Salinity Stress Mitigation Using Encapsulated Biofertilizers for Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Cyanobacterial Strain (Blue Green Algal Strain)
2.2. Culture of Rhizobacterium
2.3. Germination Experiments
- (a)
- Adding biofertilizers to soil and irrigating with saline water,
- (b)
- Presoaking in biofertilizers for one day, then irrigating with saline water,
- (c)
- Presoaking in biofertilizers for 12 h, then irrigating with saline water.
- -
- Graphene + alga + bacterium + MeSA (sample denoted 0.4 Gr-Al-B and 0.8 Gr-Al-B),
- -
- CNT + alga + bacterium + MeSA (sample denoted 0.4 CNT-Al-B and 0.8 CNT-Al-B),
- -
- GO + alga+ bacterium + MeSA (sample denoted 1 GO-Al-B and 2 GO-Al-B).
2.4. Preparation of Carbon Materials
2.4.1. Preparation of Graphene Oxide
2.4.2. Preparation of Carbon Nanotubes
2.4.3. Preparation of Graphene
2.5. Spectroscopy Techniques
2.6. Statistical Analysis
3. Results
3.1. Germination Experiments
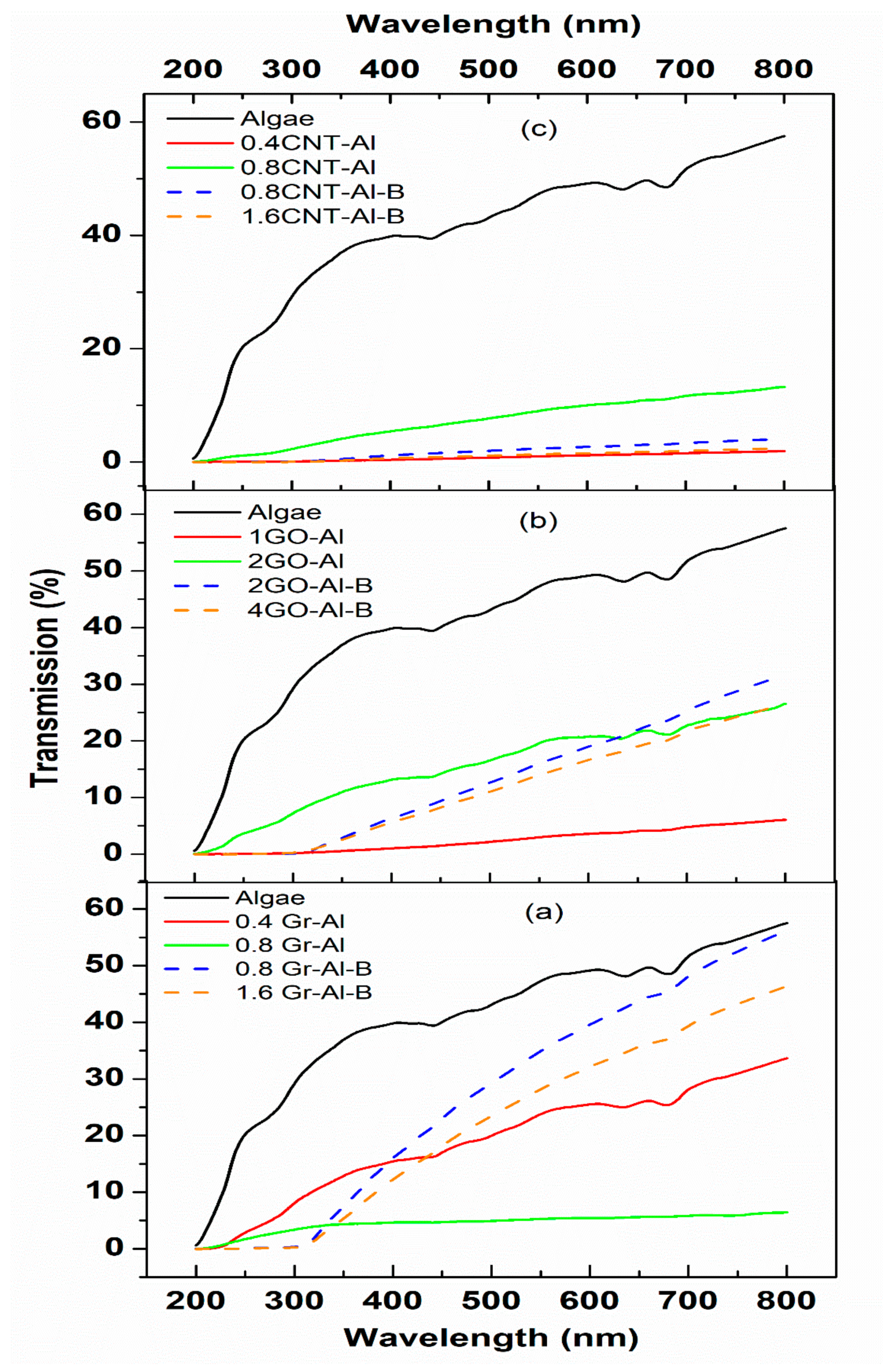
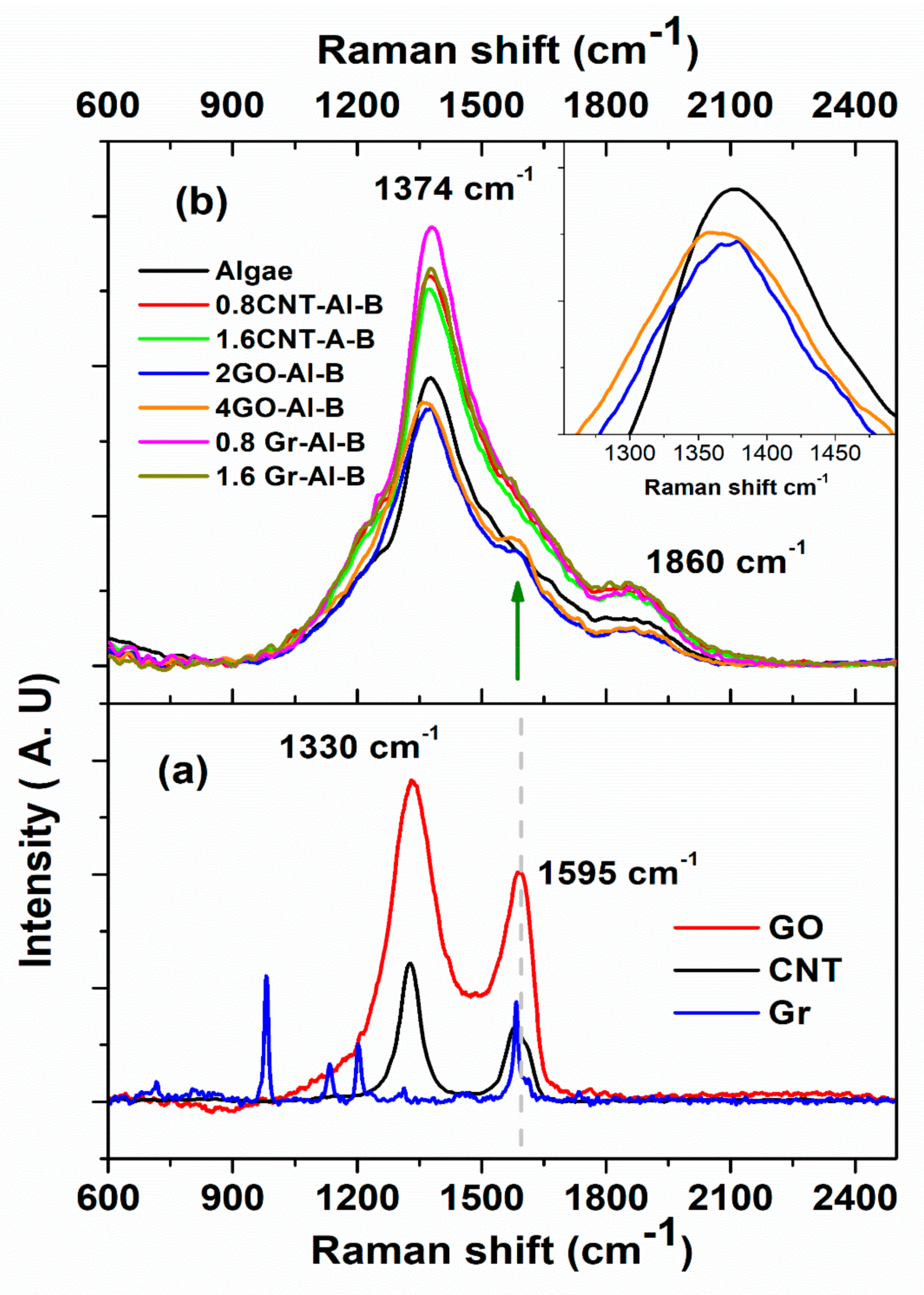
3.2. Optical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Çavuşoğlu, K.; Kabar, K. Effects of hydrogen peroxide on the germination and early seedling growth of barley under NaCl and high temperature stresses. Eurasian J. Biosci. 2010, 4, 70–79. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, A.D. Salt Stress and Phytohormone (ABA)-Induced Changes in Germination, Sugars and Enzymes of Carbohydrate Metabolism in Sorghum bicolor (L.) Moench Seeds. J. Agric. Soc. Sci. 2005, 1, 89–93. [Google Scholar]
- Patel, P.; Kajal, S.; Patel, V.R.; Patel, V.; Khristi, S.M. Impact of salt stress on nutrient uptake and growth of cowpea. Bra. J. Plant Physiol. 2010, 22, 43–48. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Brahmaprakash, G.; Kumar Sahu, P. Reviews Biofertilizers for Sustainability. J. Ind. Inst. Sci. 2012, 92, 37–62. [Google Scholar]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Rodríguez, A.; Stella, A.; Storni, M.; Zulpa, G.; Zaccaro, M. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006, 2, 7. [Google Scholar] [CrossRef]
- Sergeeva, E.; Liaimer, A.; Bergman, B. Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 2002, 215, 229–238. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ördög, V.; Van Staden, J.; Jäger, K. Cytokinin—And auxin—Like activity in Cyanophyta and microalgae. J. Appl. Phycol. 2002, 14, 215–221. [Google Scholar] [CrossRef]
- Yadav, S.; Sinha, R.P.; Tyagi, M.B.; Kumar, A. Cyanobacterial Secondary Metabolites. Int. J. Pharma Bio. Sci. 2011, 2, 144–167. [Google Scholar]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Halotolerant Rhizobacterial Strains Mitigate the Adverse Effects of NaCl Stress in Soybean Seedlings. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of methyl salicylate (MeSA), an elicitor on growth, physiology and pathology of resistant and susceptible rice varieties. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Vazirimehr, M.R.; Rigi, K. Effect Of Salicylic Acid In Agriculture. Int. J. Plant Anim. Environ. Sci. 2014, 4, 291–296. [Google Scholar]
- Wang, Y.; Voronin, G.A.; Zerda, T.W.; Winiarski, A. SiC-CNT nanocomposites: High pressure reaction synthesis and characterization. J. Phys. Condens. Matter. 2006, 18, 275–282. [Google Scholar] [CrossRef]
- Gubicza, J.; Ungár, T.; Wang, Y.; Voronin, G.; Pantea, C.; Zerda, T.W. Microstructure of diamond-SiC nanocomposites determined by X-ray line profile analysis. Diam. Relat. Mater. 2006, 15, 1452–1456. [Google Scholar] [CrossRef]
- Wang, Y.; Zerda, T.W. The mechanism of the solid-state reaction between carbon nanotubes and nanocrystalline silicon under high pressure and at high temperature. J. Phys. Condens. Matter. 2006, 18, 2995–3003. [Google Scholar] [CrossRef]
- Wang, Y.; Zerda, T.W. Microstructure evaluations of carbon nanotube/diamond/silicon carbide nanostructured composites by size–strain line-broadening analysis methods. J. Phys. Condens. Matter. 2007, 19, 356205. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.J. Influence of multi-walled carbon nanotubes on the electrochemical performance of graphene nanocomposites for supercapacitor electrodes. Electrochim. Acta 2011, 56, 1629–1635. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chong, M.H.; Rhee, K.Y.; Park, S.J. Silver-coated graphene electrode produced by electrolytic deposition for electrochemical behaviors. Curr. Appl. Phys. 2014, 14, 1212–1215. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Foo, M.E.; Gopinath, S.C.B. Feasibility of graphene in biomedical applications. Biomed. Pharm. 2017, 94, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- El Semary, N.A.; Fouda, M. Anticancer activity of Cyanothece sp. strain extracts from Egypt: First record. Asian Pac. J. Trop. Biomed. 2015, 5, 992–995. [Google Scholar] [CrossRef]
- Xin, H.; Tang, Y.; Liu, S.; Yang, X.; Xia, S.; Yin, D.; Yu, S. Impact of Graphene Oxide on Algal Organic Matter of Microcystis aeruginosa. ACS Omega 2018, 3, 16969–16975. [Google Scholar] [CrossRef]
- Nogueira, P.F.M.; Nakabayashi, D.; Zucolotto, V. The effects of graphene oxide on green algae Raphidocelis subcapitata. Aquat. Toxicol. 2015, 166, 29–35. [Google Scholar] [CrossRef]
- Handy, R.D.; van den Brink, N.; Chappell, M.; Mühling, M.; Behra, R.; Dušinská, M.; Simpson, P.; Ahtiainen, J.; Jha, A.N.; Seiter, J.; et al. Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: What have we learnt so far? Ecotoxicology 2012, 21, 933–972. [Google Scholar] [CrossRef]
- Rezgui, K.; Othmen, R.; Cavanna, A.; Ajlani, H.; Madouri, A.; Oueslati, M. The improvement of InAs/GaAs quantum dot properties capped by Graphene. J. Raman Spectrosc. 2013, 44, 1529–1533. [Google Scholar] [CrossRef]
- Othmen, R.; Rezgui, K.; Cavanna, A.; Arezki, H.; Gunes, F.; Ajlani, H.; Madouri, A.; Oueslati, M. Improvement of the quality of graphene-capped InAs/GaAs quantum dots. J. Appl. Phys. 2014, 115, 214309. [Google Scholar] [CrossRef]
- Shang, J.; Ma, L.; Li, J.; Ai, W.; Yu, T.; Gurzadyan, G.G. The Origin of Fluorescence from Graphene Oxide. Sci. Rep. 2012, 2, 792. [Google Scholar] [CrossRef]
- End de Oliveira, V.; Neves Miranda, M.A.; Carolina Silva Soares, M.; Edwards, H.G.; Fernando Cappa de Oliveira, L. Study of carotenoids in cyanobacteria by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 373–380. [Google Scholar] [CrossRef]
- Maia, L.F.; de Oliveira, V.E.; de Oliveira, M.E.R.; Fleury, B.G.; de Oliveira, L.F.C. Polyenic pigments from the Brazilian octocoral Phyllogorgia dilatata Esper, 1806 characterized by Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 161–164. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to Increasing the Salt Tolerance of Wheat and Other Cereals. In Proceedings of the Journal of Experimental Botany; Oxford Academic: Oxford, UK, 2006; Volume 57, pp. 1025–1043. [Google Scholar]
- Kader, M.A.; Lindberg, S. Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal. Behav. 2010, 5, 233–238. [Google Scholar] [CrossRef]
- Rubio, F.; Gassmann, W.; Schroeder, J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 1995, 270, 1660–1663. [Google Scholar] [CrossRef]
- Gadwal, R.; Naik, G.R. A Comparative Study on the Effect of salt stress on seed germination and early seedling growth of two Hibiscus species. IOSR J. Agric. Vet. Sci. 2014, 7, 90–96. [Google Scholar] [CrossRef]
- Lal, S.; Kumar, R. Exploration of heavy metal resistant rhizobacteria Enterobacter cloacae PC3 to enhance growth and metal remediation potential of Zea mays L. under Cd and Pb stress. Int. J. Emerg. Technol. Innov. Res. 2019, 6, 576–587. [Google Scholar]
- Lal, S.; Kumar, R.; Ahmad, S.; Dixit, V.K.; Berta, G. Exploring the survival tactics and plant growth promising traits of root-associated bacterial strains under Cd and Pb stress: A modelling based approach. Ecotoxicol. Env. Saf. 2019, 170, 267–277. [Google Scholar] [CrossRef]
- Prasanna, R.; Sood, A.; Jaiswal, P.; Nayak, S.; Gupta, V.; Chaudhary, V.; Joshi, M.; Natarajan, C. Rediscovering cyanobacteria as valuable sources of bioactive compounds (Review). Appl. Biochem. Microbiol. 2010, 46, 119–134. [Google Scholar] [CrossRef]
- Almeida, A. Physiological Performance of Wheat and Barley Seeds Treated with Bioactivator. Am. J. Exp. Agric. 2012, 2, 90–101. [Google Scholar] [CrossRef]
- Yusuf, M.; Hayat, S.; Alyemeni, M.N.; Fariduddin, Q.; Ahmad, A. Physiological Roles in Plants. In Salicylic Acid; Springer: Amsterdam, The Netherlands, 2013; pp. 15–30. [Google Scholar]
- Stout, M.J.; Thaler, J.S.; Thomma, B.P.H.J. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Larqué-Saavedra, A.; Martin-Mex, R. Effects of salicylic acid on the bioproductivity of plants. In Salicylic Acid: A Plant Hormone; Springer: Amsterdam, The Netherlands, 2007; pp. 15–23. [Google Scholar]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Žižková, E.; Kubeš, M.; Dobrev, P.I.; Přibyl, P.; Šimura, J.; Zahajská, L.; Drábková, L.Z.; Novák, O.; Motyka, V. Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Ann. Bot. 2017, 119, 151–166. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Inamuddin; Asiri, A.M. Carbon nanotube—And graphene-based advanced membrane materials for desalination. Environ. Chem. Lett. 2017, 15, 643–671. [Google Scholar] [CrossRef]
- Younis, N.S.; Bakir, E.M.; Mohamed, M.E.; El Semary, N.A. Cyanobacteria as Nanogold Factories II: Chemical Reactivity and anti-Myocardial Infraction Properties of Customized Gold Nanoparticles Biosynthesized by Cyanothece sp. Mar. Drugs 2019, 17, 402. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Eroglu, E.; Chen, X.; Smith, S.; Raston, C. Entrapment of Chlorella vulgaris cells within graphene oxide layers. RSC Adv. 2013, 3, 8180–8183. [Google Scholar] [CrossRef]
- Padorf, M.; Pourzahedi, L.; Gilbertson, L.; Lowry, G.V.; Herckes, P.; Westerhoff, P. Graphite nanoparticle addition to fertilizers reduces nitrate leaching in growth of lettuce (Lactuca sativa). Environ. Sci. 2020, 7, 127–138. [Google Scholar]
- Hafeez, M.; Ramteke, P.; Lawrence, R.; Suresh, B.G.; Kumari, S.; Singh, A.; Singla, A.; Paul, A.; Masih, S.; Masih, H.; et al. Bio-formulation of Halotolerant Phosphate Solubilizing Enterobacter cloacae HFZ-H4 Strain to Screen Different Carrier Materials and their Shelf Life Study. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2373–2380. [Google Scholar] [CrossRef][Green Version]
| Type of Seed | Salinity Treatment MPa | Biofertilizer Treatment | Radicle Length (cm) | Plumule Length (cm) | Fresh Weight (g) | Dry Weight (g) | Germination% |
|---|---|---|---|---|---|---|---|
| Barley | 0 | 0 | 0.69 (0.09) | 1.59 (0.09) | 0.09 (0.02) | 0.02 (0.01) | 50% |
| Alga | 10.05 (3.34) | 14.64 (4.10) | 0.21 (0.10) | 0.033 (0.01) | 90% | ||
| Bacterium | 9.77 (2.1) | 15.55 (3.8) | 0.17 (0.07) | 0.032 (0.00) | 100% | ||
| Bacterium + Alga | 9.30 (3.1) | 18.48 (3.04) | 0.23 (0.05) | 0.032 (0.01) | 100% | ||
| Bacterium + Alga + MeSA | 12.12 (3.6) | 14.84 (2.43) | 0.19 (0.02) | 0.0260 0.00) | 100% | ||
| −1 | 0 | 0.65 (0.09) | 0.52 (0.09) | 0.08 (0.01) | 0.037 (0.00) | 70% | |
| Alga | 1.14 (1.5) | 0.54 (0.04) | 0.09 (0.03) | 0.034 (0.00) | 80% | ||
| Bacterium | 2.0 (0.49) | 1.95 (1.09) | 0.09 (0.01) | 0.031 (0.00) | 60% | ||
| Bacterium + Alga | 2.35 (1.05) | 2.19 (0.9) | 0.10 (0.03) | 0.031 (0.00) | 70% | ||
| Bacterium + Alga + MeSA | 3.45 (1.3) | 4.7 (1.7) | 0.12 (0.05) | 0.024 (0.00) | 100% | ||
| −3 | 0 | 1.02 (0.5) | 0.15 (0.06) | 0.09 (0.03) | 0.034 (0.01) | 90% | |
| Algae | 1.22 (0.3) | 0.14 (0.02) | 0.09 (0.03) | 0.037 (0.01) | 70% | ||
| Bacteria | 1.0 (0.8) | 0.04 (0.05) | 0.078 (0.06) | 0.038 (0.01) | 60% | ||
| Bacterium + Alga | 3.62 (1.85) | 0.21 (0.01) | 0.089 (0.05) | 0.037 (0.00) | 90% | ||
| Bacterium + Alga + MeSA | 5.12 (0.88) | 1.15 (0.43) | 0.08 (0.00) | 0.36 (0.03) | 80% | ||
| −5 | 0 | 0.2 (0.03) | 0 (0) | 0.065 (0.02) | 0.039 (0.00) | 60% | |
| Alga | 1.0 (0.09) | 0. 8 (0.03) | 0.079 (0.03) | 0.0331 (0.01) | 70% | ||
| Bacterium | 0.43 (0.3) | 0.4 (0.00) | 0.081 (0.03) | 0.036 (0.00) | 60% | ||
| Bacterium + Alga | 0.29 (0.17) | 0.4 (0.00) | 0.078 (0.03) | 0.04 (0.01) | 60% | ||
| Bacterium + Alga + MeSA | 0.28 (0.20) | 0.6 (0.01) | 0.064 (0.01) | 0.03 (0.00) | 70% | ||
| Broad beans | 0 | 0 | 3.45 (1.3) | 2.57 (1.3) | 2.425 (0.9) | 1.10 (0.04) | 80% |
| Alga | 6.95 (3.3) | 7.22 (1.1) | 3.85 (0.05) | 0.77 (0.38) | 90% | ||
| Bacterium | 4.90 (1.1) | 4.98 (1.3) | 3.85 (0.05) | 0.73 (0.38) | 100% | ||
| Bacterium + Alga | 5.62 (0.78) | 7.67 (1.08) | 3.1 (1.4) | 0.72 (0.17) | 100% | ||
| Bacteria + Algae + MeSA | 7.95 (1.20) | 8.59 (3.4) | 3.8 (1. 6) | 0.70 (0.2) | 90% | ||
| −1 | 0 | 0.33 (0.06) | 0 (0) | 1.84 (0.64) | 0.98 (0.14) | 50% | |
| Alga | 0.28 (0.08) | 0.13 (0.04) | 1.75 (0.64) | 0.73 (0.15) | 40% | ||
| Bacterium | 0.55 (0.07) | 0.24 (0. 04) | 1.73 (0.18) | 0.75 (0.10) | 50% | ||
| Bacterium + Alga | 0.21 (0.02) | 0.24 (0.05) | 1.74 (0.9) | 0.75 (0.23) | 60% | ||
| Bacterium + Alga + MeSA | 1.93 (0.84) | 1.37 (0.92) | 1.88 (0.24) | 0.74 (0.04) | 40% | ||
| −3 | 0 | 0.05 (0.01) | 0 (0) | 1.5 (0.33) | 0.87 (0.20) | 10% | |
| Alga | 0 (0) | 0 (0) | 1.6 (0.30) | 0.78 (0.17) | 0 | ||
| Bacterium | 0 (0) | 0 (0) | 1.56 (0.04) | 0.75 (0.07) | 0 | ||
| Bacterium + Alga | 0.26 (0.78) | 0 (0) | 1.52 (0.60) | 0.71 (0.20) | 0 | ||
| Bacterium + Alga + MeSA | 0.26 (0.08) | 0 (0) | 1.4 (0.20) | 0.72 (0.03) | 20% | ||
| −5 | 0 | 0 (0) | 0 (0) | 1.41 (0.3) | 0.85 (0) | 0 | |
| Alga | 0 (0) | 0 (0) | 1.59 (0.14) | 0.78 (0.14) | 0 | ||
| Bacterium | 0 (0) | 0 (0) | 1.60 (0.14) | 0.77 (0.14) | 0 | ||
| Bacterium + Alga | 0 (0) | 0 (0) | 1.45 (0.19) | 0.80 (0.18) | 0 | ||
| Bacterium + Alga + MeSA | 0 (0) | 0 (0) | 1.45 (0.03) | 0.74 (0.06) | 0 |
| Type of Seed | Salinity Treatment MPa | Biofertilizer Treatment | Radicle Length (cm) | Plumule Length (cm) | Fresh Weight (g) | Dry Weight (g) | Germination % |
|---|---|---|---|---|---|---|---|
| Barley | 0 | 0 | 0 (0) | 0 (0) | 0.22 (0.0) | 0.042 (0.0) | 0% |
| Alga | 1.02 (0.17) | 2.02 (0.5) | 0.64 (0.1) | 0.06 (0.0) | 20% | ||
| Bacterium | 0.06 (0. 03) | 0.1 (0.07) | 0.07 (0.03) | 0.05 (0.02) | 20% | ||
| Bacterium + Alga | 0 (0) | 0 (0) | 0.077 (0.03) | 0.058 (0.04) | 0% | ||
| Bacterium + Alga + MeSA | 2.43 (1.2) | 3.33 (1.7) | 0.075 (0.01) | 0.049 (0.01) | 20% | ||
| −1 | 0 | 0 (0) | 0 (0) | 0.058 (0.02) | 0.041 (0.007) | 0% | |
| Alga | 0 (0) | 0 (0) | 0.065 (0.03) | 0.041 (0.01) | 0% | ||
| Bacterium | 0 (0) | 0 (0) | 0.082 (0.012) | 0.045 (0.018) | 0% | ||
| Bacterium + Alga | 0.05 (0.01) | 0 (0) | 0.067 (0.007) | 0.039 (0.007) | 10% | ||
| Bacterium + Alga + MeSA | 0.1 (0.03) | 0.09 (0.01) | 0.074 (0.03) | 0.044 (0.01) | 30% | ||
| −3 | 0 | 0 (0) | 0 (0) | 0.053 (0.007) | 0.041 (0.007) | 0% | |
| Alga | 0 (0) | 0 (0) | 0.065 (0.007) | 0.044 (0.028) | 0% | ||
| Bacterium | 0 (0) | 0 (0) | 0.070 (0.01) | 0.049 (0.01) | 0% | ||
| Bacterium + Alga | 0 (0) | 0 (0) | 0.066 (0.03) | 0.045 (0.003) | 0% | ||
| Bacterium + Alga + MeSA | 0 (0) | 0 (0) | 0.07 (0.01) | 0.04 (0.01) | 0% | ||
| −5 | 0 | 0 (0) | 0 (0) | 0.063 (0.01) | 0.045 (0.00) | 0% | |
| Alga | 0 (0) | 0 (0) | 0.065 (0.00) | 0.046 (0.00) | 0% | ||
| Bacterium | 0 (0) | 0 (0) | 0.0612 (0.007) | 0.042 (0.01) | 0% | ||
| Bacterium + Alga | 0 (0) | 0 (0) | 0.064 (0.02) | 0.05 (0.04) | 0% | ||
| Bacterium + Alga + MeSA | 0 (0) | 0 (0) | 0.06 (0.01) | 0.045 (0.01) | 0% | ||
| Broad beans | 0 | 0 | 0.69 (0.09) | 0.53 (0.05) | 1.95 (0.7) | 0.652 (0.2) | 80% |
| Alga | 1.29 (0.07) | 1.34 (0.04) | 1.72 (0.04) | 0.70 (0.04) | 100% | ||
| Bacterium | 0.82 (0.08) | 0.91 (0.08) | 1.88 (0.06) | 0.777 (0. 05) | 100% | ||
| Bacterium + Alga | 1.56 (0.94) | 1.39 (0.50) | 1.83 (0.3) | 0.74 (0.2) | 100% | ||
| Bacterium + Alga + MeSA | 1.58 (0.08) | 1.32 (0.4) | 1.73 (0.6) | 0.73 (0.09) | 90% | ||
| −1 | 0 | 0.23 (0.02) | 0(0) | 1.631 (0.8) | 0.6453 (0.08) | 60% | |
| Alga | 0.46 (0.05) | 0.20 (0.06) | 1.65 (0.2) | 0.76 (0.1) | 70% | ||
| Bacterium | 0.42 (0.18) | 0 (0) | 1.60 (0.4) | 0.66 (0.2) | 80% | ||
| Bacterium + Alga | 0.48 (0.15) | 0.15 (0.02) | 1.501 (0.01) | 0.63 (0.15) | 70% | ||
| Bacterium + Alga + MeSA | 0.52 (0.15) | 0 (0) | 1.5 (0.9) | 0.70 (0.3) | 80% | ||
| −3 | 0 | 0 (0) | 0 (0) | 1.43 (0.9) | 0.68 (0.09) | 0 | |
| Alga | 0 (0) | 0 (0) | 1.536 (0.0) | 0.784 (0.2) | 0 | ||
| Bacterium | 0 (0) | 0 (0) | 1.6 (0.0) | 0.95 (0.1) | 0 | ||
| Bacterium + Alga | 0 (0) | 0 (0) | 1.39 (0.24) | 0.94 (0.3) | 0 | ||
| Bacterium + Alga +MeSA | 0 (0) | 0 (0) | 1.559 (0.2) | 0.99 (0.19) | 0 | ||
| −5 | 0 | 0 (0) | 0 (0) | 1.41 (0.03) | 0.67 (0.03) | 0 | |
| Alga | 0 (0) | 0 (0) | 1.36 (0.6) | 1.1 (0.5) | 0 | ||
| Bacterium | 0 (0) | 0 (0) | 1.39 (0.06) | 1.10 (0.02) | 0 | ||
| Bacterium + Alga | 0 (0) | 0 (0) | 1.32 (0.43) | 1.04 (0.36) | 0 | ||
| Bacterium + Alga + MeSA | 0 (0) | 0 (0) | 1.22 (0.04) | 0.975 (0.02) | 0 |
| Salinity Treatment MPa | Type of Seed | Treatment [Nanomaterial (Concentration mg/mL)/Biofertilizer Treatment] | Radicle Length (cm) | Plumule Length (cm) | Fresh Wt. (g) | Dry Wt. (g) | Germination % |
|---|---|---|---|---|---|---|---|
| −5 | Barley | GO (1) [Bacterium + Alga + MeSA] | 4.8 | 4.2 | 0.094 | 0.047 | 60% |
| GO (2) [Bacterium + Alga + MeSA] | 5.8 | 3.0 | 0.096 | 0.047 | 70% | ||
| CNT (0.8) [Bacterium + Alga + MeSA] | 1.2 | 2.0 | 0.085 | 0.048 | 40% | ||
| GR (0.8) [Bacterium + Alga + MeSA] | 0.88 | 0.76 | 0.092 | 0.040 | 70% | ||
| Broad beans | GO (1) [Bacterium + Alga + MeSA] | 0 | 0 | 1.546 | 1.136 | 0% | |
| GO (2) [Bacterium + Alga + MeSA] | 0 | 0 | 1.724 | 1.400 | 0% | ||
| CNT (0.8) [Bacterium + Alga + MeSA] | 1.0 | 0 | 1.630 | 1.226 | 10% | ||
| GR (0.8) [Bacterium + Alga + MeSA] | 1.8 | 0 | 1.529 | 1.144 | 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Semary, N.A.H.; Alouane, M.H.H.; Nasr, O.; Aldayel, M.F.; Alhaweti, F.H.; Ahmed, F. Salinity Stress Mitigation Using Encapsulated Biofertilizers for Sustainable Agriculture. Sustainability 2020, 12, 9218. https://doi.org/10.3390/su12219218
El Semary NAH, Alouane MHH, Nasr O, Aldayel MF, Alhaweti FH, Ahmed F. Salinity Stress Mitigation Using Encapsulated Biofertilizers for Sustainable Agriculture. Sustainability. 2020; 12(21):9218. https://doi.org/10.3390/su12219218
Chicago/Turabian StyleEl Semary, Nermin Adel Hussein, Mohamed Helmi Hadj Alouane, Olfa Nasr, Munirah F. Aldayel, Fatimah H. Alhaweti, and Faheem Ahmed. 2020. "Salinity Stress Mitigation Using Encapsulated Biofertilizers for Sustainable Agriculture" Sustainability 12, no. 21: 9218. https://doi.org/10.3390/su12219218
APA StyleEl Semary, N. A. H., Alouane, M. H. H., Nasr, O., Aldayel, M. F., Alhaweti, F. H., & Ahmed, F. (2020). Salinity Stress Mitigation Using Encapsulated Biofertilizers for Sustainable Agriculture. Sustainability, 12(21), 9218. https://doi.org/10.3390/su12219218






