Towards a Global Perspective of Environmental Health: Defining the Research Grounds of an Institute of Environmental Health
Abstract
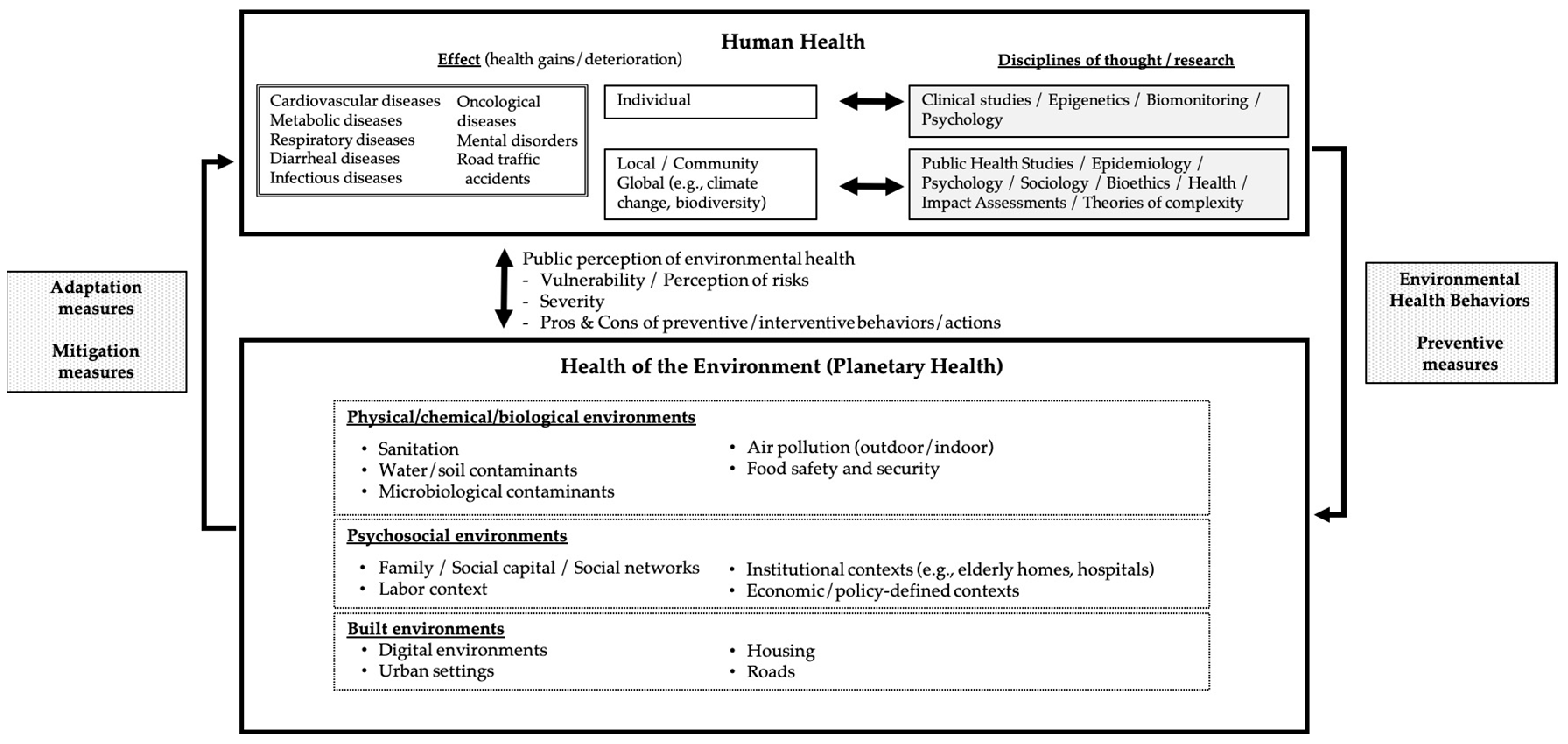
:1. Introduction
Grasping a Broader Perspective of Environmental Health
2. ISAMB’s Research Groups: Environmental Health Research Domains and Contributions
2.1. Research Group “Environment and Infectious Diseases”
2.2. Research Group “Environment and Non-Communicable Diseases”
2.3. Research Group “Supportive Environments for Public Health and Health Promotion”
2.4. Research Group “Supportive Environments for Individuals’ Lifespan Development”
2.5. Research Group “Ecogenetics and Human Health”
3. ISAMB’s Research Labs: Environmental Health Research Domains and Contributions
3.1. Environmental Health Behaviour Lab (EnviHeB Lab)
3.2. EnviHealthMicro Lab
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roser, M.; Ritchie, H.; Ortiz-Ospina, E. World Population Growth. Available online: https://ourworldindata.org/world-population-growth (accessed on 18 October 2020).
- Panorama. Panorama Perspectives: Conversations on Planetary Health—Planetary Health 101: Information and Resources, Report I. 2017. Available online: https://www.rockefellerfoundation.org/wp-content/uploads/Planetary-Health-101-Information-and-Resources.pdf (accessed on 18 October 2020).
- Jiménez Sánchez, M.; Lafuente, R. Defining and measuring environmental consciousness. Rev. Int. Sociol. 2010, 68, 731–755. [Google Scholar] [CrossRef] [Green Version]
- Rosa, C.D.; Collado, S. Experiences in nature and environmental attitudes and behaviors: Setting the ground for future research. Front. Psychol. 2019, 10, 763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Special EUROBAROMETER 340: Science and Technology. 2010. Available online: https://ec.europa.eu/commfrontoffice/publicopinion/archives/eb_special_359_340_en.htm#340 (accessed on 26 July 2020).
- Ek, R.; Johansson, N. (Eds.) Perspectives on Waste from the Social Sciences and Humanities: Opening the Bin; Cambridge Scholars Publishing: Newcastle, UK, 2020. [Google Scholar]
- National Environmental Health Association. Definitions of Environmental Health. Available online: https://www.neha.org/about-neha/definitions-environmental-health (accessed on 10 May 2020).
- Gordon, J.L. The future of environmental health, part 1. J. Environ. Health 1992, 55, 28–32. [Google Scholar]
- American Public Health Association. Environmental Health. Available online: https://www.apha.org/topics-and-issues/environmental-health (accessed on 10 May 2020).
- Whitmee, S.; Haines, A.; Beyrer, C.; Boltz, F.; Capon, A.G.; De Souza Dias, B.F.; Ezeh, A.; Frumkin, H.; Gong, P.; Head, P.; et al. Safeguarding human health in the Anthropocene epoch: Report of the Rockefeller Foundation-Lancet Commission on planetary health. Lancet 2015, 386, 1973–2028. [Google Scholar] [CrossRef]
- Guidotti, T.L. Environmental health needs a new paradigm, I. getting back in focus. Arch. Environ. Occup. Health 2018, 73, 267–269. [Google Scholar] [CrossRef] [Green Version]
- Haines, A.; Hanson, C.; Ranganathan, J. Planetary Health Watch: Integrated monitoring in the Anthropocene epoch. Lancet Planet. Health 2018, 2, e141–e143. [Google Scholar] [CrossRef] [Green Version]
- Santos, O.; Virgolino, A.; Santos, R.R.; Costa, J.; Rodrigues, A.; Vaz-Carneiro, A. Environmental health: An overview on the evolution of the concept and its definitions. In Encyclopedia of Environmental Health; Nriagu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 466–474. ISBN 9780124095489. [Google Scholar]
- Semenza, J.; Suk, J. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Lee, V.J.; Aguilera, X.; Heymann, D.; Wilder-Smith, A.; Lee, V.J.; Heymann, D.L.; Bausch, D.G.; Briand, S.; Bruschke, C.; Carmo, E.H.; et al. Preparedness for emerging epidemic threats: A Lancet Infectious Diseases Commission. Lancet Infect. Dis. 2020, 20, 17–19. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Centro de Estudos de Vetores e Doenças Infeciosas Doutor Francisco Cambournac. Relatório REVIVE 2011–2015—Culicídeos e Ixodídeos: Rede de Vigilância de Vetores; Instituto Nacional de Saúde Doutor Ricardo Jorge, IP: Lisboa, Portugal, 2016. [Google Scholar]
- Tuel, A.; Eltahir, E.A.B. Why is the Mediterranean a climate change hot spot? J. Clim. 2020, 33, 5829–5843. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Núncio, M.S.; Alves, M.J. (Eds.) Doenças Associadas a Artrópodes Vetores e Roedores – 2ª Edição; Instituto Nacional de Saúde Doutor Ricrado Jorge, IP: Lisboa, Portugal, 2019; Available online: http://www.insa.min-saude.pt/wp-content/uploads/2019/09/Doencas_artropodes_vetores_roedores.pdf (accessed on 16 October 2020).
- Osório, H.C.; Zé-Zé, L.; Amaro, F.; Alves, M.J. Mosquito surveillance for prevention and control of emerging mosquito-borne diseases in Portugal—2008–2014. Int. J. Environ. Res. Public Health 2014, 11, 11583–11596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akeda, Y. Food safety and infectious diseases. J. Nutr. Sci. Vitaminol. 2015, 61, S95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Food Safety: Climate Change and the Role of WHO; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/foodsafety/_Climate_Change.pdf (accessed on 17 October 2020).
- Uyttendaele, M.; Franz, E.; Schlüter, O. Food safety, a global challenge. Int. J. Environ. Res. Public Health 2016, 13, 67. [Google Scholar] [CrossRef]
- Bisholo, K.Z.; Ghuman, S.; Haffejee, F. Food-borne disease prevalence in rural villages in the Eastern Cape, South Africa. Afr. J. Prim. Health Care Fam. Med. 2018, 10, 1796. [Google Scholar] [CrossRef]
- Robertson, L.J.; van der Giessen, J.W.B.; Batz, M.B.; Kojima, M.; Cahill, S. Have foodborne parasites finally become a global concern? Trends Parasitol. 2013, 29, 101–103. [Google Scholar] [CrossRef]
- Salamandane, C.; Fonseca, F.; Afonso, S.; Lobo, M.L.; Antunes, F.; Matos, O. Handling of fresh vegetables: Knowledge, hygienic behavior of vendors, public health in Maputo markets, Mozambique. Int. J. Environ. Res. Public Health 2020, 17, 6302. [Google Scholar] [CrossRef]
- Morse, S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef]
- Rhodes, J.; Abdolrasouli, A.; Farrer, R.A.; Cuomo, C.A.; Aanensen, D.M.; Armstrong-James, D.; Fisher, M.C.; Schelenz, S. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg. Microbes Infect. 2018, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, A.; Ng, V.; Fisman, D. Climate change and infectious diseases in North America: The road ahead. CMAJ 2008, 178, 715–722. [Google Scholar] [PubMed] [Green Version]
- Barrozo, L.V.; Mendes, R.P.; Marques, S.A.; Benard, G.; Siqueira Silva, M.E.; Bagagli, E. Climate and acute/subacute paracoccidioidomycosis in a hyper-endemic area in Brazil. Int. J. Epidemiol. 2009, 38, 1642–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Solache, M.A.; Casadevall, A. Global warming will bring new fungal diseases for mammals. MBio 2010, 1, e00061-10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the emergence of Candida auris: Climate change, azoles, swamps, and birds. MBio 2019, 10, e01397-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz Gaitán, A.C.; Moret, A.; López Hontangas, J.L.; Molina, J.M.; Aleixandre López, A.I.; Cabezas, A.H.; Mollar Maseres, J.; Arcas, R.C.; Gómez Ruiz, M.D.; Chiveli, M.Á.; et al. Fungemia nosocomial por Candida auris: Primeros cuatro casos en Europa continental. Rev. Iberoam. Micol. 2017, 34, 23–27. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Misseri, G.; Ippolito, M.; Cortegiani, A. Global warming “heating up” the ICU through Candida auris infections: The climate changes theory. Crit. Care 2019, 23, 416. [Google Scholar] [CrossRef] [Green Version]
- Jackson, B.R.; Chow, N.; Forsberg, K.; Litvintseva, A.P.; Lockhart, S.R.; Welsh, R.; Vallabhaneni, S.; Chiller, T. On the origins of a species: What might explain the rise of Candida auris? J. Fungi 2019, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Hepatitis Report 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- UNODC. World Drug Report 2018: Executive Summary, Conclusions and Policy Implications; UNODC: Vienna, Austria, 2018. [Google Scholar]
- Smyth, B.P.; Barry, J.; Keenan, E. Irish injecting drug users and hepatitis C: The importance of the social context of injecting. Int. J. Epidemiol. 2005, 34, 166–172. [Google Scholar] [CrossRef] [Green Version]
- van Beek, I.; Buckley, R.; Stewart, M.; MacDonald, M.; Kaldor, J. Risk factors for hepatitis C infection among injecting drug users in Sydney. Genitourin. Med. 1994, 70, 321–324. [Google Scholar] [CrossRef]
- Kåberg, M.; Karlsson, N.; Discacciati, A.; Widgren, K.; Weiland, O.; Ekström, A.M.; Hammarberg, A. Significant decrease in injection risk behaviours among participants in a needle exchange programme. Infect. Dis. 2020, 52, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Lunenfeld, B. An Aging World—Demographics and challenges. Gynecol. Endocrinol. 2008, 24, 1–3. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015; Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 16 October 2020).
- United Nations. SDG 3—Ensure Healthy Lives and Promote Well-Being for All at All Ages. Available online: https://sdgs.un.org/goals/goal3 (accessed on 20 October 2020).
- Buist, A.S.; Vollmer, W.M.; Sullivan, S.D.; Weiss, K.B.; Lee, T.A.; Menezes, A.M.B.; Crapo, R.O.; Jensen, R.L.; Burney, P.G.J.B. The Burden of Obstructive Lung Disease Initiative (BOLD): Rationale and design. COPD 2005, 2, 277–283. [Google Scholar] [CrossRef] [PubMed]
- BOLD Study. Burden of Obstructive Lung Disease. Scientific Objectives. Available online: https://www.boldstudy.org/scientific-objectives (accessed on 15 October 2020).
- Sator, L.; Horner, A.; Studnicka, M.; Lamprecht, B.; Kaiser, B.; McBurnie, M.A.; Buist, A.S.; Gnatiuc, L.; Mannino, D.M.; Janson, C.; et al. Overdiagnosis of COPD in subjects with unobstructed spirometry: A BOLD analysis. Chest 2019, 156, 277–288. [Google Scholar] [CrossRef]
- Burney, P.; Jithoo, A.; Kato, B.; Janson, C.; Mannino, D.; Nizankowska-Mogilnicka, E.; Studnicka, M.; Tan, W.; Bateman, E.; KoÇabas, A.; et al. Chronic obstructive pulmonary disease mortality and prevalence: The associations with smoking and poverty-A BOLD analysis. Thorax 2014, 69, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Townend, J.; Minelli, C.; Mortimer, K.; Obaseki, D.O.; Al Ghobain, M.; Cherkaski, H.; Denguezli, M.; Gunesekera, K.; Hafizi, H.; Koul, P.A.; et al. The association between chronic airflow obstruction and poverty in 12 sites of the multinational BOLD study. Eur. Respir. J. 2017, 49, 1601880. [Google Scholar] [CrossRef] [PubMed]
- Bárbara, C.; Rodrigues, F.; Dias, H.; Cardoso, J.; Almeida, J.; Matos, M.J.; Simão, P.; Santos, M.; Ferreira, J.R.; Gaspar, M.; et al. Chronic obstructive pulmonary disease prevalence in Lisbon, Portugal: The Burden of Obstructive Lung Disease study. Rev. Port. Pneumol. 2013, 19, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.; Shakyawar, S.K.; Sharma, M.; Kaushik, S. Big data in healthcare: Management, analysis and future prospects. J. Big Data 2019, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, T.B.; Detsky, A.S. The inevitable application of big data to health care. JAMA 2013, 309, 1351–1352. [Google Scholar] [CrossRef]
- Cirillo, D.; Valencia, A. Big data analytics for personalized medicine. Curr. Opin. Biotechnol. 2019, 58, 161–167. [Google Scholar] [CrossRef]
- Floras, J.S. Sleep apnea and cardiovascular disease an enigmatic risk factor. Circ. Res. 2018, 122, 1741–1764. [Google Scholar] [CrossRef]
- Staats, R.; Barros, I.; Fernandes, D.; Grencho, D.; Reis, C.; Matos, F.; Valença, J.; Marôco, J.; de Almeida, A.B.; Bárbara, C. The importance of sleep fragmentation on the hemodynamic dipping in obstructive sleep apnea patients. Front. Physiol. 2020, 11, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staats, R.; Rodrigues, R.; Barros, A.; Bacelar-Nicolau, L.; Aguiar, M.; Fernandes, D.; Moreira, S.; Simões, A.; Silva-Santos, B.; Rodrigues, J.V.; et al. Role of CD3 + γδ-T cells in the association of obstructive sleep-disordered breathing and cancer. Sleep Breath. 2019. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Cuco, C.M.; Lavareda, C.; Miguel, F.; Ventura, M.; Almeida, S.; Pinto, P.; De Abreu, T.T.; Rodrigues, L.V.; Seixas, S.; et al. Bronchoalveolar lavage proteomics in patients with suspected lung cancer. Sci. Rep. 2017, 7, 42190. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, A.; Vaz, F.; Torres, V.M.; Valentim-Coelho, C.; Silva, R.; Prosinecki, V.; Alexandre, B.M.; Carvalho, A.S.; Matthiesen, R.; Malhotra, A.; et al. Evening and morning peroxiredoxin-2 redox/oligomeric state changes in obstructive sleep apnea red blood cells: Correlation with polysomnographic and metabolic parameters. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Rodrigues, R.; Barros, A.B.; Pejanovic, N.; Neves-Costa, A.; Pedroso, D.; Pereira, C.; Fernandes, D.; Rodrigues, J.V.; Barbara, C.; et al. Changes in expression of the CLOCK gene in obstructive sleep apnea syndrome patients are not reverted by continuous positive airway pressure treatment. Front. Med. 2017, 4, 187. [Google Scholar] [CrossRef] [Green Version]
- Staats, R.; Rodrigues, R.; Barros, A.; Bacelar-Nicolau, L.; Aguiar, M.; Fernandes, D.; Moreira, S.; Simões, A.; Silva-Santos, B.; Rodrigues, J.V.; et al. Decrease of perforin positive CD3+γδ-T cells in patients with obstructive sleep disordered breathing. Sleep Breath. 2018, 22, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.; Cavadas, B.; Ferreira, J.C.; Marques, P.I.; Monteiro, C.; Sucena, M.; Sousa, C.; Vaz Rodrigues, L.; Teixeira, G.; Pinto, P.; et al. Profiling of lung microbiota discloses differences in adenocarcinoma and squamous cell carcinoma. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Brooks, D. Telehealth technology: an emerging method of delivering pulmonary rehabilitation to patients with chronic obstructive pulmonary disease. Can Respir J. 2011, 18, 196. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the state-of-the-art and areas of application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef]
- Faria, I.; Gaspar, C.; Zamith, M.; Matias, I.; César Das Neves, R.; Rodrigues, F.; Bárbara, C. TELEMOLD project: Oximetry and exercise telemonitoring to improve long-term oxygen therapy. Telemed. e-Health 2014, 20, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.D.; Rui, C.; Ribeiro, R.M.; Caneiras, C. Novel input for designing patient-tailored pulmonary rehabilitation: Telemonitoring physical activity as a vital sign—SMARTREAB Study. J. Clin. Med. 2020, 9, 2450. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Physical Activity Strategy for the WHO European Region 2016–2025; WHO: Copenhagen, Denmark, 2015. [Google Scholar]
- European Comission. Demography Report—Short Analytical Web Note; Publications Office of the European Union; European Comission: Louxembourg, 2015. [Google Scholar]
- UNECE/European Comission. Active Ageing Index 2014: Analytical Report; 2015. Available online: https://ec.europa.eu/eip/ageing/library/2014-active-ageing-index-aai-analytical-report_en (accessed on 16 October 2020).
- World Health Organization. World Report on Ageing and Health; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Zaidi, B.; Morgan, S.P. The second demographic transition theory: A review and appraisal. Annu. Rev. Sociol. 2017, 43, 473–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reher, D.S. Economic and social implications of the demographic transition. Popul. Dev. Rev. 2011, 37, 11–33. [Google Scholar] [CrossRef]
- World Health Organization. The Ottawa Charter for Health Promotion. In Proceedings of the First International Conference on Health Promotion, Ottawa, ON, Canada, 21 November 1986. [Google Scholar]
- World Health Organization. European Food and Nutrition Action Plan 2015–2020; WHO: Copenhagen, Denmark, 2015. [Google Scholar]
- Santos, O.; Alarcão, V.; Feteira-Santos, R.; Fernandes, J.; Virgolino, A.; Sena, C.; Vieira, C.P.; Gregório, M.J.; Nogueira, P.; Graça, P.; et al. Impact of different front-of-pack nutrition labels on online food choices. Appetite 2020, 154, 104795. [Google Scholar] [CrossRef]
- Graça, P.; Silva, A.; Vieira, C.P.; Sena, C.; Gregório, M.J.; Nogueira, P.; Virgolino, A.; Fernandes, J.; Santos, O.; Santos, R.; et al. NUTR-HIA: Improving Nutrition Labelling in Portugal—Health Impact Assessment Final Report; Direção-Geral da Sáude: Lisboa, Portugal, 2019. [Google Scholar]
- Drinkwater, C.; Moffatt, S. Social prescribing. BMJ 2019, 364, l1285. [Google Scholar] [CrossRef]
- Husk, K.; Elston, J.; Gradinger, F.; Callaghan, L.; Asthana, S. Social prescribing: Where is the evidence? Br. J. Gen. Pract. 2019, 69, 6–7. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, A.J.; Critchley, J.A.; Tai, S.S.; Buckingham, K.; Westley, D.; Harridge, S.D.R.; Smith, C.; Gottlieb, J.M. Exercise Evaluation Randomised Trial (EXERT): A randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only. Health Technol. Assess. 2007, 11, 1–165. [Google Scholar] [CrossRef] [Green Version]
- The Conservation Volunteers. Green Gym. Available online: https://www.tcv.org.uk/greengym/ (accessed on 14 October 2020).
- World Health Organization; Gobierno de España; IOM International Organization for Migration. Health of Migrants—The Way forward: Report of a Global Consultation; WHO: Madrid, Spain, 2010. [Google Scholar]
- Castañeda, H.; Holmes, S.M.; Madrigal, D.S.; Young, M.E.D.T.; Beyeler, N.; Quesada, J. Immigration as a social determinant of health. Annu. Rev. Public Health 2015, 36, 375–392. [Google Scholar] [CrossRef]
- Alarcão, V.; Stefanovska-Petkovska, M.; Virgolino, A.; Santos, O.; Ribeiro, S.; Costa, A.; Nogueira, P.; Pascoal, P.M.; Pintassilgo, S.; Machado, F.L. Fertility, Migration and Acculturation (FEMINA): A research protocol for studying intersectional sexual and reproductive health inequalities. Reprod. Health 2019, 16, 140. [Google Scholar] [CrossRef]
- Reis Oliveira, C.; Gomes, N. Indicadores de Integração de Imigrantes-Relatório Estatístico Anual 2018; Alto Comissariado para as Migrações: Lisboa, Portugal, 2018. [Google Scholar]
- Viner, R.M.; Ozer, E.M.; Denny, S.; Marmot, M.; Resnick, M.; Fatusi, A.; Currie, C. Adolescence and the social determinants of health. Lancet 2012, 379, 1641–1652. [Google Scholar] [CrossRef]
- Yang, T.C.; Chen, I.C.; Choi, S.W.; Kurtulus, A. Linking perceived discrimination during adolescence to health during mid-adulthood: Self-esteem and risk-behavior mechanisms. Soc. Sci. Med. 2019, 232, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Landstedt, E.; Hammarstrom, A.; Winefield, H. How well do parental and peer relationships in adolescence predict health in adulthood? Scand. J. Public Health 2015, 43, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.G.; Simões, C. From positive youth development to youth’s engagement: The Dream Teens. Int. J. Emot. Educ. 2016, 8, 4–17. [Google Scholar]
- Israel, B.A.; Schulz, A.J.; Parker, E.A.; Becker, A.B. Review of community-based research: Assessing partnership approaches to improve public health. Annu. Rev. Public Health 1998, 19, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Horn, K.; McCracken, L.; Dino, G.; Brayboy, M. Applying community-based participatory research principles to the development of a smoking-cessation program for American Indian teens: “Telling our story. ” Health Educ. Behav. 2008, 35, 44–69. [Google Scholar] [CrossRef]
- Michie, S.; van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A. Social capital as a health asset for young people’s health and well-being. Rev. Psicol. Criança Adolesc. 2013, 1, 19–42. [Google Scholar]
- Morgan, A.; Davies, M.; Ziglio, E. Health Assets in a Global Context: Theory, Methods, Action; Springer: New York, NY, USA, 2010. [Google Scholar]
- Morgan, A.; Ziglio, E. Revitalising the evidence base for public health: An assets model. Promot. Educ. 2007, 14, 17–22. [Google Scholar] [CrossRef]
- Matos, M.G.; Equipa Aventura Social. Relatório do Estudo HBSC 2018: A Saúde Dos Adolescentes Portugueses Após a Recessão; Equipa Aventura Social: Lisboa, Portugal, 2018. [Google Scholar]
- World Health Organization Regional Office for Europe. Spotlight on Adolescent Health and Well-Being: Findings from the 2017/2018 Health Behaviour in School-Aged Children (HBSC) Survey in Europe and Canada; WHO Regional Office for Europe: Copenhagen, Denmark, 2018; Volume 1. [Google Scholar]
- Matos, M.G.; Wainwright, T.; Brebels, L.; Craciun, B.; Gabrhelík, R.; Schjodt, B.H.; Plantade-Gipch, A.; Poštuvan, V.; Stojadinovic, I.; Richards, J. Looking ahead: Challenges and opportunities for applied psychology in prevention and promotion. Eur. Psychol. 2019, 24, 1–12. [Google Scholar] [CrossRef]
- Matos, M.G.; Santos, T.; Reis, M.; Marques, A. Positive youth development: interactions between healthy lifestyle behaviours and psychosocial variables. Glob. J. Health Sci. 2018, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.G.; Camacho, I.; Reis, M.; Costa, D.; Galvão, D. Worries, coping strategies and well-being in adolescence: Highlights from HBSC study in Portugal. Vulnerable Child. Youth Stud. 2016, 11, 274–280. [Google Scholar] [CrossRef]
- Matos, M.G.; Camacho, I.; Reis, M.; Tomé, G.; Branquinho, C.; Ramiro, L. Is truth in the eyes of the beholder? Or are Portuguese schools, as viewed by Portuguese pupils, mismatching with what the educational system offers? Vulnerable Child. Youth Stud. 2018, 13, 116–126. [Google Scholar] [CrossRef]
- Branquinho, C.; Matos, M.G. The “Dream Teens” Project: After a two-year participatory action-research program. Child Indic. Res. 2019, 12, 1243–1257. [Google Scholar] [CrossRef]
- Levasseur, M.; Richard, L.; Gauvin, L.; Raymond, É. Inventory and analysis of definitions of social participation found in the aging literature: Proposed taxonomy of social activities. Soc. Sci. Med. 2010, 71, 2141–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerliu, N.; Burazeri, G.; Toçi, E.; Kempen, G.I.J.M.; Jongen, W.; Ramadani, N.; Brand, H. Social networks, social participation and self-perceived health among older people in transitional Kosovo. Eur. J. Public Health 2014, 24, 333–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukov, A.; Maas, I.; Lampert, T. Social participation in very old age: Cross-sectional and longitudinal findings from BASE. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002, 57, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Jang, S.N.; Lee, S.; Cho, S., II; Park, E.O. The relationship between social participation and self-rated health by sex and age: A cross-sectional survey. Int. J. Nurs. Stud. 2008, 45, 1042–1054. [Google Scholar] [CrossRef]
- Aroogh, M.D.; Shahboulaghi, F.M. Social participation of older adults: A concept analysis. Int. J. Community Based Nurs. Midwifery 2020, 8, 55–72. [Google Scholar]
- Teater, B. Intergenerational programs to promote active aging: the experiences and perspectives of older adults. Act. Adapt. Aging 2016, 40, 1–19. [Google Scholar] [CrossRef]
- Lai, J.; Ma, S.; Wang, Y.; Cai, Z.; Hu, J.; Wei, N.; Wu, J.; Du, H.; Chen, T.; Li, R.; et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open 2020, 3, e203976. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, L. The untold toll—The pandemic’s effects on patients without covid-19. N. Engl. J. Med. 2020, 382, 2368–2371. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Hallet, J.; Matthews, J.B.; Schnitzbauer, A.A.; Line, P.D.; Lai, P.B.S.; Otero, J.; Callegaro, D.; Warner, S.G.; Baxter, N.N.; et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br. J. Surg. 2020, 107, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Eaton, D.L. (Eds.). Gene-Environment Interactions: Fundamentals of Ecogenetics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mendes, A.I.; Mascarenhas, M.R.; Matos, S.; Sousa, I.; Ferreira, J.; Barbosa, A.P.; Bicho, M.; Jordan, P. A WNK4 gene variant relates to osteoporosis and not to hypertension in the Portuguese population. Mol. Genet. Metab. 2011, 102, 465–469. [Google Scholar] [CrossRef]
- Guerra, A.; Rego, C.; Laires, M.J.; Castro, E.M.; Silva, D.; Monteiro, C.; Silva, Z.; Lebre, A.; Bicho, M. Lipid profile and redox status in high performance rhythmic female teenagers gymnasts. J. Sports Med. Phys. Fit. 2001, 41, 505–512. [Google Scholar]
- Marinho, C.; Alho, I.; Arduíno, D.; Falcão, L.M.; Brás-Nogueira, J.; Bicho, M. GST M1/T1 and MTHFR polymorphisms as risk factors for hypertension. Biochem. Biophys. Res. Commun. 2007, 353, 344–350. [Google Scholar] [CrossRef]
- Laires, M.J.; Monteiro, C.P.; Bicho, M. Role of cellular magnesium in health and human disease. Front. Biosci. 2004, 9, 262–276. [Google Scholar] [CrossRef] [Green Version]
- Pombo, S.; Levy, P.; Bicho, M.; Ismail, F.; Neves Cardoso, J.M. Neuropsychological function and platelet monoamine oxidase activity levels in type I alcoholic patients. Alcohol. Alcohol. 2008, 43, 423–430. [Google Scholar] [CrossRef]
- Alho, I.; Costa, L.; Bicho, M.; Coelho, C. The role of low-molecular-weight protein tyrosine phosphatase (LMW-PTP ACP1) in oncogenesis. Tumor Biol. 2013, 34, 1979–1989. [Google Scholar] [CrossRef]
- Apelt, N.; Pereira da Silva, A.; Ferreira, J.; Alho, I.; Monteiro, C.; Marinho, C.; Teixeira, P.; Sardinha, L.; Laires, M.J.; Mascarenhas, M.R.; et al. ACP1 genotype, glutathione reductase activity, and riboflavin uptake affect cardiovascular risk in the obese. Metabolism 2009, 58, 1415–1423. [Google Scholar] [CrossRef]
- Almeida, E.; Alho, I.; Marques, F.; Thiran, C.; Bicho, M.; Prata, M. Haemoglobin and erythropoietin levels in polycystic kidney disease. Nephrol. Dial. Transplant. 2008, 23, 412–413. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, L.; Matos, A.; Gil, Â.; Afonso, C.; Almeida, S.; Braga, L.; Lavinha, J.; Kjollerstrom, P.; Faustino, P.; Bicho, M.; et al. Sickle cell anemia—Nitric oxide related genetic modifiers of hematological and biochemical parameters. Clin. Hemorheol. Microcirc. 2016, 64, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The global syndemic of obesity, undernutrition, and climate change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Lally, P.; Gardner, B. Promoting habit formation. Health Psychol. Rev. 2013, 7, S137–S158. [Google Scholar] [CrossRef]
- Armitage, C.J.; Conner, M. Social cognition models and health behaviour: A structured review. Psychol. Health 2000, 15, 173–189. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, W.; Friese, M.; Wiers, R.W. Impulsive versus reflective influences on health behavior: A theoretical framework and empirical review. Health Psychol. Rev. 2008, 2, 111–137. [Google Scholar] [CrossRef]
- Baumeister, R.F.; Bratslavsky, E.; Muraven, M.; Tice, D.M. Ego depletion: Is the active self a limited resource? J. Pers. Soc. Psychol. 1998, 74, 1252–1265. [Google Scholar] [CrossRef]
- Deci, E.L.; Ryan, R.M. Intrinsic Motivation and Self-Determination in Human Behavior; Springer Science & Business Media: New York, NY, USA, 1985. [Google Scholar]
- Thaler, R. Toward a positive theory of consumer choice. J. Econ. Behav. Organ. 1980, 1, 39–60. [Google Scholar] [CrossRef]
- Thaler, R.H.; Sunstein, C.R. Nudge: Improving Decisions about Health, Wealth, and Happiness; Yale University Press: New Haven, USA, 2008; ISBN 978-0-300-12223-7. [Google Scholar]
- Schwartz, S.H. Are there universal aspects in the structure and contents of human values? J. Soc. Issues 1994, 50, 19–45. [Google Scholar] [CrossRef]
- Michie, S.; Thomas, J.; Johnston, M.; Mac Aonghusa, P.; Shawe-Taylor, J.; Kelly, M.P.; Deleris, L.A.; Finnerty, A.N.; Marques, M.M.; Norris, E.; et al. The Human Behaviour-Change Project: Harnessing the power of artificial intelligence and machine learning for evidence synthesis and interpretation. Implement. Sci. 2017, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronfenbrenner, U. The Ecology of Human Development: Experiments by Nature and Design; Harvard University Press: Cambridge, MA, USA, 1979. [Google Scholar]
- Berkman, E.T. Value-based choice: An integrative, neuroscience-informed model of health goals. Psychol. Health 2018, 33, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Holtzhausen, D.; Zerfass, A. The Routledge Handbook of Strategic Communication; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Parvanta, C. A Public Health Communication Planning Framework. In Essentials of Public Health Communication; Parvanta, C., Nelson, D.E., Parvanta, S.A., Harner, R.N., Eds.; Jones & Bartlet Learning LLC: Sudbury, MA, USA, 2011; pp. 19–38. [Google Scholar]
- Richard, L.; Gauvin, L.; Raine, K. Ecological models revisited: Their uses and evolution in health promotion over two decades. Annu. Rev. Public Health 2011, 32, 307–326. [Google Scholar] [CrossRef]
- Mcleroy, K.R.; Bibeau, D.; Steckler, A.; Glanz, K. An ecological perspective on health promotion programs. Health Educ. Behav. 1988, 15, 351–377. [Google Scholar] [CrossRef]
- Thomas, S. (Ed.) Antimicrobial Resistance; Springer: Singapore, 2020; ISBN 978-981-15-3657-1. [Google Scholar]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Asaduzzaman, M. Antimicrobial resistance: An urgent need for a planetary and ecosystem approach. Lancet Planet. Health 2018, 2, e99–e100. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Sridhar, D.; Blaser, M.; Wang, M.; Woolhous, M. Achieving global targets for antimicrobial resistance. Policy Forum 2016, 353, 874–875. [Google Scholar] [CrossRef] [Green Version]
- Collignon, P. Antibiotic resistance: Are we all doomed? Intern. Med. J. 2015, 45, 1109–1115. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, E.M.; Thaden, J.T. Epidemiology and mechanisms of resistance of extensively drug resistant gram-negative bacteria. Antibiotics 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caneiras, C.; Lito, L.; Mayoralas-Alises, S.; Díaz-Lobato, S.; Melo-Cristino, J.; Duarte, A. Virulence and resistance determinants of Klebsiella pneumoniae isolated from a Portuguese tertiary university hospital centre over a 31-year period. Enferm. Infecc. Microbiol. Clin. 2019, 37, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Zhao, Y.; Zhu, D.; Gillings, M.; Penuelas, J.; Ok, Y.S.; Capon, A.; Banwart, S. Soil biota, antimicrobial resistance and planetary health. Environ. Int. 2019, 131, 105059. [Google Scholar] [CrossRef]
- Caneiras, C.; Jácome, C.; Mayoralas-Alises, S.; Ramon Calvo, J.; Almeida Fonseca, J.; Escarrabill, J.; Winck, J.C. Patient experience in home respiratory therapies: Where we are and where to go. J. Clin. Med. 2019, 8, 555. [Google Scholar] [CrossRef] [Green Version]
- Karliner, J.; Slotterback, S.; Boyd, R.; Ashby, B.; Steele, K. Health Care’s Climate Footprint: How the Health Sector Contributes to the Global Climate Crisis and Opportunities for Action. 2019. Available online: https://noharm-uscanada.org/ClimateFootprintReport (accessed on 16 October 2020).
| Main Research Line(s) | Main Scientific Disciplines Involved | |
|---|---|---|
| Research Groups | ||
| Environment and infectious diseases | Environmental determinants of infectious diseases in their broadest sense | Medicine, Veterinary Medicine, Biology, Pharmacy, Biochemistry, Epidemiology, Public Health, Environmental Health |
| Environment and non-communicable diseases (NCDs) | Environmental determinants of NCDs | Medicine, Nutrition, Psychology, Physiology, Physiotherapy, Environmental Health |
| Supportive environments for public health and health promotion | Multisectoral approach to social and environmental determinants of health | Public Health, Medicine, Nursing, Health Sociology, Health Psychology, Biostatistics, Biology, Anthropology, Nutritional Sciences, Physical Activity Sciences, Environmental Health |
| Supportive environments for individuals’ lifespan development | Relation between supportive environments and human health throughout life, from an intergenerational perspective | Health and Clinical Psychology, Environmental Psychology, Developmental Psychology, Cognitive Sciences, Behavioural Economics, Behavioural Medicine, Health Sociology, Biology, Nutritional Sciences, Physical Activity Sciences, Anthropology, Sustainability Sciences, Public Health, Biostatistics, Environmental Health |
| Ecogenetics and human health | Genetic, epigenetic and (modifiable and non-modifiable) environmental determinants of infectious diseases and NCDs | Medicine, Pharmacy, Genetics, Toxicology, Biochemistry, Ecology, Psychology, Public Health, Veterinary Medicine, Nutritional Sciences, Physical Activity Sciences, Environmental Health |
| Research Labs | ||
| Environmental Health Behaviour | Human behaviour as effect and determinant of modifications on human and environmental health | Health Psychology, Environmental Psychology, Cognitive Sciences, Behavioural Economics, Health Sociology, Biology, Veterinary Medicine, Nutritional Sciences, Physical Activity Sciences, Anthropology, Architecture, Human Geography, Biostatistics, Strategic Communication, Environmental Health |
| Environmental Health Microbiology | Environmental determinants of antimicrobial resistance and virulence in different settings | Microbiology, Public Health, Epidemiology, Biology (Molecular Genetics), Environmental Science, Medicine, Environmental Health |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virgolino, A.; Antunes, F.; Santos, O.; Costa, A.; Matos, M.G.d.; Bárbara, C.; Bicho, M.; Caneiras, C.; Sabino, R.; Núncio, M.S.; et al. Towards a Global Perspective of Environmental Health: Defining the Research Grounds of an Institute of Environmental Health. Sustainability 2020, 12, 8963. https://doi.org/10.3390/su12218963
Virgolino A, Antunes F, Santos O, Costa A, Matos MGd, Bárbara C, Bicho M, Caneiras C, Sabino R, Núncio MS, et al. Towards a Global Perspective of Environmental Health: Defining the Research Grounds of an Institute of Environmental Health. Sustainability. 2020; 12(21):8963. https://doi.org/10.3390/su12218963
Chicago/Turabian StyleVirgolino, Ana, Francisco Antunes, Osvaldo Santos, Andreia Costa, Margarida Gaspar de Matos, Cristina Bárbara, Manuel Bicho, Cátia Caneiras, Raquel Sabino, Maria Sofia Núncio, and et al. 2020. "Towards a Global Perspective of Environmental Health: Defining the Research Grounds of an Institute of Environmental Health" Sustainability 12, no. 21: 8963. https://doi.org/10.3390/su12218963
APA StyleVirgolino, A., Antunes, F., Santos, O., Costa, A., Matos, M. G. d., Bárbara, C., Bicho, M., Caneiras, C., Sabino, R., Núncio, M. S., Matos, O., Santos, R. R., Costa, J., Alarcão, V., Gaspar, T., Ferreira, J., & Carneiro, A. V. (2020). Towards a Global Perspective of Environmental Health: Defining the Research Grounds of an Institute of Environmental Health. Sustainability, 12(21), 8963. https://doi.org/10.3390/su12218963











