Abstract
Industrial latex sludge as raw material was made into sulfonated latex sludge (SLS) and latex sludge active carbon (LSC) adsorbents by sulfonate and pyrolysis treatment to remove textile dye cationic blue X-GRRL from aqueous solution. The adsorption properties of SLS and LSC for X-GRRL were studied and compared by investigating the experimental parameters such as adsorbents dosage, pH, contact time and initial concentration. The kinetics of adsorption on SLS and LSC followed the pseudo-second-order kinetic model well. The adsorption isotherm and thermodynamic studies were further used to evaluate and compare the adsorption process of X-GRRL on SLS and LSC. The maximum adsorption capacities were 1219.6 mg/g for SLS and 476.2 mg/g for LSC according to the Langmuir model, respectively. These findings not only provide a sustainable strategy to turn industrial solid waste latex sludge into useful material for environment remediation, but also develop an efficient adsorbent for the treatment of dye wastewater.
1. Introduction
Considering the scarce water resource and the essential survival demand, the issues of water contamination and management have attracted increasing attention [1]. The 2017 edition of the United Nations World Water Development Report pointed out that the improved wastewater management will generate environmental, social and economic benefits and be critical to achieve the 2030 Agenda for sustainable development [2]. To date, industrial wastewater is still regarded as one of the main water pollution sources. Therefore, much effort has been devoted to improving water quality by reducing contaminants at the source, recovering useful industrial by-products, and removing pollution from wastewater flows.
Dyes, extensively used in textiles, dyeing, printing, pulp, plastics, leather, cosmetics and other industries, are one of the most serious water pollution sources [3,4]. Every year, a large amount of dye effluent is discharged into natural water bodies, which not only exerts fatal effects on aquatic organisms, but also causes harmful threats to humans due to toxicity and the carcinogenic, mutagenic and teratogenic properties of dyes [5,6]. To reduce effluent damage and meet discharge standards, several methods, including precipitation, flocculation coagulation, photo catalytic degradation, adsorption, and biological oxidation, have been developed for removing dyes from wastewater [7,8]. However, the aromatic structures of most dyes are stable and non-degradable under heat or light, even with action of common oxidizing agents, therefore many of these methods have inherent limitations on treating dyes sewage [9,10]. Being effective and low-cost, the adsorption method is more feasible than other methods of water treatment, so adsorption is a promising and common way to remove dyes from aqueous effluents [11,12,13]. Currently, a large range of adsorbent materials based on solid waste have been researched for removing dyes from aqueous solutions, such as waste coffee grounds [14], olive cake waste [15], metal hydroxide sludge [16], waste coal gangue [17], paper mill sludge [18], waste cellulose fibers [19], tannery solid waste [20], oil shale [21].
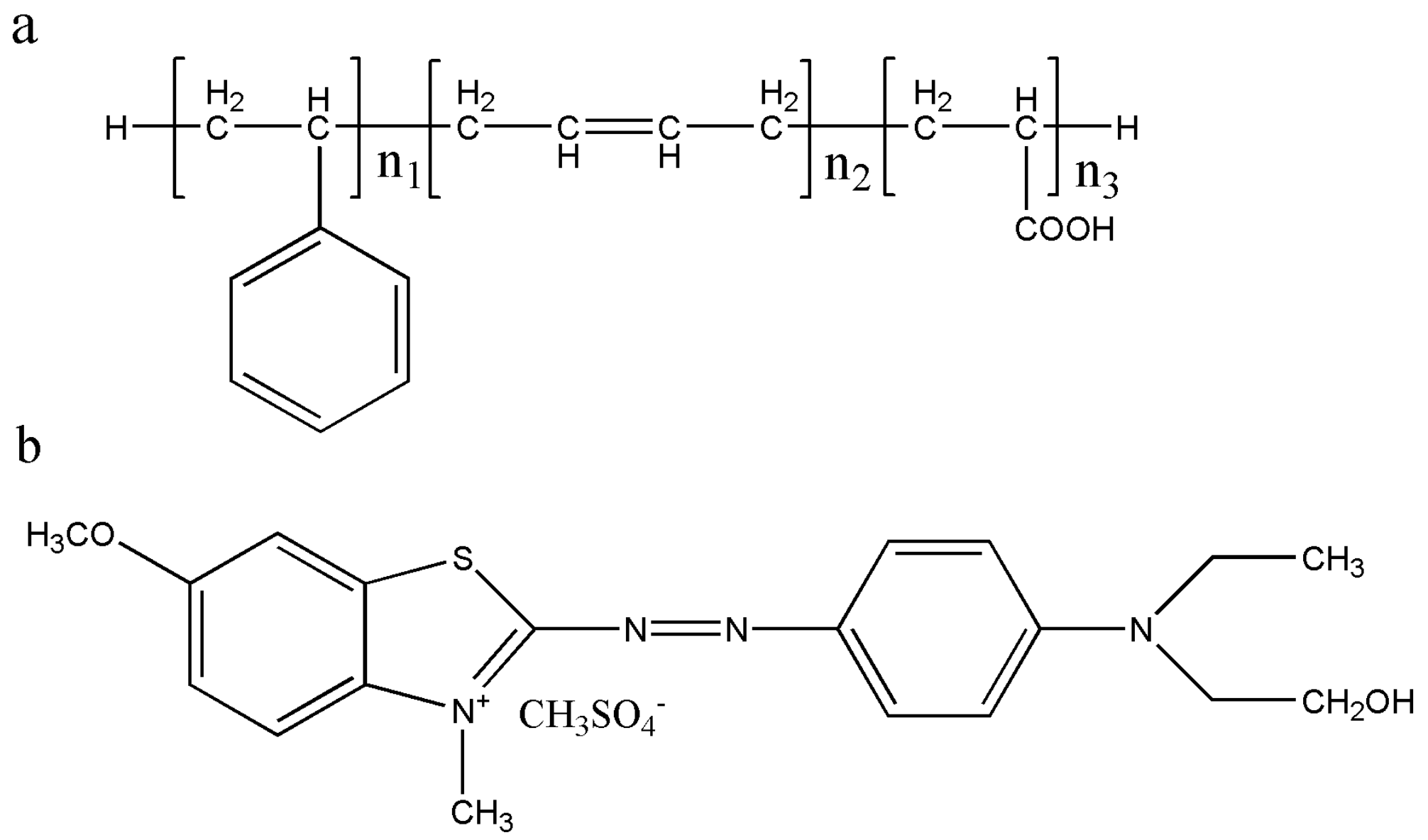
Sludge is an inevitable byproduct of wastewater in the wastewater disposal process [1,22]. The wastewater in latex plants usually contains abundant organic pollutants such as polymerization products and small amount of inorganic pollutants such as silicate. After coagulation and pressure filtration, latex wastewater is dewatered into sludge, and the output is huge. Aiming at the characteristics of high organic matter content in latex sludge, recovering and turning latex waste into useful material for resources and environmental sustainability is a critical issue. The latex sludge used in this work is mainly composed of styrene-butadiene latex, and its structural formula is shown in Figure 1a.
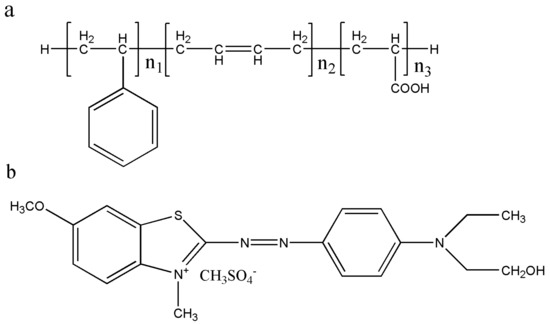
Figure 1.
Structures of (a) carboxylic styrene-butadiene latex and (b) cationic blue X-GRRL.
In this work, industrial solid waste latex sludge (LS) as a raw material was used to produce two new absorbents to remove cationic blue X-GRRL (Figure 1b) from an aqueous solution, which is one of three primary cationic dyes in acrylic textile dyeing. Sulfonated latex sludge (SLS) and latex sludge active carbon (LSC) absorbents were achieved by sulfonate and pyrolysis treatment of an industrial waste LS, respectively. Adsorption properties of X-GRRL on SLS and LSC adsorbents were investigated and compared through adjusting experimental parameters. A further isotherm model and thermodynamic process were taken toward SLS and LSC absorbents that could state maximum adsorption capacities and adsorption mechanism. These findings provide an avenue for recycling industrial solid waste latex sludge into new abundant, low-cost and highly efficient textile dye wastewater absorbents meeting environmental sustainability.
2. Materials and Methods
2.1. Materials and Reagents
The raw material latex sludge (LS) used in the study was from Guangda Construction Co., Ltd. (Jiangsu, China). The LS was obtained by an industrial coagulation and pressure filtration treatment of production wastewater. LS was dried at 105 °C to constant weight, and then crushed into powder and stored. The cationic blue X-GRRL was purchased from Runtu Co., Ltd. (Zhejiang, China). All the chemical reagents used in the experimental work were of analytical grade.
2.2. Preparation of Sulfonated Latex Sludge
10 g crushed LS and 30 mL 1,2-dichloroethane were added into the 250 mL three-necked flask and refluxed in an 80 °C water bath for 30 min to make LS fully swollen. Then, 30 mL 98 wt % sulfuric acid was slowly injected and maintained for 3 h. Afterwards, the product was rinsed with deionized water for several times and dried to a constant weight at 80 °C. Finally, the sulfonated latex sludge (SLS) was sieved to 100–200 mesh for further use.
2.3. Preparation of Latex Sludge Activated Carbon
Firstly, the crushed LS was chemically activated by 50 g/L sodium bicarbonate in a constant temperature oscillator with 60 °C for 30 min. The mass ratio of NaHCO3 to LS was 1:1. Then, the sample was dried in an oven at 105 °C for 24 h to a constant weight, and the dried samples were heated to 700 °C and kept for 1 h under N2. After that, the sample was rinsed with 10 wt % hydrochloric acid solution to eliminate soluble inorganic salts. After rinsing with distilled water and drying to a constant weight in an oven at 80 °C, the pyrolysis product latex sludge activated carbon (LSC) was obtained. The obtained LSC was sieved to 100–200 mesh adsorbent for further use.
2.4. Measurement Techniques
Fourier transform infrared (FTIR) spectra was measured by Nicolet Nexus 470 (Nicolet, Green Bay, WI, USA) after the sample was mixed with KBr at 1% and pressed into a slice. To characterize the morphology of materials, the scanning electron microscopy (SEM) was conducted by Gemini SEM-500 (Zeiss, Oberkochen, Germany). The organic elemental compositions were measured using vario EL III type elemental analyzer (Elementar, Langenselbold, Germany). The Brunauer–Emmett–Teller (BET) analysis were obtained by ASAP 2020 HD88 surface area analyzer (Micromeritics, Norcross, GA, USA) relying on N2 adsorption–desorption. The X-GRRL concentration was measured at the wavelength of 608 nm by DR5000 UV spectrometer (HACH, Loveland, CO, USA).
2.5. Batch Adsorption Experiments
Cationic blue X-GRRL was dissolved in distilled water to prepare X-GRRL stock solution (1000 mg/L), and the stock solution was further diluted to obtain the required concentration. The pH value of X-GRRL solutions was adjusted by HCl and NaOH solution of different concentrations. Adsorbents were mixed with 50 mL of X-GRRL solution, and the mixtures were shaken at 150 r/min for a certain amount of time.
The equilibrium concentrate of X-GRRL in solution was determined by UV spectrometer. The adsorption amount of X-GRRL qe (mg/g) at equilibrium was calculated using Equation (1) and the removal rate η (%) of X-GRRL was calculated using Equation (2).
where C0 (mg/L) and Ce (mg/L) are the initial concentrations and equilibrium concentrations of X-GRRL solution, respectively, m (g) is the weight of SLS or LSC adsorbent used, and V (L) is the volume of X-GRRL solution.
The effect of SLS and LSC dosages on the adsorption of X-GRRL was studied by mixing various amounts of adsorbents with 50 mL of 200 mg/L X-GRRL solution for 24 h. The amount of SLS and LSC adsorbents were 5.0–40.0 mg. The effect of pH value on the adsorption process was analyzed by carrying out the adsorption process at pH values ranging from 2 to 8. The influence of contact time was studied by adding SLS and LSC adsorbents into 50 mL X-GRRL solution (200 mg/L). The effects of the initial concentration on X-GRRL by SLS and LSC were conducted by mixing 10 mg of SLS and 30 mg of LSC with 50 mL X-GRRL solution at a series of concentrations (100–500 mg/L) for 24 h. The mass of X-GRRL adsorbed at predetermined time t was calculated by Equation (3). The above batch experiments were done at 298 K.
where Ct (mg/L) is the concentration of X-GRRL at predetermined time t.
Thermodynamic study was examined by mixing 10 mg of SLS and 30 mg of LSC into 50 mL of X-GRRL solution at 288, 303 and 318 K, respectively.
3. Results and Discussion
3.1. Characterization of Adsorbents
The LS powder was obtained by crushing the LS block. The LS block was taken directly from industrial sludge after coagulation and dehydration treatment of production wastewater of latex industry. The LS block (inset in Figure 2a) and powder (Figure 2a) show tawny. After sulfonate and pyrolysis treatment, the raw material transformed into dark brown SLS (Figure 2b) and black LSC (Figure 2c), respectively. Scanning electron microscopy (SEM) in Figure 2d,e showed that SLS displayed a similar grainy structure with LS powder, while LSC in Figure 2f showed a significantly different morphology with a higher density of porosity in a honeycomb structure. Nitrogen adsorption-desorption isotherm measurement (Table 1) concluded that LSC had a BET surface area of 313.39 m2/g and a total pore volume of 1.12 cm3/g, which is significantly higher than that of LS with 3.45 m2/g and 0.02 cm3/g. SLS showed a similar surface area of 6.13 m2/g and total pore volume with LS. The different morphology may be attributed to organic matters in LS decomposed thermally to create pore structure accompanied with gases during pyrolysis process [18].

Figure 2.
Photograph of (a) crushed latex sludge (LS), (b) sulfonated latex sludge (SLS) and (c) latex sludge active carbon (LSC), the inset in (a) is the uncrushed industrial LS. Scanning electron microscopy (SEM) images of (d) crushed LS, (e) SLS and (f) LSC.

Table 1.
Surface properties of SL, SLS and LSC.
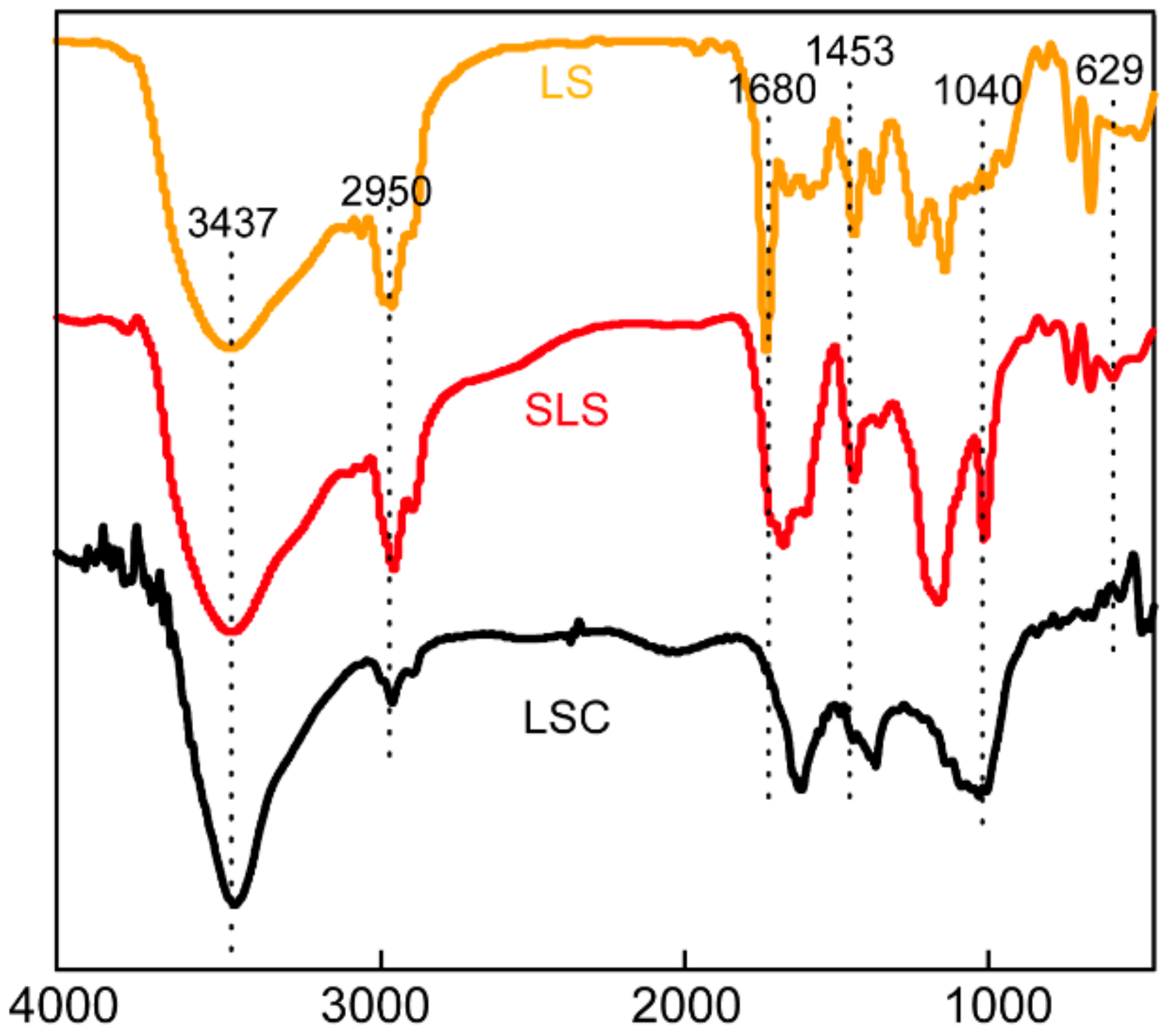
The change in surface functional groups of LS, SLS and LSC were analyzed by FTIR spectroscopy. As was shown in Figure 3, the peaks around 3437 cm−1 for all samples were due to -OH stretching vibration. The peaks around 3028 cm−1 for LS and SLS were assigned to =C-H stretching vibrations [23]. The peaks around 2950 and 2874 cm−1 for all samples were due to C-H stretching vibrations [24]. The peaks around 1734 cm−1 were ascribed to C=O stretching vibrations [10]. The peaks around 1601 cm−1 and 1453 cm−1 were assigned to C=C skeletal vibrations of aromatic rings [25]. The FTIR spectrum of SLS is similar with LS, which indicated that the basic framework structure of SLS did not changed fundamentally after modification. In the spectrum of SLS, new peaks around 1184 and 1039 cm−1 were ascribed to a stretching vibration of O=S=O, implying that -SO3H had been grafted onto SLS [25,26,27]. In the spectrum of LSC, the weakened peaks of =C-H and C-H indicated that the cracking reaction occurred at a high temperature.
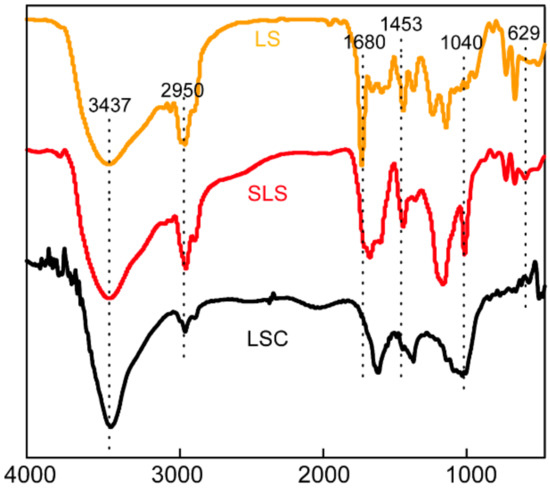
Figure 3.
Fourier transform infrared (FTIR) spectrum of LS, SLS and LSC.
The organic elemental compositions were also investigated and summarized in Table 2. SLS showed a similar atom contents of C, H, N and atom ratios of H/C and N/C with that of LS, indicating that the main C-H and C-N framework structure of LS had not changed fundamentally after sulfonate modification. A large increased O/C and S/C ratio in SLS indicated sulfonic acid group was successfully grafted onto the SLS molecular structure, which was in accordance with results of FTIR. The sulfonation degree of the SLS was calculated as 2.29 mmol/g according to the content of sulfur element. A decreased C and H content in LSC was caused by volatilization of elements during pyrolysis [28].

Table 2.
Organic elemental analysis data of LS, LSC and SLS.
3.2. Adsorption Performance in Batch Experiments
3.2.1. Effects of Adsorbent Dosage on X-GRRL Adsorption
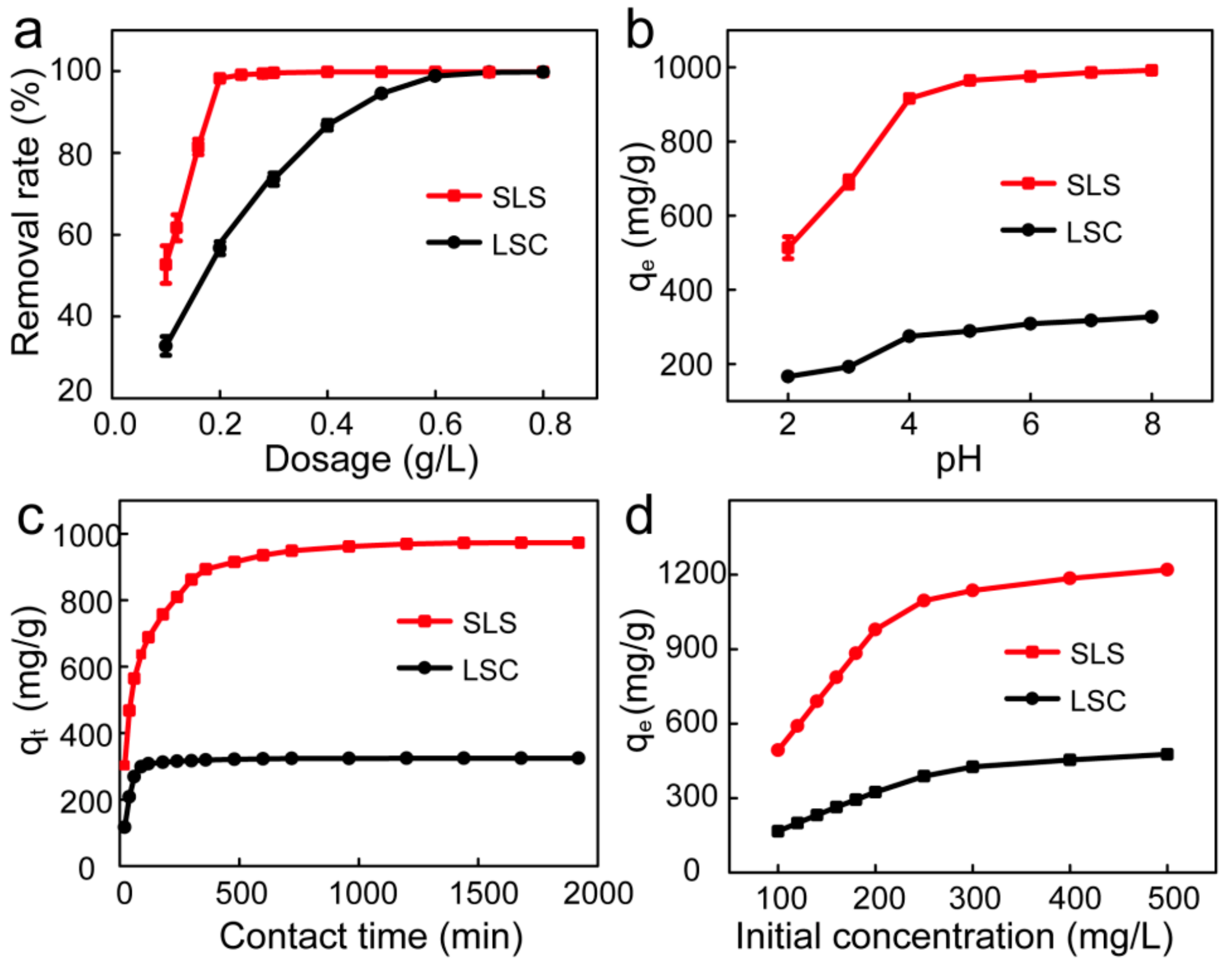
Adsorbent dosage is an essential factor of adsorption research, which can determine the most cost-efficient amount of used adsorbents to effectively remove the hazardous contaminants from the waste water [29]. The effects of SLS and LSC adsorbents dosage on the adsorption of X-GRRL were shown in Figure 4a. It shows that the percentage removal of X-GRRL on SLS and LSC showed a sharp increase with increasing adsorbents dosage from 0.10 to 0.20 g/L for SLS and 0.10 to 0.60 g/L for LSC, respectively, and then removal efficiency remained almost constant. The initial rapid increase of removal efficiency could be ascribed to more adsorption sites by increased adsorbent dosage. The following slight increase might be caused by the aggregation of adsorbent particles or the overlapping of adsorption sites, resulting in the effective surface area of the adsorption decreasing, thereby slightly increasing the amount of adsorption [17,30]. An insufficient or excessive adsorbent dosage would result in low removal efficiency or high cost, therefore 0.2 g/L SLS and 0.6 g/L LSC were selected as optimum adsorbents dosage for the next experiments.
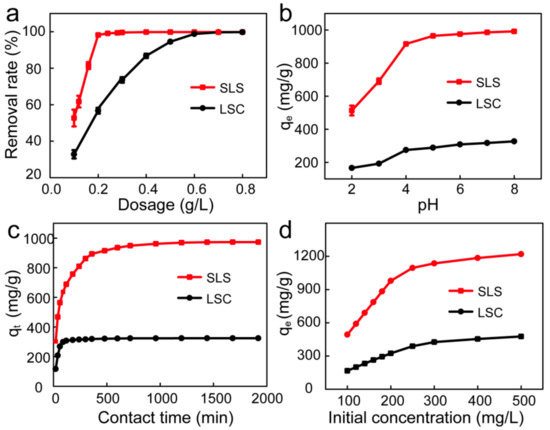
Figure 4.
Effect of different factors on X-GRRL adsorbed by SLS and LSC. (a) Adsorbent dosage, (b) pH, (c) Contact time and (d) Initial concentration.
3.2.2. Effects of Solution pH on Adsorption
The pH value of X-GRRL solution is another critical factor in adsorption studies as it will alter the surface charge of the adsorbent, thus affecting the adsorption efficiency of contaminates [31,32]. The influence of the initial solution pH value on the adsorption of X-GRRL by SLS and LSC were shown in Figure 4b. The adsorption capacity of X-GRRL on SLS and LSC adsorbents showed an upward trend with the increasing of pH value in a range from 2 to 8. This phenomenon could be attributed to the electrostatic interactions between dye ions and the carboxylic and sulfonate functional groups from absorbents. Under acidic conditions, some active sites of absorbent, such as -SO3−, were consumed by hydrogen ions, and the ionization of -COOH groups were inhibited by H+, which decreased the adsorption capacity. So as the pH increases, more electronegative active sites, including -SO3− and -COO−, were formed to combine with more positively charged dye ions. Additionally, SLS showed a far better adsorption capacity than LSC in the studied pH range, which may be due to more -SO3− and -COO− adsorption sites on SLS.
3.2.3. Effects of Contact Time on X-GRRL Adsorption
Adsorption time is also a crucial parameter to improve water treatment efficiency. Figure 4c showed the effects of adsorption time on the adsorption of X-GRRL by SLS and LSC. As was shown, adsorption was rapid in the initial 360 min for SLS and 180 min for LSC, and then became slow until the equilibrium was reached at about 960 and 480 min for SLS and LSC, respectively. In the initial stage, a large number of vacant adsorption sites were freely available, so a fast adsorption occurred, whereas in the following stage, a slow X-GRRL adsorption was ascribed to the decreased availability of the remaining active adsorption sites and a long-range diffusion of X-GRRL dye molecules into the absorbent. Compared with LSC, adsorption of X-GRRL on SLS took more time to reach equilibrium, while the adsorption capacity of SLS was more than three times as much as LSC.
3.2.4. Effects of Initial Concentration on X-GRRL Adsorption
The influence of initial X-GRRL concentration on adsorption by SLS and LSC was shown in Figure 4d; the adsorption capacities for X-GRRL increased from 166.2 to 476.8 mg/g onto LSC and 493.0 to 1220.0 mg/g onto SLS, when the initial concentration of X-GRRL increased from 100 to 500 mg/L, respectively. The increasing trend of adsorption capacity may be ascribed to the higher driving force for mass transfer at a higher initial X-GRRL concentration [33,34].
3.3. Kinetics Studies
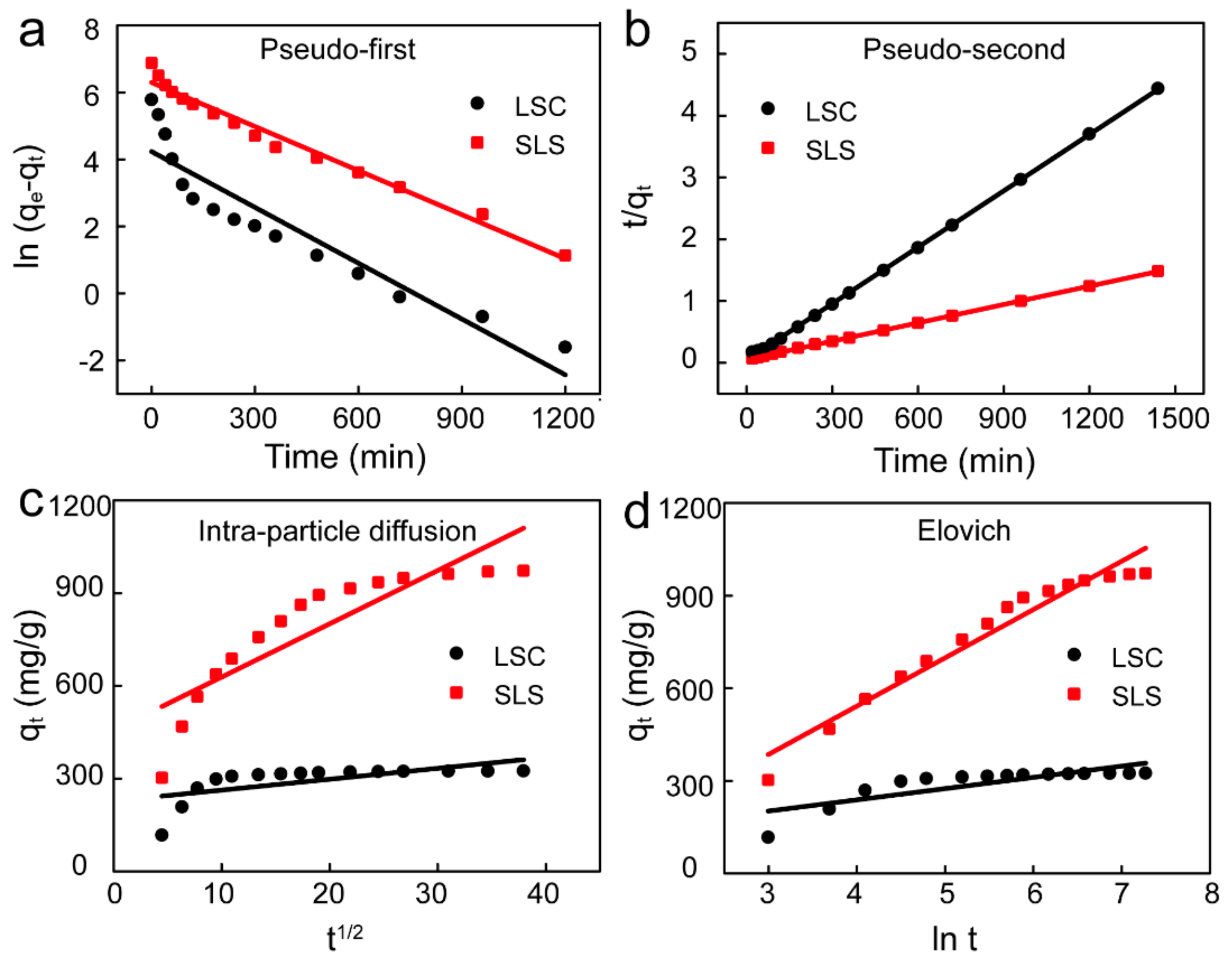
To further analyze the adsorption process and rapid adsorption rate, the data from adsorption process of X-GRRL onto SLS and LSC absorbents was fitted by four kinetic models of pseudo-first order, pseudo-second order, intra-particle diffusion and Elovich model in non-linear forms [35,36,37]. These models were shown by Equations (4)–(7), respectively. The fitting curves of four models were shown in Figure 5 and the parameters of the fitting results were summarized in Table 3.
where qt (mg/g) is the adsorption capability at time t, t (min) is the adsorption time, K1 (/min), K2 (/min) and Ki (mg/(g·min0.5)) are the pseudo-first order, pseudo-second order and intra-particle diffusion adsorption rate constants, C (mg/g) is the intercept of intra-particle diffusion adsorption, α (mg/(g·min)) and β (g/mg) are the initial adsorption coefficient and the desorption coefficient of Elovich model.
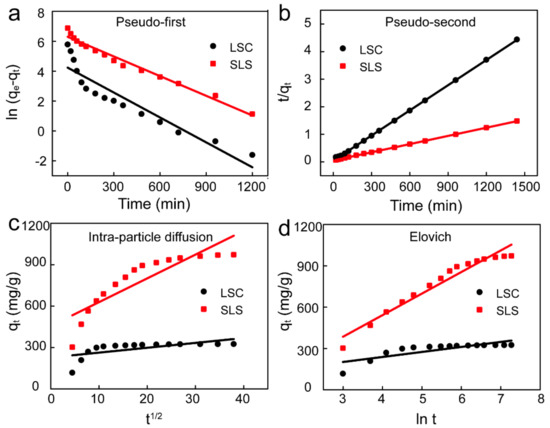
Figure 5.
Adsorption kinetic fitting curves of X-GRRL adsorbed by SLS and LSC. (a) Pseudo-first-order, (b) Pseudo-second-order, (c) Intra-particle diffusion and (d) Elovich model.

Table 3.
Adsorption parameters of four kinetic models of X-GRRL adsorbed by SLS and LSC.
It could be seen that the correlation coefficient (R2) of the pseudo-second order model was highest and higher than 0.999, indicating the adsorption of X-GRRL onto LSC and SLS fitted the pseudo-second order kinetic model well [38]. Furthermore, the calculated qe by pseudo-second order kinetic model was much closer to the experimentally determined qe. This result suggests that what occurred in the adsorption process of X-GRRL on SLS and LSC adsorbents, and the interaction between the X-GRRL molecules and the adsorbents was the rate limiting step [39]. These results are consistent with those of pH and dosage.
3.4. Adsorption Isotherms
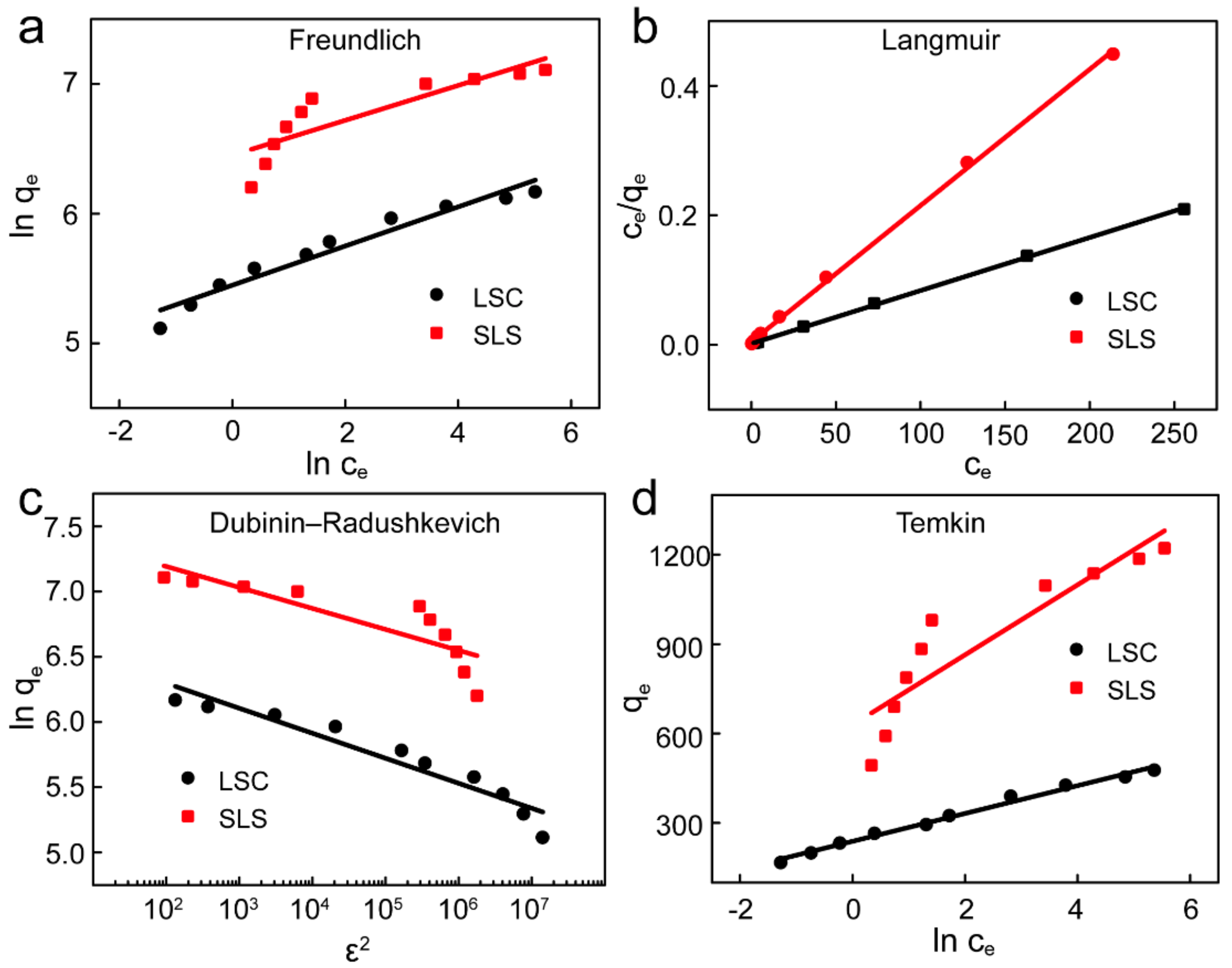
To accurately describe the equilibrium phase of the adsorption process and provide information on the interaction of X-GRRL with SLS and LSC adsorbents, adsorption isotherm models, such as Freundlich, Langmuir, Temkin and Dubinin-Radushkevich (D-R) adsorption isotherm models, were used to fit relevant experimental data [5,7,40,41]. The Langmuir model assumes that the adsorption process is single-layer homogeneous adsorption in monolayer surface, while the Freundlich isotherm assumes that the adsorption process occurs on a heterogeneous surface of absorbents [42]. The Temkin isotherm model considers for the heterogeneous surface of adsorbents, and the decreasing of adsorption heat is linear. Finally, the D–R isotherm can be used to determine whether the properties of the adsorption process are physical or chemical. These model equations can be expressed as Equations (8)–(13), respectively. The fitting curves of four models were shown in Figure 6 and the parameters of fitting results were summarized in Table 4.
where qm (mg/g) is the maximum adsorption capacity in the experiment, Kf (L/mg) is Freundlich model constant associated with the adsorption capacity, Kl (L/mg) is Langmuir model constant related to the adsorption site, n is the adsorption strength of adsorbents, bT is Temkin isotherm constant, T (K) is the absolute temperature of the working solution, R is the universal gas constant, β (mol2/kJ2) is D–R isotherm model constant, Ea (kJ/mol) is the free energy of adsorbents, and ε is the D–R isotherm model constant.
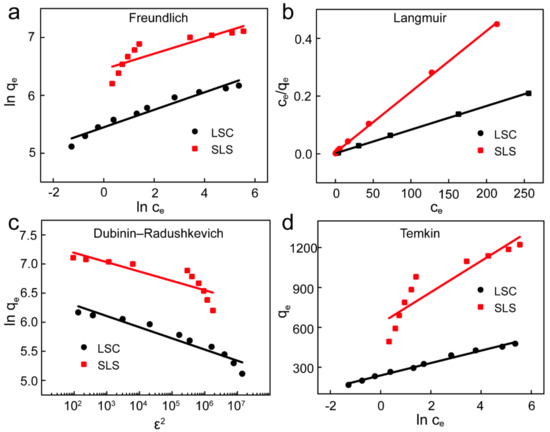
Figure 6.
Adsorption isotherms of X-GRRL onto SLS and LSC. (a) Freundlich, (b) Langmuir, (c) D-R and (d) Temkin model.

Table 4.
Fitting parameters of four isotherm models of X-GRRL adsorbed by SLS and LSC.
The basic characteristic of the Langmuir model can be expressed by the dimensionless equilibrium factor RL [38]. It was shown by Equation (14). The value of RL indicates that the isotherm is irreversible (RL = 0), linear (RL = 1), favorable (occurs spontaneously) (0 < RL <1), and unfavorable (RL > 1).
where C0 (mg/L) is the initial concentration of X-GRRL.
For SLS, the R2 of the Langmuir was the highest and higher than 0.999 and other models were lower than 0.9, indicating that the adsorption process of SLS followed Langmuir isotherm well and other three models cannot accurately describe the adsorption process of X-GRRL on SLS. The results also suggested that the adsorption took place on the homogeneous surface of SLS with equal binding sites [5]. A saturation point was reached after attaining equilibrium and further adsorption would not be occurred at the same sites [32,43]. RL indicated that SLS was favorable adsorbent for X-GRRL adsorption.
For LSC, the higher correlation coefficients of Freundlich and Langmuir indicated that the mechanism of adsorption was a compounded process rather than an ideal monolayer adsorption. On the other hand, high R2 of Temkin and the D–R isotherm model also confirmed this view, suggesting electrostatic interaction and molecular force simultaneously were important mechanisms, which affected interactions between the X-GRRL and LSC.
3.5. Thermodynamic Study
Parameters including enthalpy (ΔH0), Gibbs free energy (ΔG0) and entropy (ΔS0) evaluate the thermodynamics of adsorption. KL is the distribution coefficient at temperature T, and the value can be calculated from Equation (15). The value of the ΔG0 (J/mol) at different absolute temperatures can be obtained from Equation (16). The values of ΔS0 and ΔH0 can be obtained from the slope and the intercept of the plots of lnKL against 1/T by Equation (17) [43].
where R (8.314 J/(mol·K)) is the gas constant and KL is the distribution coefficient at temperature T.
At the initial concentration of 200 mg/L, the determined values of the thermodynamic parameters were given in Table 5. The negative values of ΔG0 supported that the adsorption of X-GRRL onto SLS and LSC adsorbents was spontaneous. The positive values of ΔH0 and ΔS0 suggested that the adsorption process of the dye on SLS and LSC was endothermic and the disorder at the solid–liquid interface was increasing, respectively [44]. This phenomenon was ascribed to the adsorption process in the solid-liquid system and can be seen as a combination of the desorption process of the solvent molecules (water) adsorbed previously and the adsorption process of the adsorbates molecules. The bigger X-GRRL molecules displaced more water molecules in their adsorption onto SLS and LSC, leading to endothermic adsorption processes in both SLS and LSC. The absolute values of ΔH0 were between 20 and 0 kJ/mol, which indicated that the adsorption of X-GRRL onto LSC was mainly physical adsorption mechanism [32,45]. The absolute value of ΔH0 was higher than 30 kJ/mol, which suggested that the adsorption of X-GRRL onto SLS was dominated by a chemical mechanism.

Table 5.
Thermodynamic parameters of X-GRRL adsorbed by SLS and LSC adsorbent at different temperatures.
4. Conclusions
In this work, industrial latex sludge as raw material was made into SLS and LSC absorbents by sulfonate and pyrolysis treatment. SLS and LSC absorbents were used to remove textile dye X-GRRL. The adsorption capacities and mechanism of SLS and LSC absorbents were studied and compared. The results showed that the adsorption efficiency of X-GRRL onto both SLS and LSC was greatly improved with the increasing of adsorption time, pH and initial concentration. The process followed the pseudo-second-order rate equation and were both endothermic. However, the adsorption process of X-GRRL on SLS occurred on a homogeneous surface by monolayer adsorption and was a chemical adsorption by electrostatic interactions, while the adsorption on LSC was a hybrid process and dominated by a physisorption mechanism. Furthermore, SLS showed a higher adsorption X-GRRL dye capacity than LSC. According to the Langmuir model, the maximum adsorption capacities were 1219.6 mg/g for SLS and 476.2 mg/g for LSC, respectively. Therefore, sulfonate treatment was an efficient way to recycle industrial solid waste latex sludge into low-cost, high-efficiency textile dye wastewater absorbent, which not only reduces latex sludge but also turns solid waste into a useful material for environmental remediation.
Author Contributions
Conceptualization, R.L. and J.S.; methodology, H.W.; software, B.Z.; validation, H.W., J.S. and B.Z.; formal analysis, W.H.; investigation, H.W.; resources, R.L.; data curation, H.W.; writing—original draft preparation, H.W.; writing—review and editing, J.S.; visualization, H.W.; supervision, R.L.; project administration, R.L.; funding acquisition, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Major Technological Innovation Project of Key Technology Research and Development Program of Shandong province, China, grant number 2019JZZY020301 and 2019JZZY010507. The APC was funded by Major Technological Innovation Project of Key Technology Research and Development Program of Shandong province, China, grant number 2019JZZY020301 and 2019JZZY010507.
Acknowledgments
This work was supported by the Major Technological Innovation Project of Key Technology Research and Development Program of Shandong province, China (Grant No. 2019JZZY020301 and 2019JZZY010507).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hu, W.; Xie, Y.; Lu, S.; Li, P.; Xie, T.; Zhang, Y.; Wang, Y. One-step synthesis of nitrogen-doped sludge carbon as a bifunctional material for the adsorption and catalytic oxidation of organic pollutants. Sci. Total. Environ. 2019, 680, 51–60. [Google Scholar] [CrossRef] [PubMed]
- The United Nations World Water Development Report 2017: Wastewater, The Untapped Resource; UNESCO: Paris, France, 2017; Available online: http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/wwdr/2017-wastewater-the-untapped-resource/ (accessed on 11 March 2017).
- Cheng, R.; Ou, S.; Li, M.; Li, Y.; Xiang, B. Ethylenediamine modified starch as biosorbent for acid dyes. J. Hazard. Mater. 2009, 172, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Shin, J.; Choi, E.; Jang, E.; Gil Hong, S.; Lee, S.R.; Ravindran, B. Adsorption Characteristics of Ammonium Nitrogen and Plant Responses to Biochar Pellet. Sustainability 2018, 10, 1331. [Google Scholar] [CrossRef]
- Ma, J.; Cui, B.; Dai, J.; Li, D. Mechanism of adsorption of anionic dye from aqueous solutions onto organobentonite. J. Hazard. Mater. 2011, 186, 1758–1765. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Tehrani-Bagha, A. Cationic ester-containing gemini surfactants as retarders in acrylic dyeing. Colloids Surfaces Physicochem. Eng. Asp. 2015, 479, 52–59. [Google Scholar] [CrossRef]
- Gao, Y.; Deng, S.-Q.; Jin, X.; Cai, S.-L.; Zheng, S.-R.; Zhang, W.-G. The construction of amorphous metal-organic cage-based solid for rapid dye adsorption and time-dependent dye separation from water. Chem. Eng. J. 2019, 357, 129–139. [Google Scholar] [CrossRef]
- Jung, B.K.; Jun, J.W.; Hasan, Z.; Jhung, S.H. Adsorptive removal of p-arsanilic acid from water using mesoporous zeolitic imidazolate framework-8. Chem. Eng. J. 2015, 267, 9–15. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; Alothman, Z.A.; AlShehri, S.M.; Ghfar, A.A. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism. J. Clean. Prod. 2017, 156, 426–436. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Lin, Y.-C.; Ho, S.-H.; Zhou, Y.; Ren, N.-Q. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Liu, H.; Zhang, L.; Zhang, J.; Fu, C.; Shi, X.; Chen, X.; Mijowska, E.; Chen, M.-J.; Wang, D.-Y. Large-scale converting waste coffee grounds into functional carbon materials as high-efficient adsorbent for organic dyes. Bioresour. Technol. 2019, 272, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Toumi, K.; Bergaoui, M.; Khalfaoui, M.; Benguerba, Y.; Erto, A.; Dotto, G.L.; Amrane, A.; Nacef, S.; Ernst, B. Computational study of acid blue 80 dye adsorption on low cost agricultural Algerian olive cake waste: Statistical mechanics and molecular dynamic simulations. J. Mol. Liq. 2018, 271, 40–50. [Google Scholar] [CrossRef]
- Santos, S.C.; Vilar, V.J.; Boaventura, R. Waste metal hydroxide sludge as adsorbent for a reactive dye. J. Hazard. Mater. 2008, 153, 999–1008. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Hu, Y.; Yan, S.; Yang, J.-L. Adsorption removal of cationic dyes from aqueous solutions using ceramic adsorbents prepared from industrial waste coal gangue. J. Environ. Manag. 2019, 234, 245–252. [Google Scholar] [CrossRef]
- Jaria, G.; Silva, C.P.; Oliveira, J.A.; Santos, S.M.; Gil, M.V.; Otero, M.; Calisto, V.; Esteves, V.I. Production of highly efficient activated carbons from industrial wastes for the removal of pharmaceuticals from water—A full factorial design. J. Hazard. Mater. 2019, 370, 212–218. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X.S. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Uematsu, Y.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removing Sr(II) and Cs(I) from the Aqueous Phase Using Basil Seed and Elucidating the Adsorption Mechanism. Sustainability 2020, 12, 2895. [Google Scholar] [CrossRef]
- Acar, E.T.; Ortaboy, S.; Atun, G. Adsorptive removal of thiazine dyes from aqueous solutions by oil shale and its oil processing residues: Characterization, equilibrium, kinetics and modeling studies. Chem. Eng. J. 2015, 276, 340–348. [Google Scholar] [CrossRef]
- Cai, H.; Liu, J.; Kuo, J.; Buyukada, M.; Evrendilek, F. Thermal characteristics, kinetics, gas emissions and thermodynamic simulations of (co-)combustions of textile dyeing sludge and waste tea. J. Clean. Prod. 2019, 239, 118113. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Ma, Q.; Li, Y.; Zhu, Z. Chemical treatment of CNTs in acidic KMnO4 solution and promoting effects on the corresponding Pd–Pt/CNTs catalyst. J. Mol. Catal. Chem. 2012, 356, 114–120. [Google Scholar] [CrossRef]
- Larous, S.; Meniai, A.-H. Adsorption of Diclofenac from aqueous solution using activated carbon prepared from olive stones. Int. J. Hydrogen Energy 2016, 41, 10380–10390. [Google Scholar] [CrossRef]
- Macías-García, A.; Sanz-Calcedo, J.G.; Carrasco-Amador, J.P.; Segura-Cruz, R. Adsorption of Paracetamol in Hospital Wastewater through Activated Carbon Filters. Sustainability 2019, 11, 2672. [Google Scholar] [CrossRef]
- Wu, K.; Guo, L.; Xu, W.; Xu, H.; Aguilar, Z.P.; Xu, G.; Lai, W.; Xiong, Y.; Wan, Y. Sulfonated polystyrene magnetic nanobeads coupled with immunochromatographic strip for clenbuterol determination in pork muscle. Talanta 2014, 129, 431–437. [Google Scholar] [CrossRef]
- Yu, B.; Li, Z.; Cong, H.; Li, G.; Peng, Q.; Yang, C. Synthesis and application of sulfonated polystyrene/ferrosoferric oxide/diazoresin nanocomposite microspheres for highly selective removal of dyes. Mater. Des. 2017, 135, 333–342. [Google Scholar] [CrossRef]
- Low, S.K.; Tan, M.C.; Chin, N.L. Effect of ultrasound pre-treatment on adsorbent in dye adsorption compared with ultrasound simultaneous adsorption. Ultrason. Sonochem. 2018, 48, 64–70. [Google Scholar] [CrossRef]
- Mittal, H.; Parashar, V.; Mishra, S.; Mishra, A. Fe3O4 MNPs and gum xanthan based hydrogels nanocomposites for the efficient capture of malachite green from aqueous solution. Chem. Eng. J. 2014, 255, 471–482. [Google Scholar] [CrossRef]
- Xu, S.; Yu, W.; Liu, S.; Xu, C.; Li, J.; Zhang, Y. Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism. Sustainability 2018, 10, 4250. [Google Scholar] [CrossRef]
- Tahir, M.A.; Bhatti, H.N.; Iqbal, M. Solar Red and Brittle Blue direct dyes adsorption onto Eucalyptus angophoroides bark: Equilibrium, kinetics and thermodynamic studies. J. Environ. Chem. Eng. 2016, 4, 2431–2439. [Google Scholar] [CrossRef]
- Shoukat, S.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Mango stone biocomposite preparation and application for crystal violet adsorption: A mechanistic study. Microporous Mesoporous Mater. 2017, 239, 180–189. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total. Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kim, J.-H.; Guo, X.; Park, H.-S. Adsorption equilibrium and kinetics of polyvinyl alcohol from aqueous solution on powdered activated carbon. J. Hazard. Mater. 2008, 153, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Uzun, I. Kinetics of the adsorption of reactive dyes by chitosan. Dye. Pigment. 2006, 70, 76–83. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Xiao, B.; Dai, Q.; Yu, X.; Yu, P.; Zhai, S.; Liu, R.; Guo, X.; Liu, J.; Chen, H. Effects of sludge thermal-alkaline pretreatment on cationic red X-GRL adsorption onto pyrolysis biochar of sewage sludge. J. Hazard. Mater. 2018, 343, 347–355. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, C.; Cheng, W.; Wang, X. Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J. Hazard. Mater. 2014, 280, 399–408. [Google Scholar] [CrossRef]
- Aşçı, Y.; Nurbaş, M.; Açıkel, Y.S. A comparative study for the sorption of Cd(II) by soils with different clay contents and mineralogy and the recovery of Cd(II) using rhamnolipid biosurfactant. J. Hazard. Mater. 2008, 154, 663–673. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Frankl. Inst. 1917, 183, 102–105. [Google Scholar] [CrossRef]
- Yildiz, I.; Yildiz, I.; Alyammahi, A.; Obaidalla, F.; AlMehairbi, M.; Alkhajeh, S.; Alhammadi, T.A. Adsorptive removal capacity of gravel for metal cations in the absence/presence of competitive adsorption. Environ. Sci. Pollut. Res. 2017, 25, 7530–7540. [Google Scholar] [CrossRef]
- Mushtaq, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Eriobotrya japonica seed biocomposite efficiency for copper adsorption: Isotherms, kinetics, thermodynamic and desorption studies. J. Environ. Manag. 2016, 176, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Srivastava, V.; Banerjee, S.; Weng, C.-H.; Sharma, Y.C. Adsorption characteristics of modified sand for the removal of hexavalent chromium ions from aqueous solutions: Kinetic, thermodynamic and equilibrium studies. Catena 2013, 100, 120–127. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H.; Wang, C.; Zhang, J.; Zhang, Z. Adsorption of hexavalent chromium from aqueous solutions by graphene modified with cetyltrimethylammonium bromide. J. Colloid Interface Sci. 2013, 394, 183–191. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).