Trade-Offs between Sugarcane Straw Removal and Soil Organic Matter in Brazil
Abstract
:1. Introduction
2. Materials and Methods
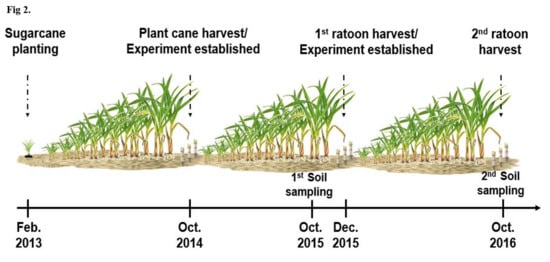
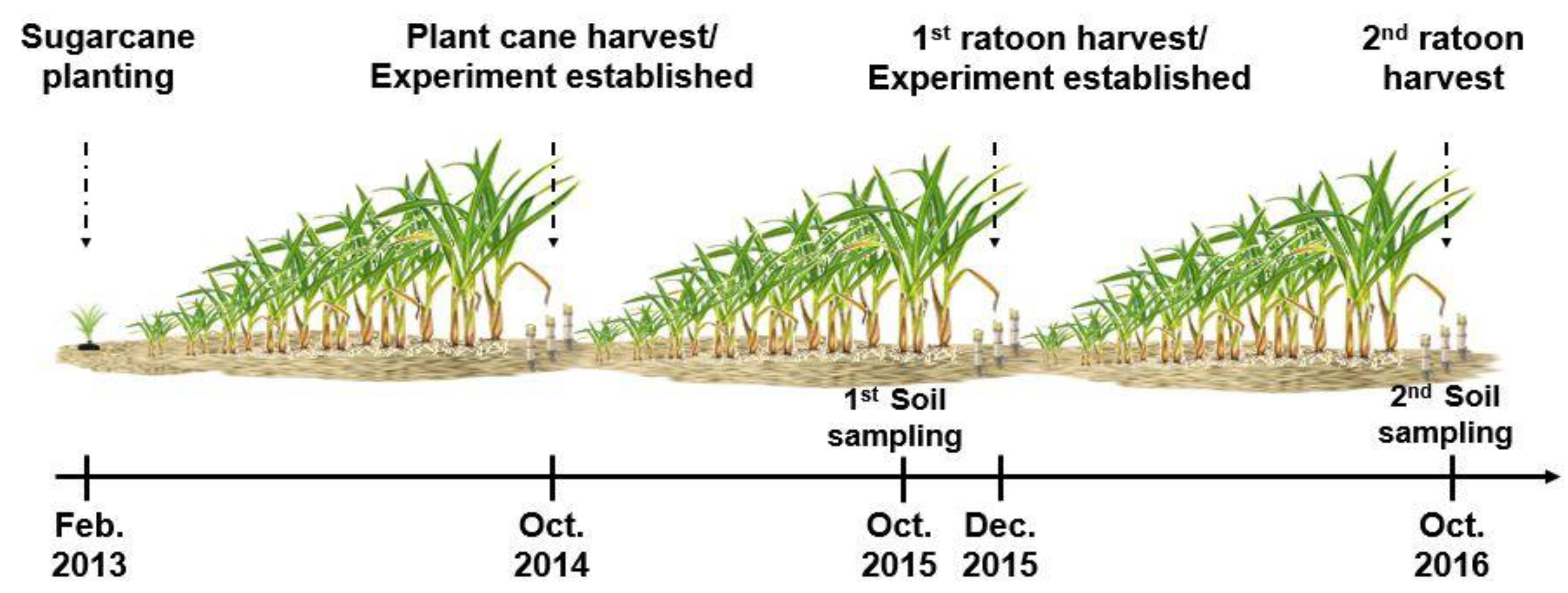
2.1. Study Area and Experimental Design
2.2. Soil Sampling and Analysis
2.3. Statistical Analysis
3. Results
3.1. Soil Carbon and Nitrogen Concentration and Stocks
3.2. Soil Carbon and Nitrogen Indexes
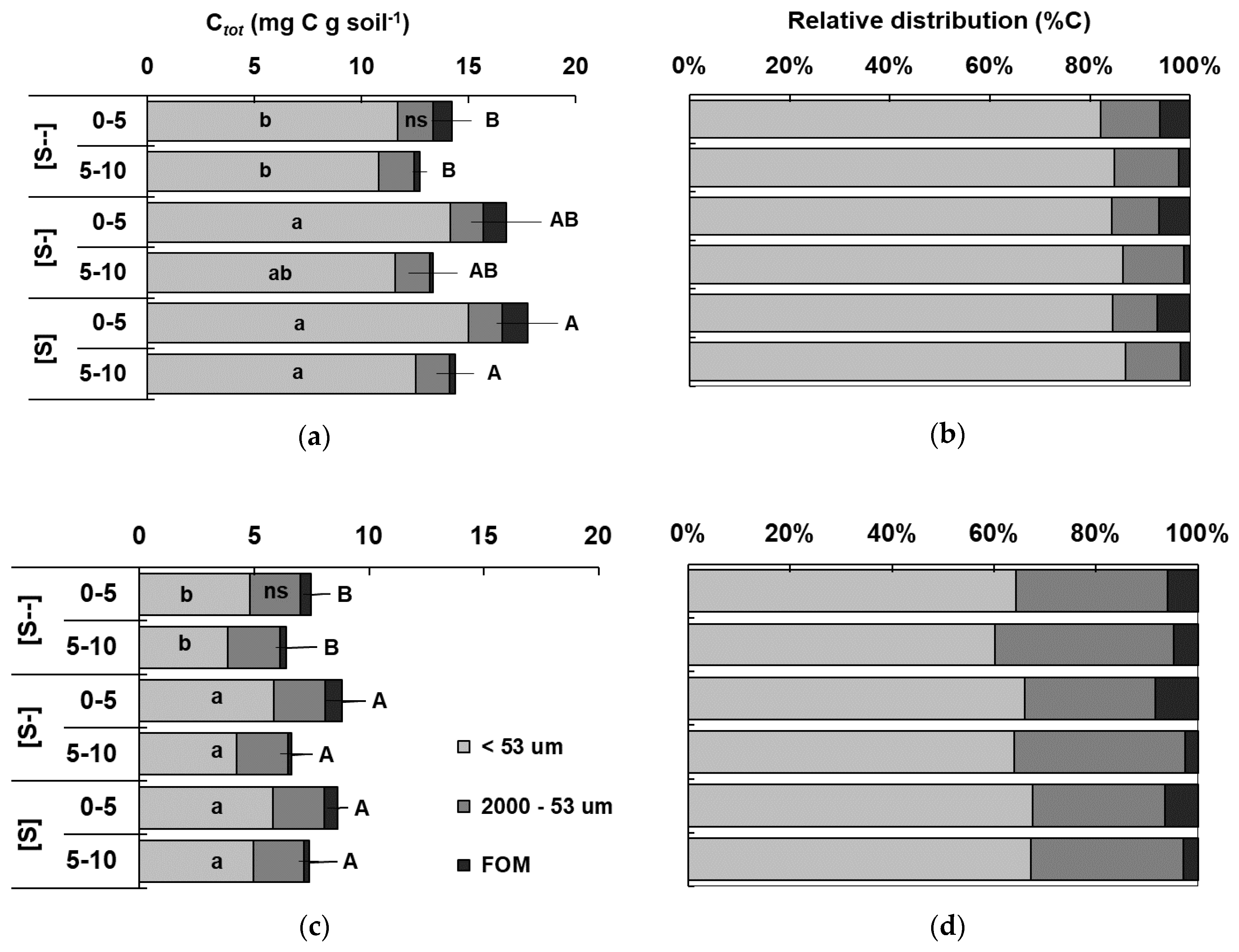
3.3. Soil Physical Fractionation of Soil Organic Matter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging renewable and sustainable energy technologies: State of the art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Global Energy & CO2 Status Report 2019. Available online: https://www.iea.org/reports/global-energy-co2-status-report-2019 (accessed on 7 August 2020).
- Heck, V.; Gerten, D.; Lucht, W.; Popp, A. Biomass-based negative emissions difficult to reconcile with planetary boundaries. Nat. Clim. Chang. 2018, 8, 151–155. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Cana-de-Açúcar. Available online: http://www.conab.gov.br (accessed on 30 July 2020).
- Carvalho, J.L.N.; Nogueirol, R.C.; Menandro, L.M.S.; Bordonal, R.D.O.; Borges, C.D.; Cantarella, H.; Franco, H.C.J. Agronomic and environmental implications of sugarcane straw removal: A major review. GCB Bioenergy 2017, 9, 1181–1195. [Google Scholar] [CrossRef]
- Barbosa, C.M.G.; Terra-Filho, M.; De Albuquerque, A.L.P.; Di Giorgi, D.; Grupi, C.; Negrão, C.E.; Rondon, M.U.P.B.; Martinez, D.G.; Marcourakis, T.; Dos Santos, F.A.; et al. Burnt Sugarcane Harvesting—Cardiovascular Effects on a Group of Healthy Workers, Brazil. PLoS ONE 2012, 7, e46142. [Google Scholar] [CrossRef]
- Aguiar, D.A.; Rudorff, B.F.T.; Silva, W.F.; Adami, M.; Mello, M.P. Remote Sensing Images in Support of Environmental Protocol: Monitoring the Sugarcane Harvest in São Paulo State, Brazil. Remote Sens. 2011, 3, 2682–2703. [Google Scholar] [CrossRef] [Green Version]
- Bordonal, R.D.O.; Carvalho, J.L.N.; Lal, R.; De Figueiredo, E.B.; De Oliveira, B.G.; La Scala, N. Sustainability of sugarcane production in Brazil. A review. Agron. Sustain. Dev. 2018, 38, 13. [Google Scholar] [CrossRef] [Green Version]
- Cherubin, M.R.; Oliveira, D.M.S.; Feigl, B.J.; Pimentel, L.G.; Lisboa, I.P.; Gmach, M.R.; Varanda, L.L.; Morais, M.C.; Satiro, L.S.; Popin, G.V.; et al. Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: A review. Sci. Agric. 2018, 75, 255–272. [Google Scholar] [CrossRef] [Green Version]
- Menandro, L.M.S.; Cantarella, H.; Franco, H.C.J.; Kölln, O.T.; Pimenta, M.T.B.; Sanches, G.M.; Rabelo, S.C.; Carvalho, J.L.N. Comprehensive assessment of sugarcane straw: Implications for biomass and bioenergy production. Biofuels Bioprod. Biorefin. 2017, 11, 488–504. [Google Scholar] [CrossRef]
- Galdos, M.V.; Cantarella, H.; Hastings, A.; Hillier, J.; Smith, P. Environmental sustainability aspects of second generation ethanol production from sugarcane. In Advances of Basic Science for Second Generation Bioethanol from Sugarcane; Buckeridge, M., De Souza, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 177–195. [Google Scholar]
- Fortes, C.; Trivelin, P.C.O.; Vitti, A.C. Long-term decomposition of sugarcane harvest residues in Sao Paulo state, Brazil. Biomass Bioenergy 2012, 42, 189–198. [Google Scholar] [CrossRef]
- De Sousa, G.B.; Filho, M.V.M.; Matias, S.S.R. Soil, organic matter and nutrients losses by water erosion in a slope with sugarcane straw, in Guariba, State of São Paulo. Eng. Agric. 2012, 32, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.A.; Telles, T.S.; Machado, W.; Hungria, M.; Filho, J.T.; Guimarães, M.D.F. Effects of sugarcane harvesting with burning on the chemical and microbiological properties of the soil. Agric. Ecosyst. Environ. 2012, 155, 1–6. [Google Scholar] [CrossRef]
- dos Anjos, J.C.R.; de Andrade Júnior, A.S.; Bastos, E.A.; Noleto, D.H.; de Brito Melo, F.; De Brito, R.R. Water storage in a Plinthaqualf cultivated with sugarcane under straw levels. Pesqui. Agropecu. Bras. 2017, 52, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, S.T.R.; Barbosa, L.C.; Menandro, L.M.S.; Scarpare, F.V.; Reichardt, K.; De Moraes, L.O.; Hernandes, T.A.D.; Franco, H.C.J.; Carvalho, J.L.N. Straw Removal Effects on Soil Water Dynamics, Soil Temperature, and Sugarcane Yield in South-Central Brazil. BioEnergy Res. 2019, 12, 749–763. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Hudiburg, T.W.; Franco, H.C.J.; DeLucia, E.H. Contribution of above- and belowground bioenergy crop residues to soil carbon. GCB Bioenergy 2017, 9, 1333–1343. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, R. Managing Soils and Ecosystems for Mitigating Anthropogenic Carbon Emissions and Advancing Global Food Security. Bioscience 2010, 60, 708–721. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Soil Organic Carbon: The Hidden Potential; FAO: Rome, Italy, 2017. [Google Scholar]
- Hoffland, E.; Kuyper, T.W.; Comans, R.N.; Creamer, R. Eco-functionality of organic matter in soils. Plant Soil 2020, 455, 1–22. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.U.; Lang, B.; Von Lützow, M.; Marin-Spiotta, E.; Van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Crop Residue Removal Impacts on Soil Productivity and Environmental Quality. Crit. Rev. Plant Sci. 2009, 28, 139–163. [Google Scholar] [CrossRef]
- Rowe, R.L.; Keith, A.M.; Elias, D.M.O.; McNamara, N.P. Soil carbon stock impacts following reversion of Miscanthus × giganteus and short rotation coppice willow commercial plantations into arable cropping. GCB Bioenergy 2020, 12, 680–693. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi-Filho, A.; Andrade, D.S.; Dick, R.P. Microbial biomass in soils under different tillage and crop rotation systems. Biol. Fertil. Soils 2003, 38, 15–20. [Google Scholar] [CrossRef]
- Lupwayi, N.; Rice, W.; Clayton, G. Soil microbial diversity and community structure under wheat as influenced by tillage and crop rotation. Can. J. Soil Sci. 1999, 79, 273–280. [Google Scholar] [CrossRef]
- Fischer, H.; Ingwersen, J.; Kuzyakov, Y. Microbial uptake of low-molecular-weight organic substances out-competes sorption in soil. Eur. J. Soil Sci. 2010, 61, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2019, 26, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparling, G. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Ladha, J.K.; Lefroy, R.D. Carbon management for sustainability of an intensively managed rice-based cropping system. Biol. Fertil. Soils 2002, 36, 215–223. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Liu, X.; Zhao, X.; Lu, D.; Zhou, J.; Li, C. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice–wheat cropping system. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Lisboa, I.P.; Cherubin, M.R.; Cerri, C.E.P.; Cerri, D.G.; Cerri, C.E.P. Guidelines for the recovery of sugarcane straw from the field during harvesting. Biomass Bioenergy 2017, 96, 69–74. [Google Scholar] [CrossRef]
- Vance, E.; Brookes, P.; Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Sparling, G.; West, A. A direct extraction method to estimate soil microbial C: Calibration in situ using microbial respiration and 14C labelled cells. Soil Biol. Biochem. 1988, 20, 337–343. [Google Scholar] [CrossRef]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Lee, J.; Hopmans, J.W.; Rolston, D.E.; Baer, S.G.; Six, J. Determining soil carbon stock changes: Simple bulk density corrections fail. Agric. Ecosyst. Environ. 2009, 134, 251–256. [Google Scholar] [CrossRef]
- Signor, D.; Zani, C.F.; Paladini, A.A.; Deon, M.D.; Cerri, C.E.P. Estoques de carbono e qualidade da matéria orgânica do solo em áreas cultivadas com cana-de-açúcar. Rev. Bras. Cienc. Solo 2014, 38, 1402–1410. [Google Scholar] [CrossRef] [Green Version]
- Christensen, B.T. Physical Fractionation of Soil and Organic Matter in Primary Particle Size and Density Separates. Adv. Soil Sci. 1992, 20, 1–77. [Google Scholar] [CrossRef]
- Smith, W.; Grant, B.; Campbell, C.; McConkey, B.G.; Desjardins, R.L.; Kröbel, R.; Malhi, S. Crop residue removal effects on soil carbon: Measured and inter-model comparisons. Agric. Ecosyst. Environ. 2012, 161, 27–38. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Otto, R.; Franco, H.C.J.; Trivelin, P.C. Input of sugarcane post-harvest residues into the soil. Sci. Agric. 2013, 70, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Thorburn, P.; Meier, E.; Collins, K.; Robertson, F. Changes in soil carbon sequestration, fractionation and soil fertility in response to sugarcane residue retention are site-specific. Soil Tillage Res. 2012, 120, 99–111. [Google Scholar] [CrossRef]
- Xu, H.; Sieverding, H.; Kwon, H.; Clay, D.; Stewart, C.; Johnson, J.M.F.; Qin, Z.; Karlen, D.L.; Wang, M. A global meta-analysis of soil organic carbon response to corn stover removal. GCB Bioenergy 2019, 11, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Robertson, F.A.; Thorburn, P. Decomposition of sugarcane harvest residue in different climatic zones. Aust. J. Soil Res. 2007, 45, 1–11. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Soil structure and organic carbon relationships following 10 years of wheat straw management in no-till. Soil Tillage Res. 2007, 95, 240–254. [Google Scholar] [CrossRef]
- Silva, A.G.B.; Lisboa, I.P.; Cherubin, M.R.; Cerri, C.E.P. How Much Sugarcane Straw is Needed for Covering the Soil? BioEnergy Res. 2019, 12, 858–864. [Google Scholar] [CrossRef]
- Silva, A.P.; Babujia, L.C.; Dos Santos, J.C.F.; Ralisch, R.; Hungria, M.; Guimarães, M.D.F. Soil structure and its influence on microbial biomass in different soil and crop management systems. Soil Tillage Res. 2014, 142, 42–53. [Google Scholar] [CrossRef]
- Tormena, C.A.; Karlen, D.L.; Logsdon, S.; Cherubin, M.R. Visual Soil Structure Effects of Tillage and Corn Stover Harvest in Iowa. Soil Sci. Soc. Am. J. 2016, 80, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Castioni, G.A.; Cherubin, M.R.; Menandro, L.M.S.; Sanches, G.M.; Bordonal, R.D.O.; Barbosa, L.C.; Franco, H.C.J.; Carvalho, J.L.N. Soil physical quality response to sugarcane straw removal in Brazil: A multi-approach assessment. Soil Tillage Res. 2018, 184, 301–309. [Google Scholar] [CrossRef]
- Akinsete, S.J.; Nkongolo, N. Soil Carbon and Nitrogen Fractions of a Grassland in Central Missouri, USA. Commun. Soil Sci. Plant Anal. 2016, 47, 1128–1136. [Google Scholar] [CrossRef]
- Brandani, C.B.; Abbruzzini, T.F.; Conant, R.T.; Cerri, C.E.P. Soil organic and organomineral fractions as indicators of the effects of land management in conventional and organic sugar cane systems. Soil Res. 2016, 55, 145. [Google Scholar] [CrossRef]
- Pimentel, L.G.; Cherubin, M.R.; Oliveira, D.M.; Cerri, C.E.; Cerri, C.C. Decomposition of sugarcane straw: Basis for management decisions for bioenergy production. Biomass Bioenergy 2019, 122, 133–144. [Google Scholar] [CrossRef]
- Vasconcelos, A.L.S.; Cherubin, M.R.; Feigl, B.J.; Cerri, C.E.; Gmach, M.R.; Siqueira-Neto, M. Greenhouse gas emission responses to sugarcane straw removal. Biomass Bioenergy 2018, 113, 15–21. [Google Scholar] [CrossRef]
- Oliveira, D.M.D.S.; Williams, S.; Cerri, C.E.P.; Paustian, K. Predicting soil C changes over sugarcane expansion in Brazil using the DayCent model. GCB Bioenergy 2017, 9, 1436–1446. [Google Scholar] [CrossRef] [Green Version]
- Di Lonardo, D.P.; De Boer, W.; Zweers, H.; Van Der Wal, A. Effect of the amount of organic trigger compounds, nitrogen and soil microbial biomass on the magnitude of priming of soil organic matter. PLoS ONE 2019, 14, e0216730. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.M.; Assunção, S.A.; De Oliveira, A.P.P.; Dos Anjos, L.H.C.; Pereira, M.G.; Lima, E. Carbon fractions and soil fertility affected by tillage and sugarcane residue management an Xanthic Udult. Semin. Ciênc. Agrar. 2017, 38, 2921. [Google Scholar] [CrossRef] [Green Version]



| Local | Clay | Silt | Sand | C | Bulk Density | pH | Ca | Mg | K | BS | CEC | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | g cm−3 | mmolc kg−1 | mg kg−1 | |||||||||
| Oxisol | 330 | 60 | 610 | 11.3 | 1.32 | 5.2 | 26.1 | 7.7 | 9.3 | 68.8 | 62.6 | 29.3 |
| Ultisol | 112 | 23 | 865 1 | 6.2 2 | 1.51 | 5.2 3 | 9.3 | 2.9 | 3.3 4 | 51.1 | 30.2 | 17.4 5 |
| Treatments | Oxisol | Ultisol |
|---|---|---|
| Total removal [S--] | 0 | 0 |
| Moderate removal [S-] | 8.7 | 10.2 |
| No removal [S] | 18.9 | 16.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, M.C.; Siqueira-Neto, M.; Guerra, H.P.; Satiro, L.S.; Soltangheisi, A.; Cerri, C.E.P.; Feigl, B.J.; Cherubin, M.R. Trade-Offs between Sugarcane Straw Removal and Soil Organic Matter in Brazil. Sustainability 2020, 12, 9363. https://doi.org/10.3390/su12229363
Morais MC, Siqueira-Neto M, Guerra HP, Satiro LS, Soltangheisi A, Cerri CEP, Feigl BJ, Cherubin MR. Trade-Offs between Sugarcane Straw Removal and Soil Organic Matter in Brazil. Sustainability. 2020; 12(22):9363. https://doi.org/10.3390/su12229363
Chicago/Turabian StyleMorais, Maristela C., Marcos Siqueira-Neto, Henrique P. Guerra, Lucas S. Satiro, Amin Soltangheisi, Carlos E. P. Cerri, Brigitte J. Feigl, and Maurício R. Cherubin. 2020. "Trade-Offs between Sugarcane Straw Removal and Soil Organic Matter in Brazil" Sustainability 12, no. 22: 9363. https://doi.org/10.3390/su12229363
APA StyleMorais, M. C., Siqueira-Neto, M., Guerra, H. P., Satiro, L. S., Soltangheisi, A., Cerri, C. E. P., Feigl, B. J., & Cherubin, M. R. (2020). Trade-Offs between Sugarcane Straw Removal and Soil Organic Matter in Brazil. Sustainability, 12(22), 9363. https://doi.org/10.3390/su12229363