Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
 MW = 194.2; pKa = 14; Solubility = 21.6 g L−1; EC50 (D. Magna) = 182 mg L−1.
MW = 194.2; pKa = 14; Solubility = 21.6 g L−1; EC50 (D. Magna) = 182 mg L−1.2.1. Batch Adsorption Tests
2.2. Preparation of the Catalysts
2.3. Photocatalysis with TiO2 and Sep–TiO2 Nanocomposite
2.4. Post-Treatment of the Samples by High-Frequency Ultrasound
2.5. Analytical
3. Results
3.1. Characterization of the Nanocomposite
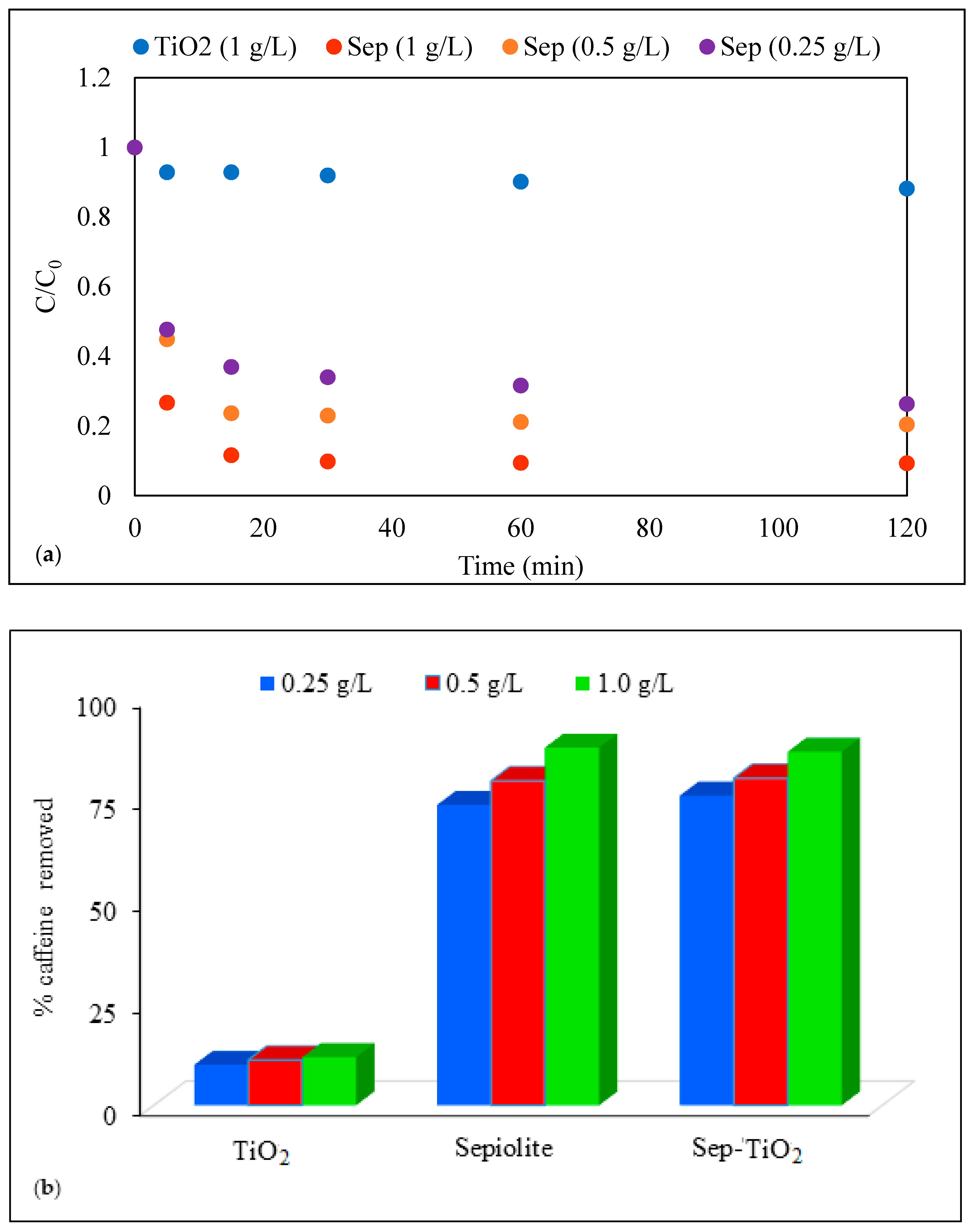
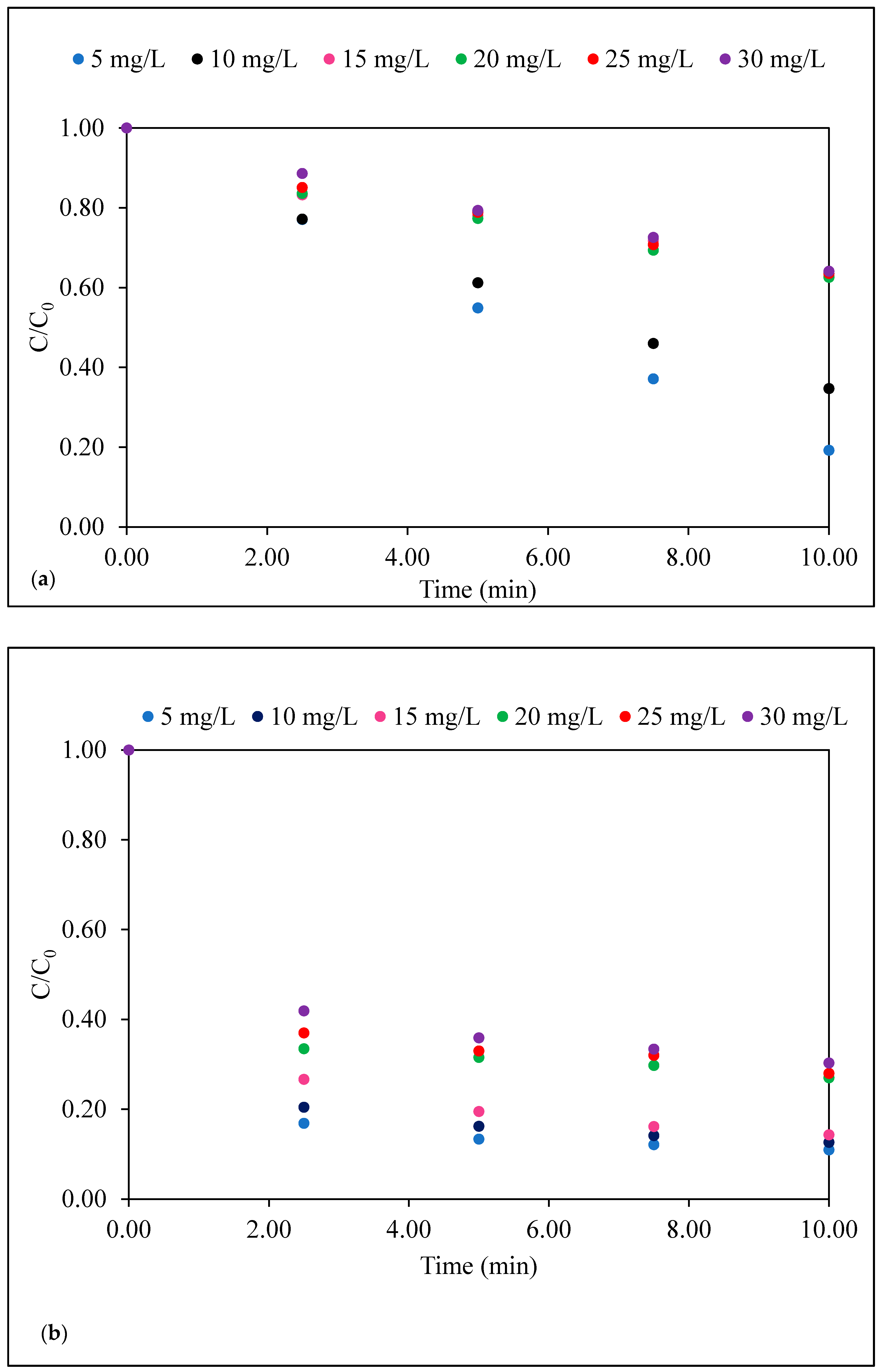
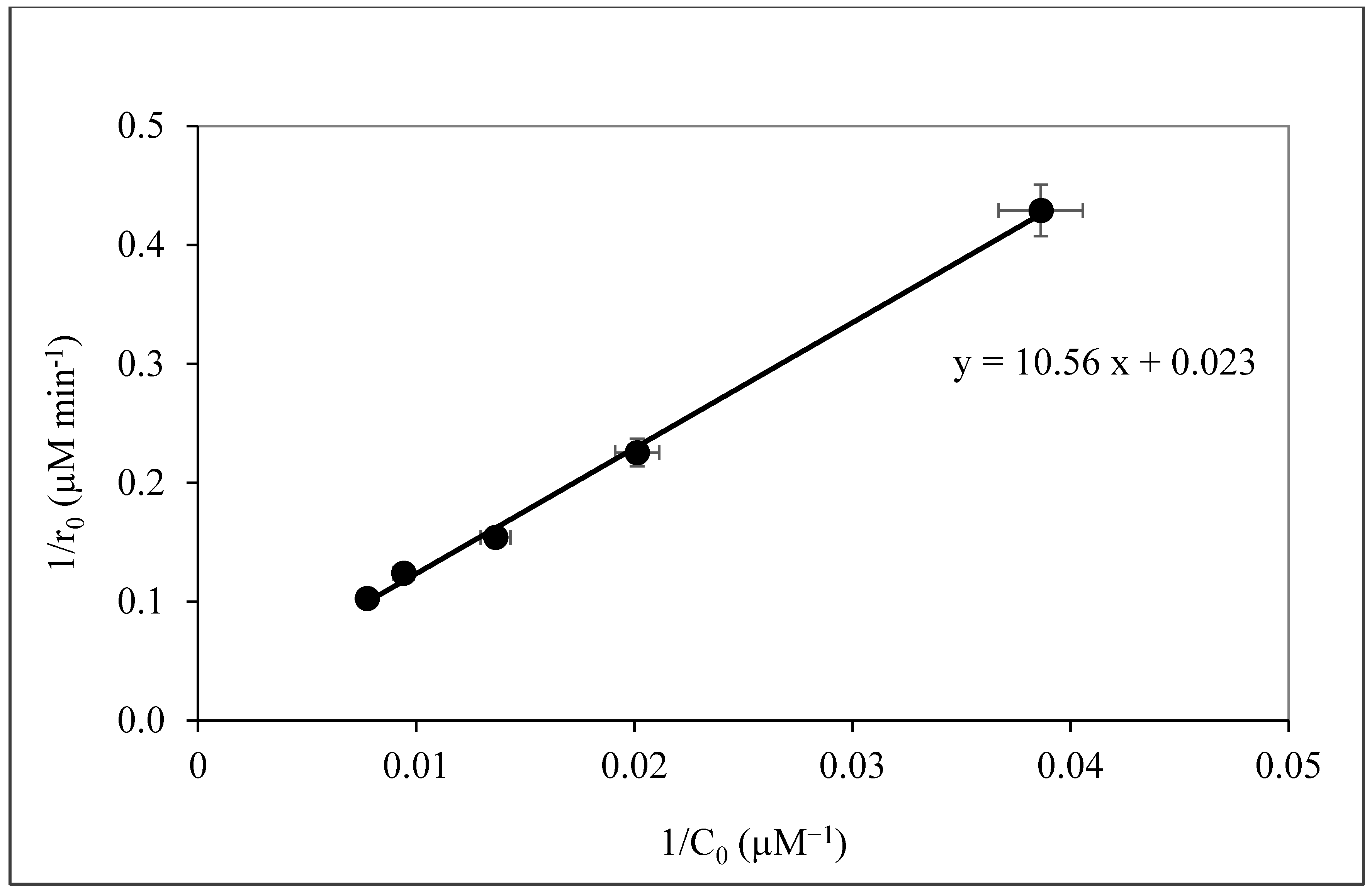
3.2. Dark Adsorption of Caffeine on Sepiolite, Pristine TiO2 (P-25) and Sep–TiO2 Nanocomposite
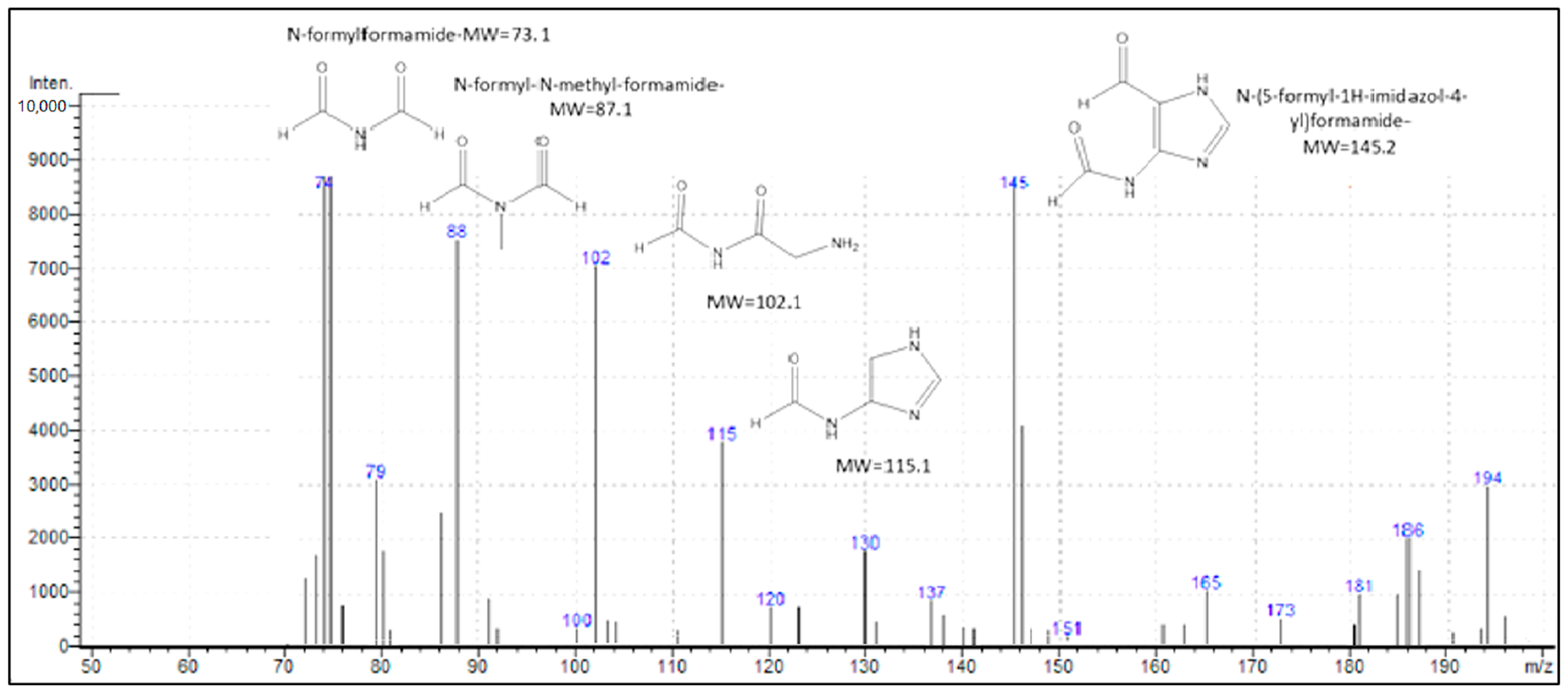
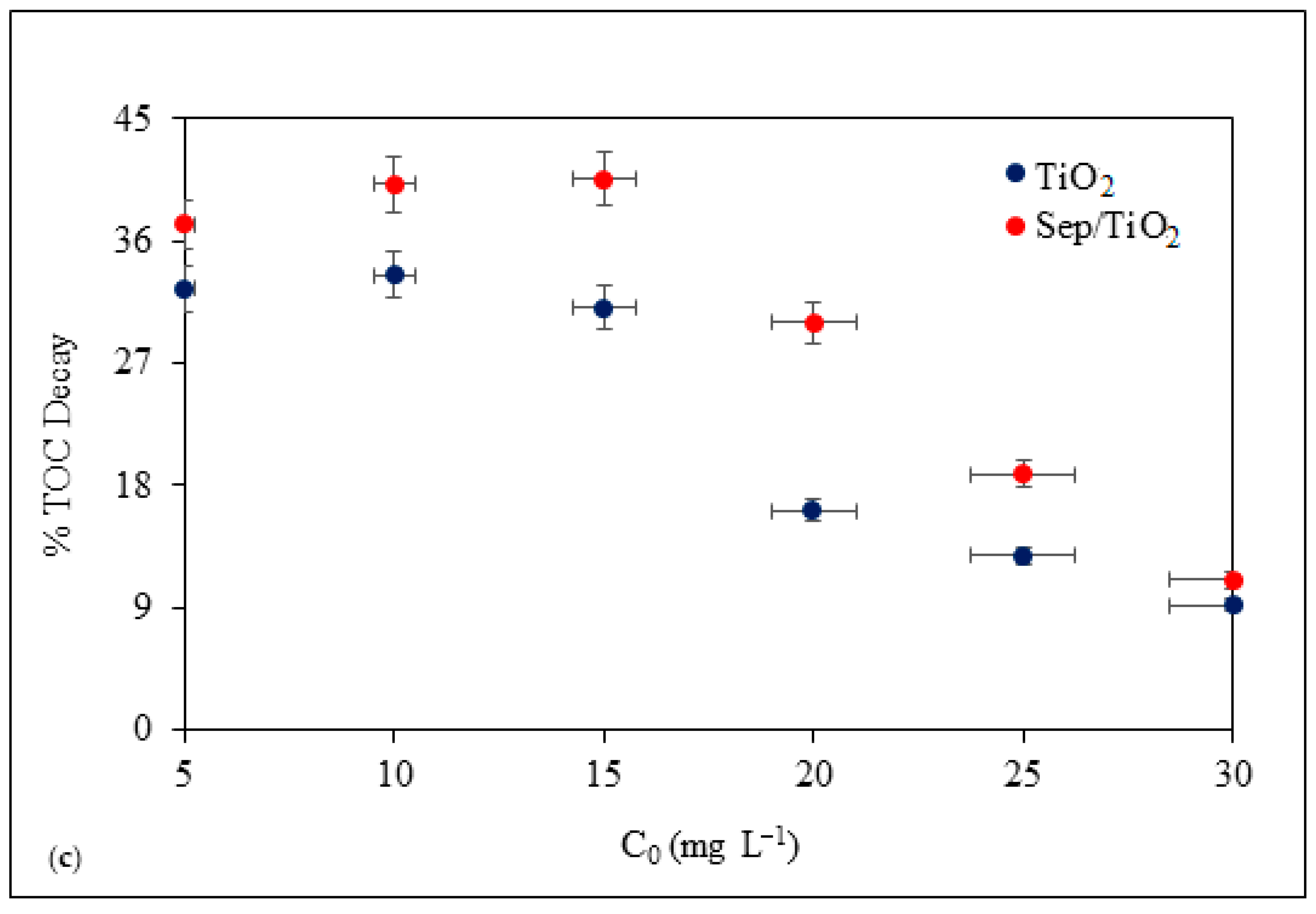
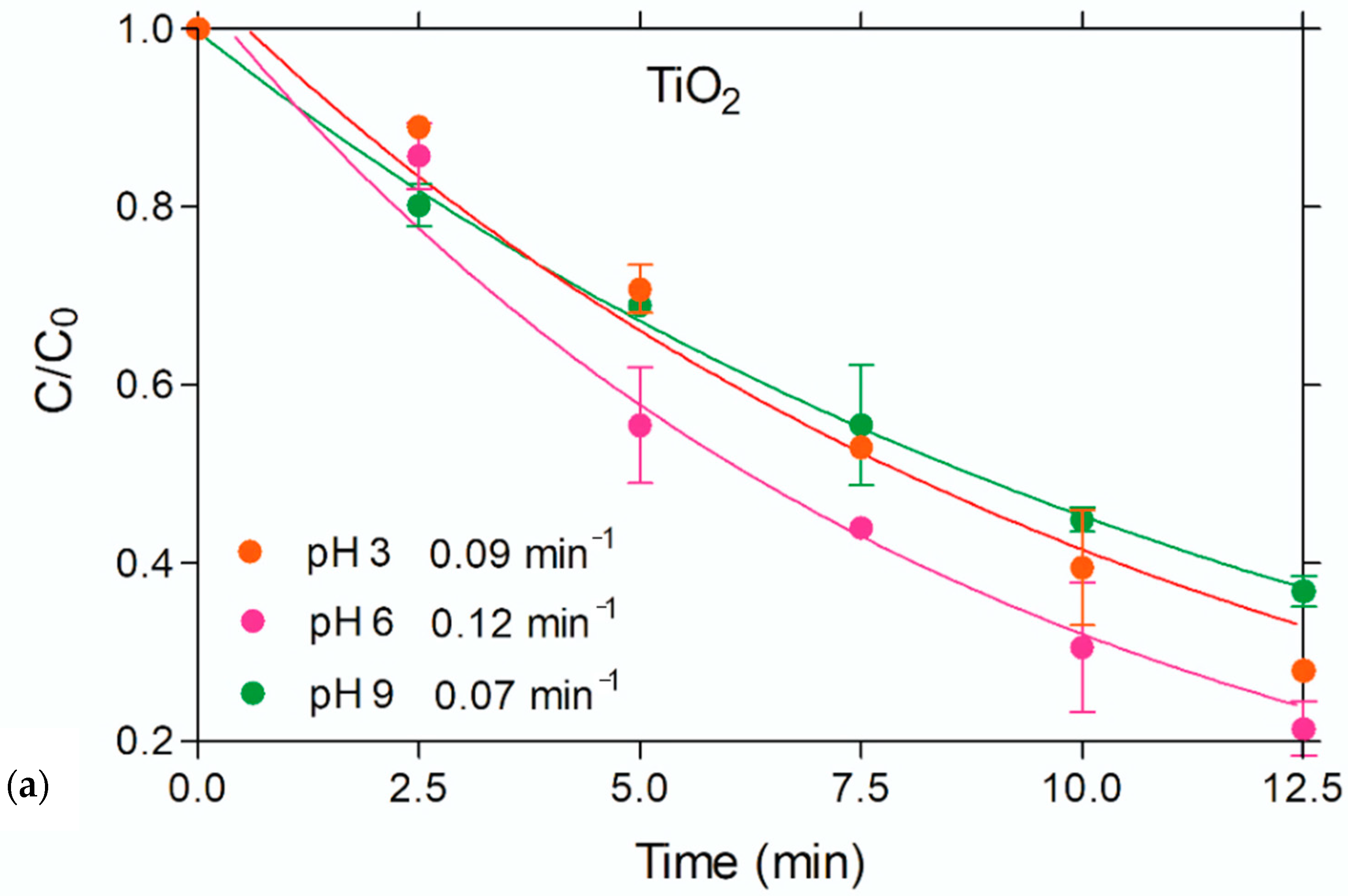
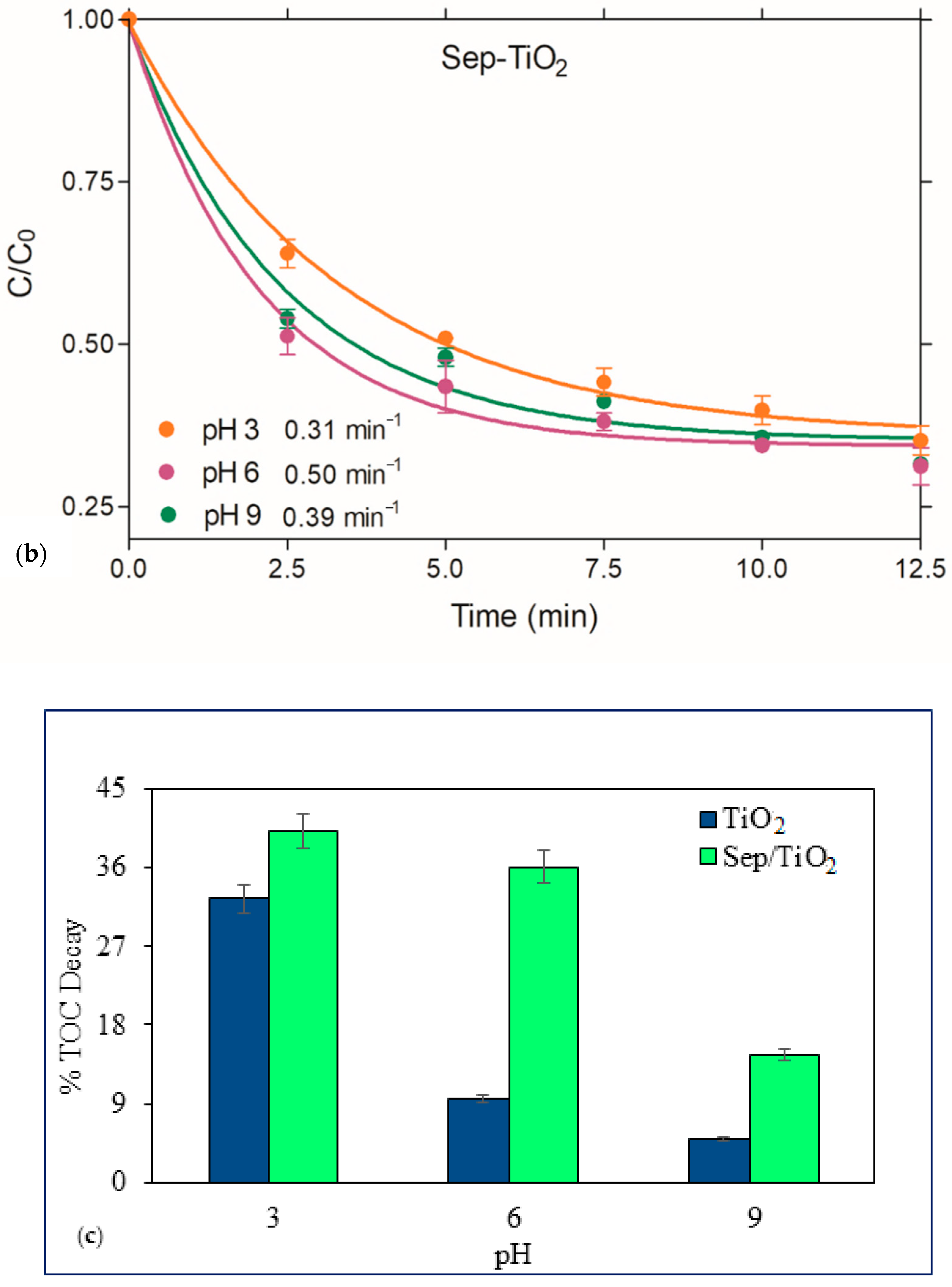
3.3. Photocatalysis
- (i)
- Strong adsorption of the parent and daughter molecules on the surface of the nanocomposite
- (ii)
- Formation of acidic sites (generated by the Si–OH groups on sepiolite) on the surface of the catalyst that easily capture the photo-generated holes to produce •OH.
- (iii)
- More rapid capture of photo-generated electrons by O2 to yield superoxide radicals, which upon reaction with H2O2 produce additional •OH.
3.4. Post-Treatment by Sonication
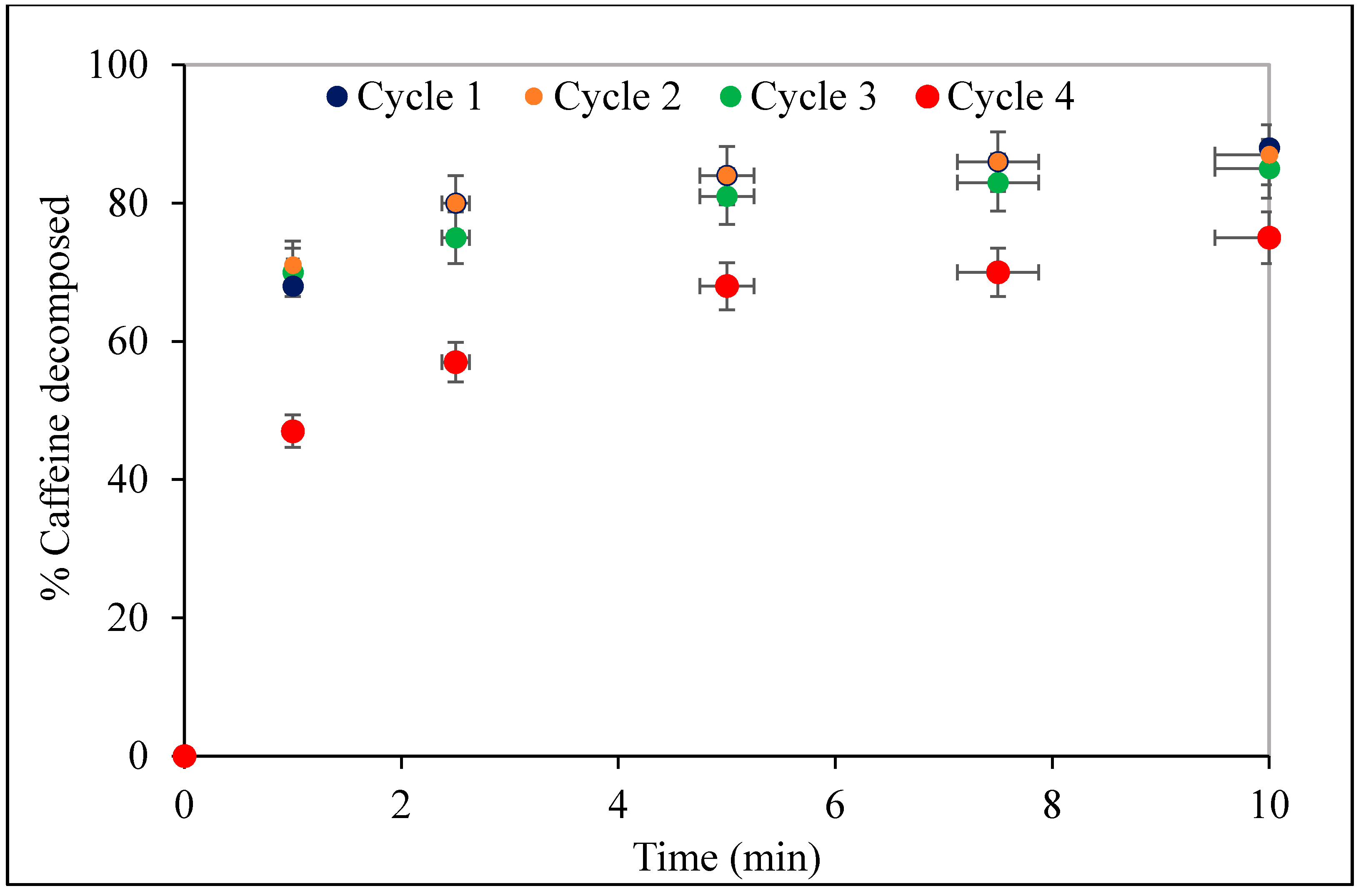
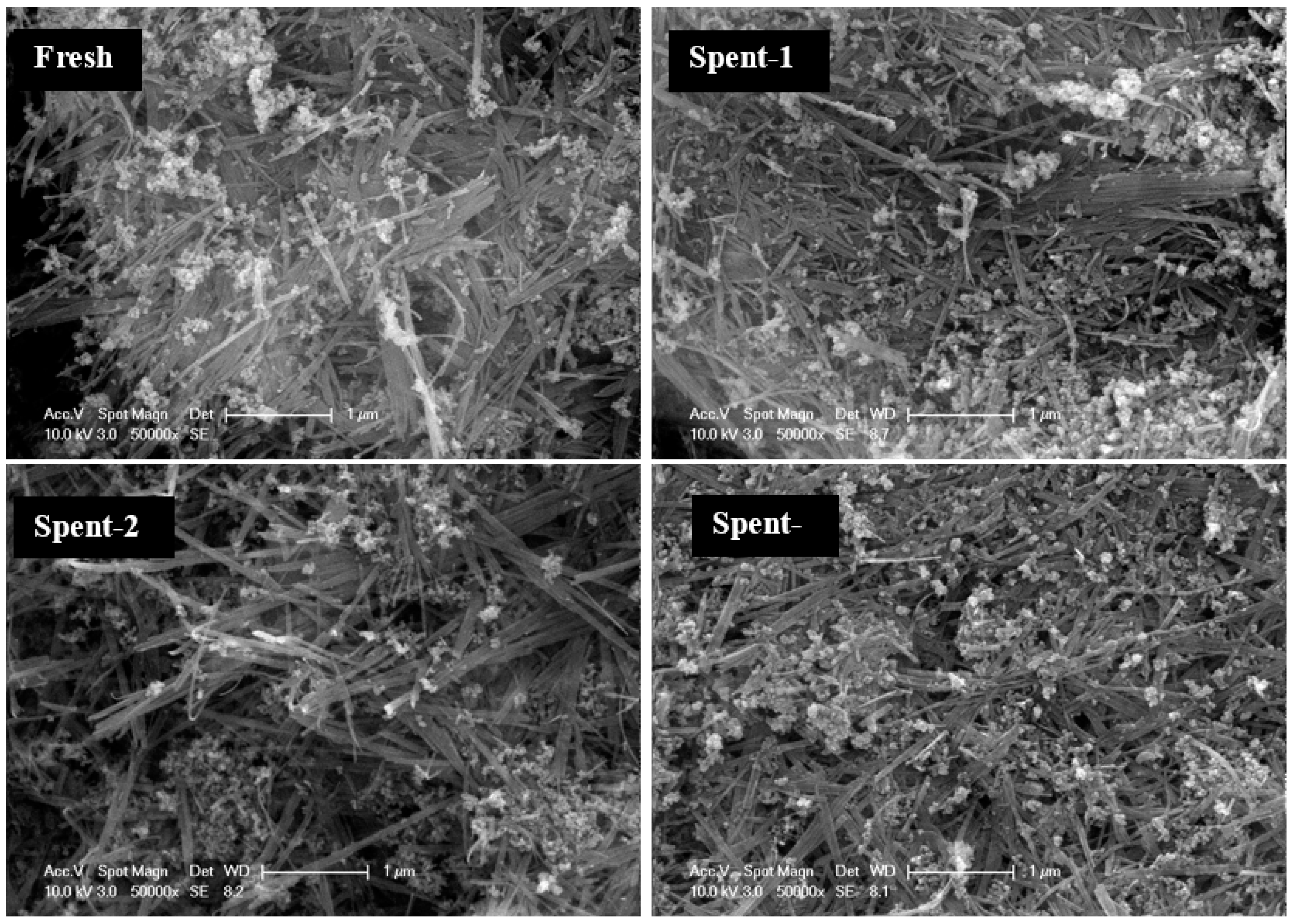
3.5. Stability of the Nanocomposite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zalkeska-Radziwill, M.; Affek, K.; Rybak, J. Ecotoxicity of chosen pharmaceuticals in relation to micro-organisms risk assessment. Desalin. Water Treat. 2014, 52, 3908–3917. [Google Scholar] [CrossRef]
- Bruton, T.; Alboloushi, A.; De La Garza, B.; Kim, B.O.; Halden, R.U. Fate of caffeine in the environment and ecotoxicological considerations. In Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 257–273. [Google Scholar]
- Gardinali, P.R.; Zhao, X. Trace determination of caffeine in surface water samples by liquid chromatography—Atmospheric pressure chemical ionization—Mass spectrometry (LC-APCI-MS). Environ. Int. 2002, 28, 521–528. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Ovejero, G.; Rodriguez, A.; Álvarez, S.; Galán, J.; Garcia, J. Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon. Chem. Eng. J. 2014, 240, 443–453. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Ovejero, G.; Rodríguez, A.; Álvarez, S.; García, J. Study of natural clay adsorbent sepiolite for the removal of caffeine from aqueous solutions: Batch and fixed-bed column operation. Water Air Soil Pollut. 2013, 224, 1466. [Google Scholar] [CrossRef]
- Kim, M.; Guerra, P.; Shah, A.; Parsa, M.; Alaee, M.; Smyth, S.A. Removal of pharmaceuticals and personal care products in a membrane bioreactor wastewater treatment plant. Water Sci. Technol. 2014, 69, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Cuevas, S.; Oller, I.; Ruiz-Delgado, A.; Cabrera-Reina, A.; Cornejo-Ponce, L.; Malato, S. EDDS as complexing agent for enhancing solar advanced oxidation processes in natural water: Effect of iron species and different oxidants. J. Hazard. Mater. 2019, 372, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ganzenko, O.; Trellu, C.; Papirio, S.; Oturan, N.; Huguenot, D.; Van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Bioelectro-Fenton: Evaluation of a combined biological advanced oxidation treatment for pharmaceutical wastewater. Environ. Sci. Pollut. Res. 2018, 25, 20283–20292. [Google Scholar] [CrossRef]
- Shu, Z.; Singh, A.; Klamerth, N.; McPhedran, K.; Chelme-Ayala, P.; Bolton, J.R.; Belosevic, M.; El Din, M.G. Application Of The UV/H2O2 Advanced Oxidation Process For Municipal Reuse Water: Bench-And Pilot-scale Studies. WIT Trans. Ecol. Environ. 2016, 209, 233–244. [Google Scholar]
- Ashokkumar, M. An Overview on Semiconductor Particulate of Hydrogen Systems For. Int. J. Hydrog. Energy 1998, 23, 427–438. [Google Scholar] [CrossRef]
- Kamat, P.V.; Meisel, D. Nanoparticles in advanced oxidation processes. Curr. Opin. Colloid Interface Sci. 2002, 7, 282–287. [Google Scholar] [CrossRef]
- Seo, W.J.; Sung, Y.T.; Kim, S.B.; Lee, Y.B.; Choe, K.H.; Choe, S.H.; Sung, J.Y.; Kim, W.N. Effects of Ultrasound on the Synthesis and Properties of Polyurethane Foam/Clay Nanocomposites. J. Appl. Polym. Sci. 2006, 102, 3764–3773. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. Dev. Clay Sci. 2013, 5, 1–19. [Google Scholar]
- Coruh, S.; Geyikci, F.; Coruh, U. Removal of Cu2+ from copper flotation waste leachant using sepiolite: Full factorial design approach. Acta Geodyn. Geomater. 2013, 10, 453–458. [Google Scholar] [CrossRef]
- Carrado, K.A.; Winans, R.E. Electrochemical performance of sepiolite-derived carbon with lithium transition metal oxide positive electrodes. Electrochem. Carbon Mater. Proc. Int. Symp. 2004, 2000, 162. [Google Scholar]
- Novikova, L.; Roessner, F.; Belchinskaya, L.; AlSawalha, M.; Krupskaya, V. Study of surface acid-base properties of natural clays and zeolites by the conversion of 2-methylbut-3-yn-2-ol. Appl. Clay Sci. 2014, 101, 229–236. [Google Scholar] [CrossRef]
- Reinholdt, M.X.; Hubert, F.; Faurel, M.; Tertre, E.; Razafitianamaharavo, A.; Francius, G.; Prêt, D.; Petit, S.; Béré, E.; Pelletier, M. Morphological properties of vermiculite particles in size-selected fractions obtained by sonication. Appl. Clay Sci. 2013, 77, 18–32. [Google Scholar] [CrossRef]
- Sabah, E.; Turan, M. Adsorption mechanism of cationic surfactants onto acid- and heat-activated sepiolites. Water Res. 2002, 36, 3957–3964. [Google Scholar] [CrossRef]
- Lemić, J.; Tomašević-Čanović, M.; Djuričić, M.; Stanić, T. Surface modification of sepiolite with quaternary amines. J. Colloid Interface Sci. 2005, 292, 11–19. [Google Scholar] [CrossRef]
- Karamanis, D.; Ökte, A.N.; Vardoulakis, E.; Vaimakis, T. Applied Clay Science Water vapor adsorption and photocatalytic pollutant degradation with TiO2–sepiolite nanocomposites. Appl. Clay Sci. 2011, 53, 181–187. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Belver, C.; Bedia, J.; Amara, A.B.H.; Ruiz-Hitzky, E. ZnO/sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl. Clay Sci. 2018, 156, 104–109. [Google Scholar] [CrossRef]
- Chen, Y.; Dionysiou, D.D. Bimodal mesoporous TiO2–P25 composite thick films with high photocatalytic activity and improved structural integrity. Appl. Catal. B Environ. 2008, 80, 147–155. [Google Scholar] [CrossRef]
- Dvininov, E.; Popovici, E.; Pode, R.; Cocheci, L.; Barvinschi, P.; Nica, V. Synthesis and characterization of TiO2-pillared Romanian clay and their application for azoic dyes photodegradation. J. Hazard. Mater. 2009, 167, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Suárez, S.; Yates, M.; Petre, A.L.; Martín, J.A.; Avila, P.; Blanco, J. Development of a new Rh/TiO2–sepiolite monolithic catalyst for N2O decomposition. Appl. Catal. B Environ. 2006, 64, 302–311. [Google Scholar] [CrossRef]
- Zhang, G.; Xiong, Q.; Xu, W.; Guo, S. Synthesis of bicrystalline TiO2 supported sepiolite fibers and their photocatalytic activity for degradation of gaseous formaldehyde. Appl. Clay Sci. 2014, 102, 231–237. [Google Scholar] [CrossRef]
- Suárez, S.; Coronado, J.M.; Portela, R.; Martín, J.C.; Yates, M.; Avila, P.; Sánchez, B. On the preparation of TiO2−sepiolite hybrid materials for the photocatalytic degradation of TCE: Influence of TiO2 distribution in the mineralization. Environ. Sci. Technol. 2008, 42, 5892–5896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Yang, J. Preparation of TiO2/Sepiolite Composite and its adsorption and photo-catalysis property to simulated dye water. In 2009 International Conference on Energy and Environment Technology; IEEE: Piscataway, NJ, USA, 2009; Volume 3, pp. 465–468. [Google Scholar]
- Kamaya, Y.; Fukaya, Y.; Suzuki, K. Acute toxicity of benzoic acids to the crustacean Daphnia magna. Chemosphere 2005, 59, 255–261. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Fundamentals and Applications in Chemistry and Biology, ACS Professional Reference Book; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Todorova, N.; Giannakopoulou, T.; Karapati, S.; Petridis, D.; Vaimakis, T.; Trapalis, C. Applied Surface Science Composite TiO2/clays materials for photocatalytic NOx oxidation. Appl. Surf. Sci. 2014, 319, 113–120. [Google Scholar] [CrossRef]
- Dalmázio, I.; Santos, L.S.; Lopes, R.P.; Eberlin, M.N.; Augusti, R. Advanced oxidation of caffeine in water: On-line and real-time monitoring by electrospray ionization mass spectrometry. Environ. Sci. Technol. 2005, 39, 5982–5988. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Zhang, J.; Xie, S.; Wang, X.; Ji, Z. Honeycomb-like micro-mesoporous structure TiO2/sepiolite composite for combined chemisorption and photocatalytic elimination of formaldehyde. Microporous Mesoporous Mater. 2017, 248, 234–245. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Rozas, O.; Mansilla, H.D.; Gracia-Pinilla, M.A.; Meléndrez, M.F.; Anandan, S. Sonophotocatalytic degradation of Acid Blue 113 in the presence of rare earth nanoclusters loaded TiO2 nanophotocatalysts. Sep. Purif. Technol. 2014, 133, 407–414. [Google Scholar] [CrossRef]
- Xiao, N.; Li, D.; Cui, G.; Li, N.; Li, Q.; Wu, G. Electrochimica Acta Adsorption behavior of triblock copolymer suppressors during the copper electrodeposition. Electrochim. Acta 2014, 116, 284–291. [Google Scholar] [CrossRef]
- Ince, N.H.; Tezcanli, G.; Belen, R.; Apikyan, İ. Ultrasound as a catalyzer of aqueous reaction systems: The state of the art and environmental applications. Appl. Catal. B Environ. 2001, 29, 167–176. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Singh, V. Visible light induced sonophotocatalytic degradation of Reactive Red dye 198 using dye sensitized TiO2. Ultrason. Sonochem. 2007, 14, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Ogino, C.; Dadjour, M.F.; Murata, T. Sonocatalytic degradation of methylene blue with TiO2 pellets in water. Ultrason. Sonochem. 2007, 14, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ziylan-Yavas, A.; Mizukoshi, Y.; Maeda, Y.; Ince, N.H. Applied Catalysis B: Environmental Supporting of pristine TiO2 with noble metals to enhance the oxidation and mineralization of paracetamol by sonolysis and sonophotolysis. Appl. Catal. B Environ. 2015, 172–173, 7–17. [Google Scholar] [CrossRef] [Green Version]



| Characteristic | TiO2 | Sepiolite | Sepiolite/TiO2 |
|---|---|---|---|
| Size | 30–40 nm | 5 µm | 23.50–91.28 nm |
| Specific surface area (m2/g) | 35–65 | 160.77 | 210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savun-Hekimoğlu, B.; Eren, Z.; Ince, N.H. Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite. Sustainability 2020, 12, 10314. https://doi.org/10.3390/su122410314
Savun-Hekimoğlu B, Eren Z, Ince NH. Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite. Sustainability. 2020; 12(24):10314. https://doi.org/10.3390/su122410314
Chicago/Turabian StyleSavun-Hekimoğlu, Başak, Zeynep Eren, and Nilsun H. Ince. 2020. "Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite" Sustainability 12, no. 24: 10314. https://doi.org/10.3390/su122410314
APA StyleSavun-Hekimoğlu, B., Eren, Z., & Ince, N. H. (2020). Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite. Sustainability, 12(24), 10314. https://doi.org/10.3390/su122410314







