study of adsorption parameters for removal of Cr(VI) using Magnolia leaf biomass by response surface methodology. Sustain. Water Resour. Manag. 2019, 5, 1627–1639. [Google Scholar] [CrossRef]
- Sanati, A.M.; Kamari, S.; Ghorbani, F. Application of response surface methodology for optimization of cadmium adsorption from aqueous solutions by Fe3O4@SiO2@APTMS core–shell magnetic nanohybrid. Surf. Interfaces 2019, 17, 100374. [Google Scholar] [CrossRef]
- Petrović, M.S.; Šoštarić, T.; Stojanović, M.D.; Milojković, J.; Mihajlović, M.; Stanojević, M.; Stanković, S. Removal of Pb2+ ions by raw corn silk (Zea mays L.) as a novel biosorbent. J. Taiwan Inst. Chem. Eng. 2016, 58, 407–416. [Google Scholar] [CrossRef]
- Ben Amar, M.; Walha, K.; Salvadó, V. Evaluation of Olive Stones for Cd(II), Cu(II), Pb(II) and Cr(VI) Biosorption from Aqueous Solution: Equilibrium and Kinetics. Int. J. Environ. Res. 2020, 14, 193–204. [Google Scholar] [CrossRef]
- Kwan, T.H.; Pleissner, D.; Lau, K.Y.; Venus, J.; Pommeret, A.; Lin, C.S.K. Techno-economic analysis of a food waste valorization process via microalgae cultivation and co-production of plasticizer, lactic acid and animal feed from algal biomass and food waste. Bioresour. Technol. 2015, 198, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour. Technol. 2016, 202, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Hu, Y.; Li, S.; Huang, J.; Nie, Q.; Zhao, H.; Tang, J. Simultaneous dark fermentative hydrogen and ethanol production from waste bread in a mixed packed tank reactor. J. Clean. Prod. 2017, 141, 608–611. [Google Scholar] [CrossRef] [Green Version]
- Bulgariu, L.; Bulgariu, D. Functionalized soy waste biomass—A novel environmental-friendly biosorbent for the removal of heavy metals from aqueous solution. J. Clean. Prod. 2018, 197, 875–885. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.; Kanchi, S.; Bisetty, K.; Skelton, A.A.; Honarparvar, B. Green synthesis, characterization and electrochemical sensing of silymarin by ZnO nanoparticles: Experimental and DFT studies. J. Electroanal. Chem. 2018, 808, 160–172. [Google Scholar] [CrossRef]
- Tham, Y.; Latif, P.A.; Abdullah, A.M.; Shamala-Devi, A.; Taufiq-Yap, Y. Performances of toluene removal by activated carbon derived from durian shell. Bioresour. Technol. 2011, 102, 724–728. [Google Scholar] [CrossRef]
- Latib, E.H.A.; Mustfha, M.S.; Sufian, S.; Shaari, K.Z.K. Methylene Blue Dye Adsorption to Durian Shell Activated Carbon. Key Eng. Mater. 2013, 594–595, 350–355. [Google Scholar] [CrossRef]
- Naushad, M.; Alqadami, A.A.; Alothman, Z.A.; Alsohaimi, I.H.; Algamdi, M.S.; Aldawsari, A.M. Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J. Mol. Liq. 2019, 293, 111442. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, M.; Amiri, M.; Bagheri, F. Optimization of the lead removal from aqueous solution using two starch based adsorbents: Design of experiments using response surface methodology (RSM). J. Environ. Chem. Eng. 2019, 7, 102793. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Yang, M.; Yu, F.; Ma, J. Response surface methodological evaluation and optimization for adsorption removal of ciprofloxacin onto graphene hydrogel. J. Mol. Liq. 2019, 284, 124–130. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Adlii, A.; Bakry, B.M. Green fabrication of bentonite/chitosan@cobalt oxide composite (BE/CH@Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr (VI) from water. Int. J. Boil. Macromol. 2019, 126, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Yan, X.; Wang, X. Chitosan coated polyacrylonitrile nanofibrous mat for dye adsorption. Int. J. Boil. Macromol. 2019, 135, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Kumar, R. Adsorption studies of hazardous malachite green onto treated ginger waste. J. Environ. Manag. 2010, 91, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Vlysidis, A.; Binns, M.; Webb, C.; Theodoropoulos, C. A techno-economic analysis of biodiesel biorefineries: Assessment of integrated designs for the co-production of fuels and chemicals. Energy 2011, 36, 4671–4683. [Google Scholar] [CrossRef]
- Han, W.; Yan, Y.; Gu, J.; Shi, Y.; Tang, J.; Li, Y. Techno-economic analysis of a novel bioprocess combining solid state fermentation and dark fermentation for H2 production from food waste. Int. J. Hydrogen Energy 2016, 41, 22619–22625. [Google Scholar] [CrossRef] [Green Version]
- Ljunggren, M.; Zacchi, G. Techno-economic evaluation of a two-step biological process for hydrogen production. Biotechnol. Prog. 2009, 26, 496–504. [Google Scholar] [CrossRef]
- Yunus, Z.M.; Al-Gheethi, A.; Othman, N.; Hamdan, R.; Ruslan, N.N. Removal of heavy metals from mining effluents in tile and electroplating industries using honeydew peel activated carbon: A microstructure and techno-economic analysis. J. Clean. Prod. 2020, 251, 119738. [Google Scholar] [CrossRef]



| Run | ||||||
|---|---|---|---|---|---|---|
| 1 | 4.00 | 30.00 | 0.10 | 10.00 | 80.65 | 57.52 |
| 2 | 8.00 | 30.00 | 0.10 | 10.00 | 77.36 | 63.82 |
| 3 | 8.00 | 30.00 | 1.00 | 15.00 | 72.81 | 78.09 |
| 4 | 8.00 | 30.00 | 0.10 | 20.00 | 72.01 | 55.14 |
| 5 | 8.00 | 30.00 | 0.10 | 20.00 | 77.24 | 59.79 |
| 6 | 4.00 | 30.00 | 1.00 | 20.00 | 82.07 | 62.06 |
| 7 | 4.00 | 30.00 | 0.10 | 10.00 | 76 | 78.22 |
| 8 | 6.00 | 30.00 | 1.00 | 10.00 | 71.04 | 86.18 |
| 9 | 6.00 | 30.00 | 0.55 | 15.00 | 72.82 | 79.54 |
| 10 | 8.00 | 30.00 | 1.00 | 15.00 | 77.61 | 80.6 |
| 11 | 4.00 | 135.00 | 0.55 | 15.00 | 72.78 | 30.11 |
| 12 | 4.00 | 135.00 | 1.00 | 10.00 | 70.26 | 68.33 |
| 13 | 8.00 | 135.00 | 1.00 | 20.00 | 72.07 | 53.08 |
| 14 | 8.00 | 135.00 | 1.00 | 20.00 | 72.54 | 52.02 |
| 15 | 6.00 | 135.00 | 0.10 | 15.00 | 40.03 | 18.08 |
| 16 | 4.00 | 135.00 | 0.10 | 20.00 | 50 | 10.64 |
| 17 | 8.00 | 135.00 | 0.55 | 15.00 | 56.28 | 29.53 |
| 18 | 6.00 | 135.00 | 1.00 | 15.00 | 57.64 | 58.85 |
| 19 | 8.00 | 240.00 | 0.10 | 10.00 | 46 | 28.54 |
| 20 | 8.00 | 240.00 | 0.10 | 20.00 | 38.82 | 26.23 |
| 21 | 8.00 | 240.00 | 1.00 | 10.00 | 51.22 | 56.74 |
| 22 | 4.00 | 240.00 | 0.10 | 10.00 | 40.61 | 24.88 |
| 23 | 4.00 | 240.00 | 1.00 | 20.00 | 69.34 | 48.65 |
| 24 | 8.00 | 240.00 | 1.00 | 10.00 | 62.86 | 67.94 |
| 25 | 6.00 | 240.00 | 0.55 | 15.00 | 52.05 | 32.46 |
| Term | Coefficient | Standard Error | F Value | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| Model | 57.76 | 35.79 | 3.37 | 5.26 | 11.35 | 11.55 | 0.0002 | 0.0002 |
| −1.09 | 2.13 | 1.31 | 2.05 | 0.69 | 1.08 | 0.4246 | 0.3234 | |
| −12.41 | −14.64 | 1.43 | 2.23 | 75.67 | 43.05 | <0.0001 | <0.0001 | |
| 7.73 | 15.55 | 1.25 | 1.96 | 38.04 | 62.86 | <0.0001 | <0.0001 | |
| 0.20 | −6.73 | 1.43 | 2.24 | 0.020 | 9.04 | 0.8895 | 0.0132 | |
| 7.55 | −2.66 | 3.27 | 5.11 | 5.33 | 0.27 | 0.0436 | 0.6146 | |
| 3.90 | 16.90 | 2.58 | 4.04 | 2.28 | 17.54 | 0.1619 | 0.0019 | |
| −7.97 | 3.31 | 4.08 | 6.38 | 3.83 | 0.27 | 0.0790 | 0.6154 | |
| 4.20 | 1.41 | 4.04 | 6.32 | 1.08 | 0.050 | 0.3230 | 0.8275 | |
| −0.26 | 0.87 | 1.60 | 2.51 | 0.025 | 0.12 | 0.8766 | 0.7353 | |
| −1.63 | −1.42 | 1.42 | 2.22 | 1.32 | 0.41 | 0.2772 | 0.5354 | |
| 1.32 | 5.00 | 1.49 | 2.32 | 0.79 | 4.64 | 0.3937 | 0.0566 | |
| 4.65 | 4.65 | 1.48 | 2.31 | 9.91 | 4.04 | 0.0104 | 0.0721 | |
| 0.36 | 1.37 | 1.58 | 2.48 | 0.053 | 0.31 | 0.8234 | 0.5920 | |
| 2.83 | −0.48 | 1.36 | 2.13 | 4.33 | 0.052 | 0.0641 | 0.8250 | |
| Source | Degree of Freedom | Sum of Squares | Mean Square | F Value | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Model | 14 | 4248.69 | 10578.36 | 303.48 | 755.60 | 11.35 | 11.55 | 0.0002 significant | 0.0002 significant |
| Residual error | 10 | 267.35 | 654.04 | 26.74 | 65.40 | ||||
| Lack-of-fit | 5 | 163.49 | 362.55 | 32.70 | 72.51 | 1.57 | 1.24 | 0.3154 non-significant | 0.4083 non-significant |
| Pure error | 5 | 103.86 | 291.49 | 20.77 | 58.30 | ||||
| Total | 24 | 4516.04 | 11232.40 | ||||||
| Responses | Predicted Results | Experimental Results | ||||
|---|---|---|---|---|---|---|
| 8.00 | 30.00 | 1.00 | 15.00 | 77.61 | 74.26 | |
| 80.60 | 78.72 |
| No | Item Code * | Item | Percentage of FCE | Cost |
|---|---|---|---|---|
| 1 | P-1/WSH-101 | Washing tank | 62.5% | 10,000.00 |
| 2 | P-2/BCFBD-101 | Basket centrifuge | ||
| 3 | P-1/ST-10 | Straight flow steam Turbine Generator | ||
| 4 | P-1/TDR-101 | Tray Drying | ||
| 5 | P-2/SFR-101 | Shake flasks | ||
| 6 | P-1/HX-101 | Heat Exchange | 1000.00 | |
| 7 | P-1/CL-101 | Clarification | 2000.00 | |
| 8 | P-1/DF-101 | Dia-filtration | 3000.00 | |
| 9 | P-2/C-102 PBA | Adsorption unit | 4000.00 | |
| Total Equipment purchase cost | 20,000.00 | |||
| 10 | Equipment installation | 3.13 | 1000.00 | |
| 11 | Process piping | 3.13 | 1000.00 | |
| 12 | Instrumentation and controls | 6.25 | 2000.00 | |
| 13 | Electrical systems | 3.13 | 1000.00 | |
| 14 | Buildings | 15.63 | 5000.00 | |
| 15 | Yard improvements | 3.13 | 1000.00 | |
| 16 | Construction | 3.13 | 1000.00 | |
| TOTAL | 32,000.00 | |||
| Component | Price | Unit | Quantity | Cost (USD) | |
|---|---|---|---|---|---|
| Raw material (chemicals) | Cassava peels | Free | USD/kg | 48 ton/year | 0 |
| Utilities | Electricity | 0.04 | USD/kWh | 100,000 | 4000.00 |
| Water | 0.01 | USD/m3 | 100,000 | 1000.00 | |
| Other costs | Labour | 6000.00 | USD/employee | 1 | 6000.00 |
| Maintenance | 2 | % of FCE | 640.00 | ||
| Insurance | 1 | % of FCE | 320.00 | ||
| Total | 11,960.00 | ||||
| (a) | |||
| Price | Quantity | Value | |
| Durian shell | USD 261.81/Ton | 240 Ton | 62,834.00 |
| Annual revenue | 62,834.00 | ||
| Annual operation cost | 11,960.00 | ||
| Local tax | 9425.00 | ||
| Annual profitability | 41,499.24 | ||
| (b) | |||
| Cost Factor | Physical | Chemical | Durian Shell Adsorbent |
| Heat and energy requirement | 2 | 1 | 0 |
| Chemicals (acid/alkali/ammonia) | 0 | 2 | 0 |
| pH neutralisation | 0 | 2 | 0 |
| Detoxification/conditioning | 1 | 2 | 0 |
| Special reactor construction | 2 | 2 | 0 |
| Total | 5 | 9 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopalakrishnan, Y.; Al-Gheethi, A.; Abdul Malek, M.; Marisa Azlan, M.; Al-Sahari, M.; Radin Mohamed, R.M.S.; Alkhadher, S.; Noman, E. Removal of Basic Brown 16 from Aqueous Solution Using Durian Shell Adsorbent, Optimisation and Techno-Economic Analysis. Sustainability 2020, 12, 8928. https://doi.org/10.3390/su12218928
Gopalakrishnan Y, Al-Gheethi A, Abdul Malek M, Marisa Azlan M, Al-Sahari M, Radin Mohamed RMS, Alkhadher S, Noman E. Removal of Basic Brown 16 from Aqueous Solution Using Durian Shell Adsorbent, Optimisation and Techno-Economic Analysis. Sustainability. 2020; 12(21):8928. https://doi.org/10.3390/su12218928
Chicago/Turabian StyleGopalakrishnan, Yashni, Adel Al-Gheethi, Marlinda Abdul Malek, Mawar Marisa Azlan, Mohammed Al-Sahari, Radin Maya Saphira Radin Mohamed, Sadeq Alkhadher, and Efaq Noman. 2020. "Removal of Basic Brown 16 from Aqueous Solution Using Durian Shell Adsorbent, Optimisation and Techno-Economic Analysis" Sustainability 12, no. 21: 8928. https://doi.org/10.3390/su12218928
APA StyleGopalakrishnan, Y., Al-Gheethi, A., Abdul Malek, M., Marisa Azlan, M., Al-Sahari, M., Radin Mohamed, R. M. S., Alkhadher, S., & Noman, E. (2020). Removal of Basic Brown 16 from Aqueous Solution Using Durian Shell Adsorbent, Optimisation and Techno-Economic Analysis. Sustainability, 12(21), 8928. https://doi.org/10.3390/su12218928








