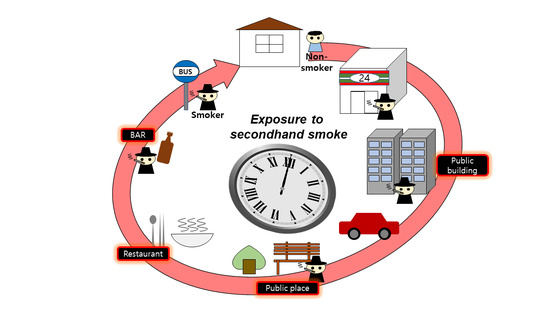
1. Introduction
Secondhand smoke (SHS) is defined as a mixture of the smoke from the burning of tobacco products and smoke exhaled by smokers [
1]. SHS exposure can cause adverse health effects such as respiratory disease, cardiovascular disease, and lung cancer [
2,
3]. In particular, exposure to SHS has become an important health issue due to its association with sudden infant death syndrome (SIDS) [
4,
5]. The World Health Organization (WHO) has estimated that, globally, more than 6 million people die from smoking, and the mortality rate from SHS exposure has reached 900,000 [
6,
7].
The WHO has organized the Framework Convention on Tobacco Control (FCTC) and encourages countries to implement education and awareness programs regarding the risks of SHS with the aim of a “Smoke Free World” [
8]. According to the FCTC, fifteen developed and developing countries have successfully implemented smoke-free policies in indoor environments such as homes, workplaces, and public places during the last decade [
9,
10].
In Europe, under the theme of “lifting the smokescreen”, the importance of SHS monitoring was recognized, and the results are being utilized to assess smoke-free policies [
11]. In Canada, the Smoking Regulatory Index (a new way to measure public health performance) was developed and utilized to assess as an indicator of how well people are protected from SHS and the effectiveness of the policies to minimize SHS exposure [
12]. Since the enactment of the National Health Promotion Act in Korea in 1995, indoor non-smoking areas have been gradually expanded, while a few outdoor areas have been designated as non-smoking areas through revisions of the corresponding laws in 2010 and 2012 [
13,
14]. As a result, the exposure rate of non-smokers to indoor SHS has significantly decreased [
15].
However, as most indoor environments were designated as non-smoking areas, smokers had to shift their designated smoking areas from indoors to outdoors [
16]. Consequently, non-smokers are more likely to be exposed to SHS at entrances or terraces of buildings with a smoking ban [
17]. Hence, several countries are expanding non-smoking areas to outdoor locations of hospitals, kindergartens, restaurants, sports facilities, and public transportation [
18,
19]. However, smoking is still prevalent at the entrances of buildings such as restaurants, bars, shops, and universities in Korea [
13]. Sureda et al. investigated the concentration of particulate matter with diameter less than 2.5 μm (PM
2.5) as an indicator of SHS at entrances and corridors of buildings with a smoking ban and compared the results, which showed that there was no significant difference in concentrations at either of the two places [
20].
Previous studies have mainly used questionnaires and biological sampling for SHS indicators [
21,
22]. However, these methods provide limited information on specific SHS exposure duration and microenvironments. Therefore, this study attempted to understand the temporal and spatial characteristics of SHS exposure using time activity patterns, and evaluated the relationship between these characteristics and the biological sampling results.
4. Discussion
Considering the health effects of SHS, various policies, including the prohibition of indoor smoking, are being implemented worldwide. The pattern of SHS exposure is changing from indoor to outdoor environments. Therefore, this study assessed the degree of SHS exposure in outdoor environments, taking into account the pattern of people’s time activities. The methodology used in this study and the results derived might be used in non-smoking policy.
The results of SHS exposures were compared with previous study conducted several countries. A total of 97% of the participants responded that they had been exposed to SHS at least once during the last week. The SHS exposure rates were 92% for outdoors, 62% at public places, 39% at workplaces, and 36% at home. In comparison with a study by Eriksen et al., SHS exposure rates were the highest after China, Bangladesh, Egypt, Vietnam, Greece, and Indonesia [
9]. These SHS exposure rates were higher than the Korea National Health and Nutrition Examination Survey (home: 4.7%, workplaces: 12.7%, and public places: 21.1%) [
22]. The results indicate that SHS exposure rates obtained by a simple questionnaire may be subjective and overestimated depending on the assessment method. Non-smokers can perceive not only the smell of cigarettes directly, but also smokers’ breathing and body odor as SHS exposure. Therefore, it is necessary to clarify the route of SHS exposure.
Based on the questionnaire, the microenvironment where the participants were most exposed to SHS was outdoors. Kaufman et al. and Sureda et al. have reported that smoking areas are shifting from indoors to outdoors [
16,
20]. In the results of the time activity survey conducted in this study, participants spent approximately 8% of their daily time outdoors. Time activity studies previously conducted by Klepis et al. and Yang et al. indicated that people spent 5% of their time outdoors, which is approximately 1 to 2 h per day [
39,
40,
41]. As the time spent outdoors was relatively short, the effectiveness of the outdoor smoking ban has been widely debated [
42,
43]. However, Lopez et al. reported that the concentration of nicotine and other substances from SHS in the air can be higher in terraces or corridors of buildings with a smoking ban, as compared to their concentrations outdoors. In addition, building types and ventilation conditions could also affect the concentration of airborne SHS indicators [
20,
44]. Therefore, building users may be intermittently exposed to high concentrations of SHS when they enter or exit buildings, which may have a negative psychological effect.
The number of cigarette butts found, smokers observed, and self-reported SHS exposure showed similar patterns over time, which were also similar to the participants’ time activity patterns for outdoors. This indicates that the participants were frequently exposed to SHS outdoors while moving from one space to another. Several studies have reported that exposure at entrances and exits of buildings has been an issue, and some countries are expanding non-smoking areas to these outdoor locations [
16,
18,
20,
44].
The classification of non-smokers exposed to SHS, based on existing literature, showed that most of their exposures to SHS were low. However, according to the classification by CLSI, 64–78% of non-smokers were exposed to SHS. Therefore, further studies are required to set the criteria for biological samples for SHS exposure. In addition, Hecht et al. reported that concentrations of NNAL and NNAL-Gluc in some human subjects could be detected even after 281 days since smoking cessation [
45]. Therefore, these factors should be considered when selecting non-smokers in further SHS exposure assessment studies.
The number of smokers observed during a week showed a marginal correlation with the concentrations of cotinine and NNAL, and the number of self-reported exposures to SHS had a significant positive correlation with the concentrations of cotinine. Although the correlations between time spent at each location and concentrations of biomarkers were marginal, the results showed that the time activity and SHS exposure patterns could be individually used to assess non-smokers’ exposure to SHS. As most residential areas prohibit smoking, non-smokers would have a lower chance of SHS exposure as they stay at home for longer durations. Likewise, time spent at other indoor areas would also decrease SHS exposure and biomarker concentrations. However, non-smokers’ SHS exposure and biomarker concentrations would be higher if they frequently move within different microenvironments, which would increase the time spent outdoors. In addition, their exposure to SHS would increase as smokers would smoke at entranceways, exits, or terraces of buildings with a smoking ban [
46]. There was no significant correlation between the concentration of cotinine and time activities. This can be explained by the half-life of cotinine being 18–24 h, which cannot be compared with weekly activities [
47].
In this study, the main microenvironment for SHS exposure was outdoors, especially entrances to restaurants, bars, clubs, and places related to transportation. In addition, it was also found that people were mainly exposed to SHS during peak movement hours, such as rush hour while commuting and lunch hours. In Korea, smoking is prohibited indoors; however, smokers can be seen in areas near entrances and exits of buildings such as restaurants, bars, malls, and clubs. Even though the duration of SHS exposure in such a microenvironment was relatively low, the facility users could be exposed to high concentrations of SHS, causing discomfort. Therefore, management and countermeasures are required to control the exposure to SHS in such microenvironments. The number of participants in this study was relatively low, and the types of public places visited by each participant were limited. In addition, their time spent in some of the microenvironments was very short. Hence, there were limitations in analyzing the relationship between the time spent in every microenvironment and the respective concentrations of biomarkers. Therefore, in future studies, a longer-term study period and larger-scale study of the population would be required to obtain a detailed exposure assessment and the relationship between the times spent in microenvironments and the concentrations of biomarkers for SHS exposure. The sample size and of 100 non-smokers may not be representative to assess the exposure to SHS. However, this study suggested the methodology by combining questionnaires and biomarkers with time activity patterns. The results of this study could be used as a basis for establishing an expansion to the non-smoking area policies, designation of outdoor smoking areas, non-smoking while walking, and outdoor smoking during rush hour, which would help reduce SHS exposure.