Cyperus laevigatus L. Enhances Diesel Oil Remediation in Synergism with Bacterial Inoculation in Floating Treatment Wetlands
Abstract
:1. Introduction
2. Methodology
2.1. Diesel Oil
2.2. Preparation of Mixed Bacterial Culture
2.3. Manufacturing of Floating Treatment Wetlands
2.4. Experimental Design
2.5. Plant Biomass
2.6. Hydrocarbons Assessment
2.7. Water Quality Parameter Analyses
2.8. Persistence of Bacterial Culture
2.9. Evaluation of Toxicity
2.10. Statistical Analyses
3. Results and Discussion
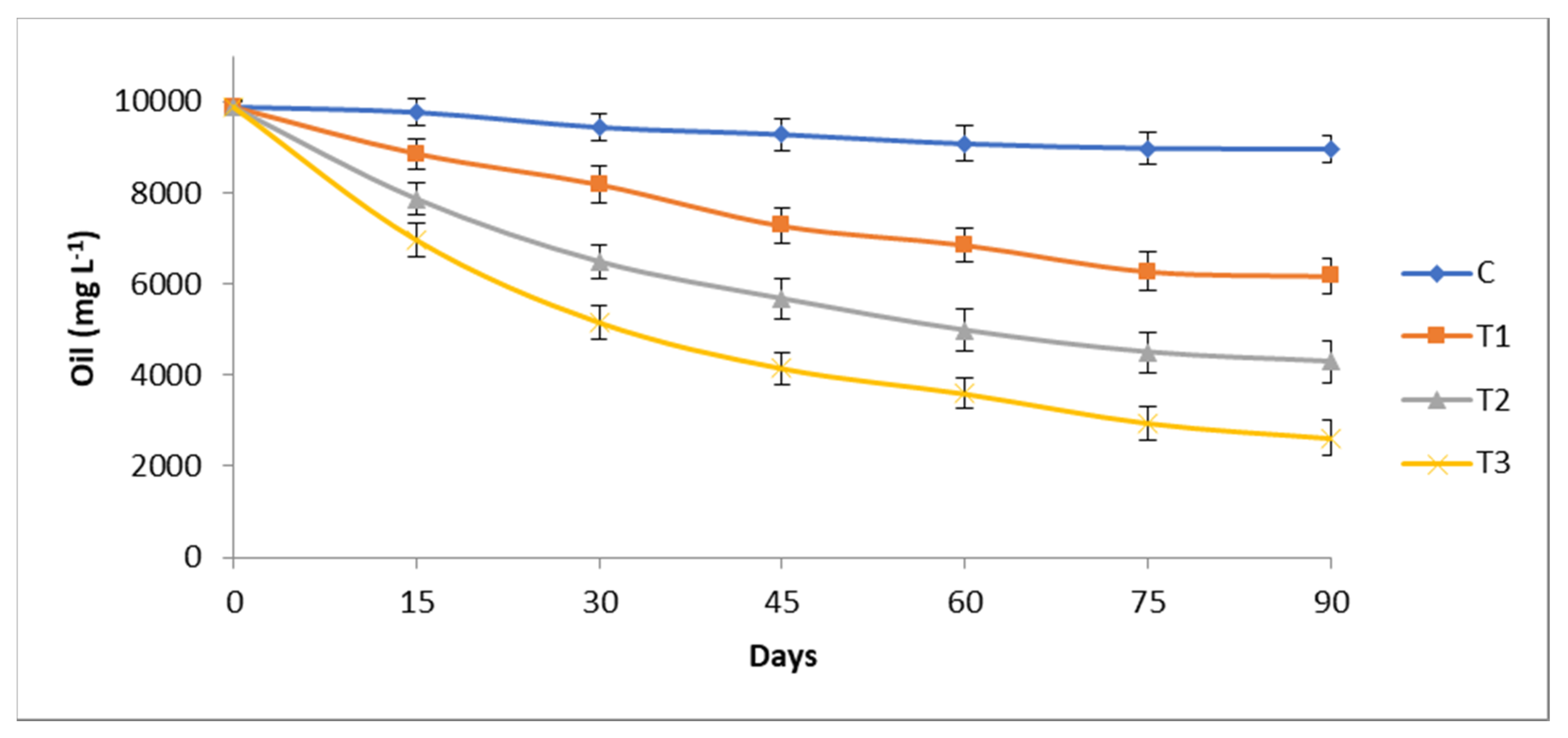
3.1. Hydrocarbons Degradation
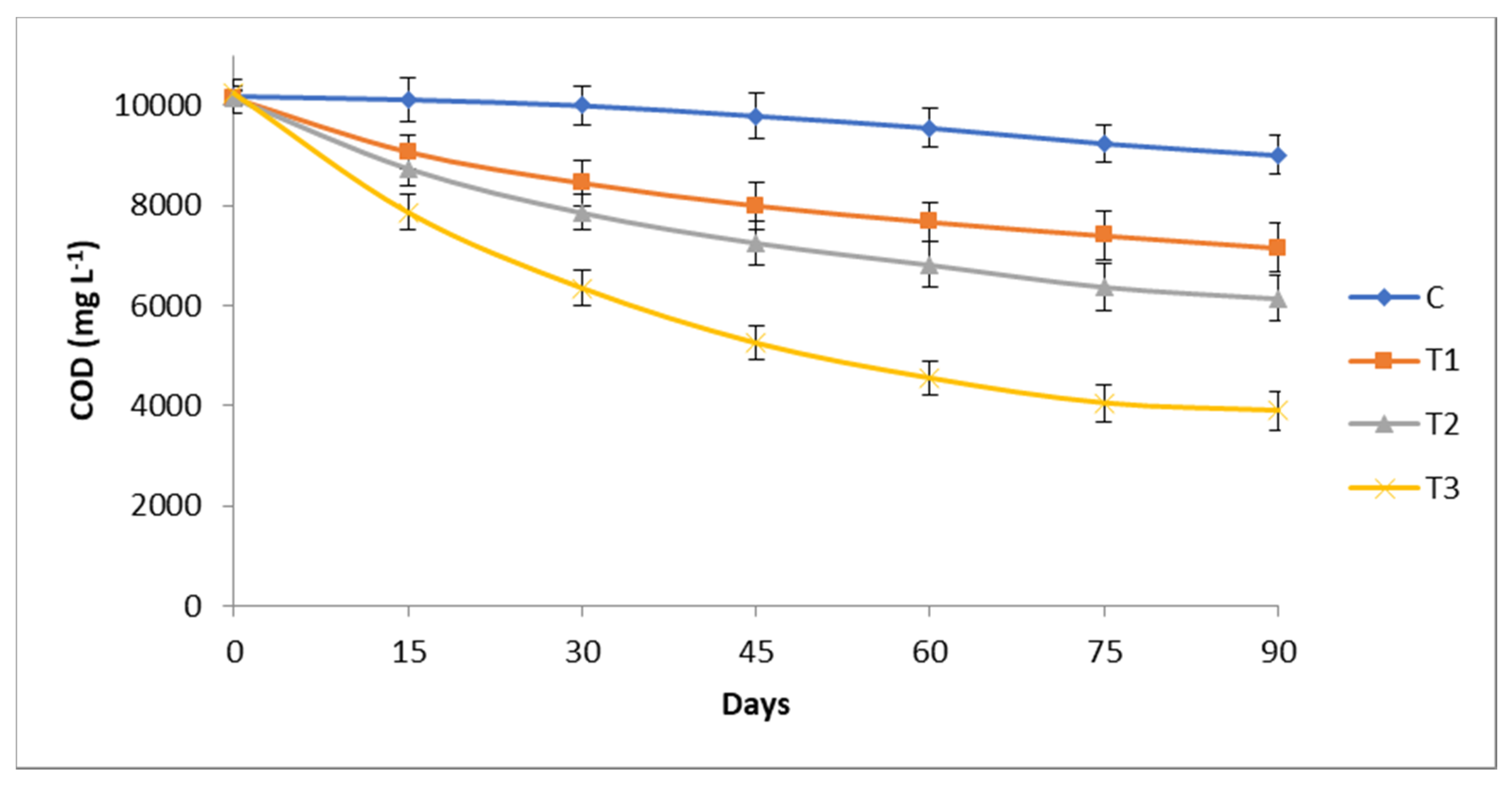
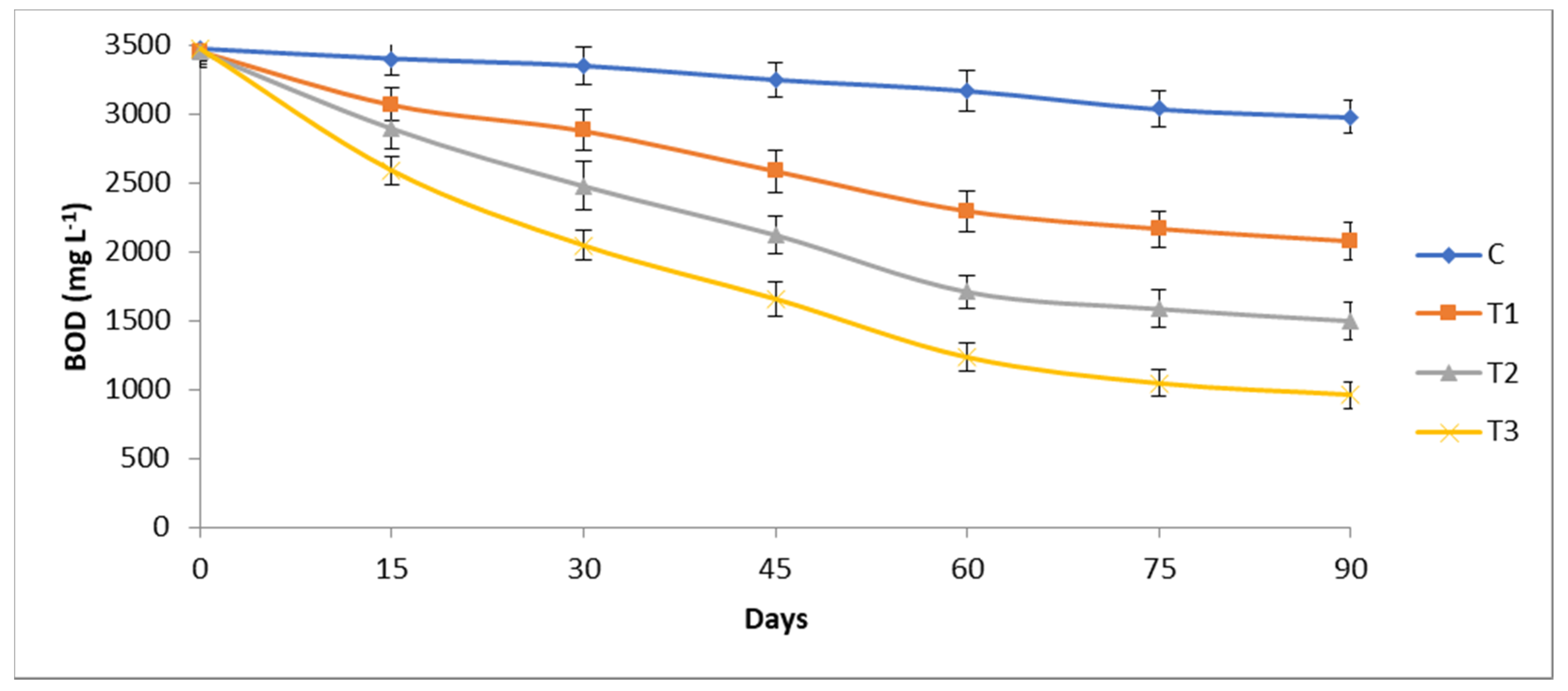
3.2. Chemical and Biological Oxygen Demand
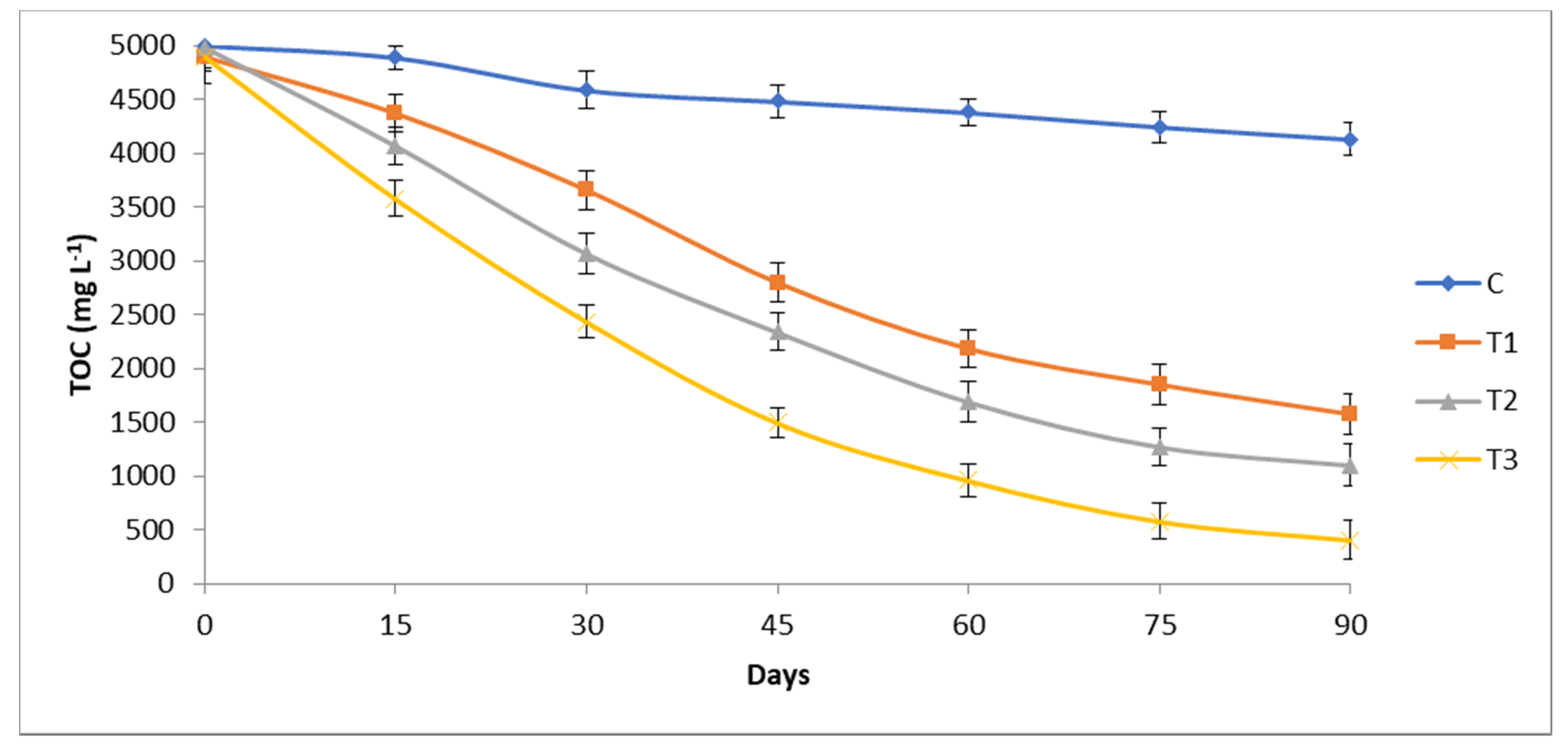
3.3. Total Organic Carbon and Phenol Reduction
3.4. Removal of Solids
3.5. Persistence of Microbial Population
3.6. Plant Height and Biomass
3.7. Reduction of Toxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.; Hao, H.; Yang, X.; Xiang, L.; Zhao, F.; Jiang, H.; He, Z. Purification of refinery wastewater by different perennial grasses growing in a floating bed. J. Plant Nutr. 2012, 35, 93–110. [Google Scholar] [CrossRef]
- Tony, M.A.; Purcell, P.J.; Zhao, Y. Oil refinery wastewater treatment using physicochemical, Fenton and Photo-Fenton oxidation processes. J. Environ. Sci. Health 2012, 47, 435–440. [Google Scholar] [CrossRef]
- Daflon, S.D.A.; Guerra, I.L.; Reynier, M.V.; Botta, C.R.; Campos, J.C. Toxicity identification and evaluation of a refinery wastewater from Brazil (Phase I). Ecotoxicol. Environ. Contam. 2015, 10, 41–45. [Google Scholar] [CrossRef]
- Shahriari, T.; Karbassi, A.; Reyhani, M. Treatment of oil refinery wastewater by electrocoagulation–flocculation (Case Study: Shazand Oil Refinery of Arak). Int. J. Environ. Sci. Technol. 2018, 16, 4159–4166. [Google Scholar] [CrossRef]
- Hernández-Francisco, E.; Peral, J.; Blanco-Jerez, L. Removal of phenolic compounds from oil refinery wastewater by electrocoagulation and Fenton/photo-Fenton processes. J. Water Proc. Eng. 2017, 19, 96–100. [Google Scholar] [CrossRef]
- Agarry, S.E.; Oghenejoboh, K.M.; Latinwo, G.K.; Owabor, C.N. Biotreatment of petroleum refinery wastewater in vertical surface-flow constructed wetland vegetated with Eichhornia crassipes: Lab-scale experimental and kinetic modelling. Environ. Technol. 2018, 1–21. [Google Scholar] [CrossRef]
- Mandri, T.; Lin, J. Isolation and characterization of engine oil degrading indigenous microrganisms in Kwazulu-Natal, South Africa. Afr. J. Biotechnol. 2007, 7, 1927–1932. [Google Scholar]
- Mustapha, H.I.; Van Bruggen, J.; Lens, P. Vertical subsurface flow constructed wetlands for polishing secondary Kaduna refinery wastewater in Nigeria. Ecol. Eng. 2015, 84, 588–595. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Chen, Z.; Reiche, N.; Vymazal, J.; Kuschk, P. Treatment of water contaminated by volatile organic compounds in hydroponic root mats. Ecol. Eng. 2017, 98, 339–345. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Siddique, M.; Ali, S.; Tahseen, R.; Afzal, M. Potentialities of floating wetlands for the treatment of polluted water of river Ravi, Pakistan. Ecol. Eng. 2019, 133, 167–176. [Google Scholar] [CrossRef]
- Tara, N.; Arslan, M.; Hussain, Z.; Iqbal, M.; Khan, Q.M.; Afzal, M. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J. Clean. Prod. 2019, 217, 541–548. [Google Scholar] [CrossRef]
- Chen, Z.; Kuschk, P.; Reiche, N.; Borsdorf, H.; Kästner, M.; Köser, H. Comparative evaluation of pilot scale horizontal subsurface-flow constructed wetlands and plant root mats for treating groundwater contaminated with benzene and MTBE. J. Hazard. Mater. 2012, 209, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Sample, D.J. Assessment of the nutrient removal effectiveness of floating treatment wetlands applied to urban retention ponds. J. Environ. Manag. 2014, 137, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Headley, T.R.; Tanner, C.C. Constructed wetlands with floating emergent macrophytes: An innovative stormwater treatment technology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2261–2310. [Google Scholar] [CrossRef]
- Tanner, C.C.; Headley, T.R. Components of floating emergent macrophyte treatment wetlands influencing removal of stormwater pollutants. Ecol. Eng. 2011, 37, 474–486. [Google Scholar] [CrossRef]
- Chen, Z.; Cuervo, D.P.; Müller, J.A.; Wiessner, A.; Köser, H.; Vymazal, J.; Kästner, M.; Kuschk, P. Hydroponic root mats for wastewater treatment—A review. Environ. Sci. Pollut. Res. 2016, 23, 15911–15928. [Google Scholar] [CrossRef]
- Faulwetter, J.; Burr, M.D.; Cunningham, A.B.; Stewart, F.M.; Camper, A.K.; Stein, O.R. Floating treatment wetlands for domestic wastewater treatment. Water Sci. Technol. 2011, 64, 2089–2095. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Dastidar, M.G.; Sreekrishnan, T. Bioremediation of Chromium Complex Dye by Growing Aspergillus flavus. In Water Quality Management; Springer: New York, NY, USA, 2018; pp. 81–92. [Google Scholar]
- Sessitsch, A.; Mitter, B.; Compant, S.; Trognitz, F.; Brader, G. The plant microbiome: Ecology and functioning of bacterial endophytes and how plants can benefit. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 8–13 April 2018; p. 2201. [Google Scholar]
- Gomes, M.; Gonzales-Limache, E.; Sousa, S.; Dellagnezze, B.; Sartoratto, A.; Silva, L.; Gieg, L.; Valoni, E.; Souza, R.; Torres, A. Exploring the potential of halophilic bacteria from oil terminal environments for biosurfactant production and hydrocarbon degradation under high-salinity conditions. Int. Biodeterior. Biodegrad. 2018, 126, 231–242. [Google Scholar] [CrossRef]
- Lopes, P.R.M.; Montagnolli, R.N.; Cruz, J.M.; Claro, E.M.T.; Bidoia, E.D. Biosurfactants in Improving Bioremediation Effectiveness in Environmental Contamination by Hydrocarbons. In Microbial Action on Hydrocarbons; Springer: New York, NY, USA, 2018; pp. 21–34. [Google Scholar]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef]
- Fatima, K.; Afzal, M.; Imran, A.; Khan, Q.M. Bacterial rhizosphere and endosphere populations associated with grasses and trees to be used for phytoremediation of crude oil contaminated soil. Bull. Environ. Contam. Toxicol. 2015, 94, 314–320. [Google Scholar] [CrossRef]
- Arslan, M.; Afzal, M.; Amin, I.; Iqbal, S.; Khan, Q.M. Nutrients can enhance the abundance and expression of alkane hydroxylase CYP153 gene in the rhizosphere of ryegrass planted in hydrocarbon-polluted soil. PLoS ONE 2014, 9, e111208. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Enhancement of oil field-produced wastewater remediation by bacterially-augmented floating treatment wetlands. Chemosphere 2019, 217, 576–583. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int. J. Phytoremediat. 2012, 14, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.J.; Tahseen, R.; Siddique, M.; Ali, S.; Iqbal, S.; Afzal, M. Remediation of polluted river water by floating treatment wetlands. Water Supply 2019, 19, 967–977. [Google Scholar] [CrossRef]
- Shehzadi, M.; Afzal, M.; Khan, M.U.; Islam, E.; Mobin, A.; Anwar, S.; Khan, Q.M. Enhanced degradation of textile effluent in constructed wetland system using Typha domingensis and textile effluent-degrading endophytic bacteria. Water Res. 2014, 58, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.; Banat, I.M.; Joshi, S.J. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal. Agric. Biotechnol. 2018, 14, 23–32. [Google Scholar]
- Varjani, S.J.; Joshi, R.R.; Kumar, P.S.; Srivastava, V.K.; Kumar, V.; Banerjee, C.; Kumar, R.P. Polycyclic aromatic hydrocarbons from petroleum oil industry activities: Effect on human health and their biodegradation. In Waste Bioremediation; Springer: New York, NY, USA, 2018; pp. 185–199. [Google Scholar]
- Jambon, I.; Thijs, S.; Weyens, N.; Vangronsveld, J. Harnessing plant-bacteria-fungi interactions to improve plant growth and degradation of organic pollutants. J. Plant Interact. 2018, 13, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Borthakur, A. A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean. Prod. 2018, 196, 1669–1680. [Google Scholar] [CrossRef]
- Turkovskaya, O.; Muratova, A. Plant–Bacterial Degradation of Polyaromatic Hydrocarbons in the Rhizosphere. Trends Biotechnol. 2019, 37, 926–930. [Google Scholar] [CrossRef]
- Kumari, S.; Regar, R.K.; Manickam, N. Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, J.; Cui, N.; Dai, Y.; Kong, L.; Wu, J.; Cheng, S. Enhancing the water purification efficiency of a floating treatment wetland using a biofilm carrier. Environ. Sci. Pollut. Res. 2016, 23, 7437–7443. [Google Scholar] [CrossRef] [PubMed]
- Adeboye, P.T.; Bettiga, M.; Olsson, L. The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. Amb Express 2014, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Dhote, M.; Kumar, A.; Jajoo, A.; Juwarkar, A. Assessment of hydrocarbon degradation potentials in a plant–microbe interaction system with oil sludge contamination: A sustainable solution. Int. J. Phytoremediat. 2017, 19, 1085–1092. [Google Scholar] [CrossRef]
- Pawlik, M.; Cania, B.; Thijs, S.; Vangronsveld, J.; Piotrowska-Seget, Z. Hydrocarbon degradation potential and plant growth-promoting activity of culturable endophytic bacteria of Lotus corniculatus and Oenothera biennis from a long-term polluted site. Environ. Sci. Pollut. Res. 2017, 24, 19640–19652. [Google Scholar] [CrossRef]
- Kösesakal, T.; Ünal, M.; Kulen, O.; Memon, A.; Yüksel, B. Phytoremediation of petroleum hydrocarbons by using a freshwater fern species Azolla filiculoides Lam. Int. J. Phytoremediat. 2016, 18, 467–476. [Google Scholar] [CrossRef]
- Darajeh, N.; Idris, A.; Truong, P.; Abdul Aziz, A.; Abu Bakar, R.; Che Man, H. Phytoremediation potential of vetiver system technology for improving the quality of palm oil mill effluent. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-B.; Liu, W.-L.; Pan, X.-C.; Guan, M.; Liu, S.-Y.; Ge, Y.; Chang, J. Comparison of effects of plant and biofilm bacterial community parameters on removal performances of pollutants in floating island systems. Ecol. Eng. 2014, 73, 58–63. [Google Scholar] [CrossRef]
- Mitter, E.K.; Kataoka, R.; de Freitas, J.R.; Germida, J.J. Potential use of endophytic root bacteria and host plants to degrade hydrocarbons. Int. J. Phytoremediat. 2019, 21, 928–938. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with bacteria in floating treatment wetlands positively modulates the phytoremediation of oil field wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef]
- Ye, C.; Chen, D.; Hall, S.J.; Pan, S.; Yan, X.; Bai, T.; Guo, H.; Zhang, Y.; Bai, Y.; Hu, S. Reconciling multiple impacts of nitrogen enrichment on soil carbon: Plant, microbial and geochemical controls. Ecol. Lett. 2018, 21, 1162–1173. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tang, C.; Severi, J.; Butterly, C.R.; Baldock, J.A. Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol. 2016, 211, 864–873. [Google Scholar] [CrossRef]
- Saleem, H.; Rehman, K.; Arslan, M.; Afzal, M. Enhanced degradation of phenol in floating treatment wetlands by plant-bacterial synergism. Int. J. Phytoremediat. 2018, 20, 692–698. [Google Scholar] [CrossRef]
- Ijaz, A.; Imran, A.; ul Haq, M.A.; Khan, Q.M.; Afzal, M. Phytoremediation: Recent advances in plant-endophytic synergistic interactions. Plant Soil 2016, 405, 179–195. [Google Scholar] [CrossRef]
- Cui, L.; Ouyang, Y.; Lou, Q.; Yang, F.; Chen, Y.; Zhu, W.; Luo, S. Removal of nutrients from wastewater with Canna indica L. under different vertical-flow constructed wetland conditions. Ecol. Eng. 2016, 36, 1083–1088. [Google Scholar] [CrossRef]
- Bordoloi, N.; Konwar, B. Bacterial biosurfactant in enhancing solubility and metabolism of petroleum hydrocarbons. J. Hazard. Mater. 2009, 170, 495–505. [Google Scholar] [CrossRef]
- Ijaz, A.; Iqbal, Z.; Afzal, M. Remediation of sewage and industrial effluent using bacterially assisted floating treatment wetlands vegetated with Typha domingensis. Water Sci. Technol. 2016, 74, 2192–2201. [Google Scholar] [CrossRef] [Green Version]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2019, 249, 71–131. [Google Scholar]
- Pal, S.; Kundu, A.; Banerjee, T.D.; Mohapatra, B.; Roy, A.; Manna, R.; Sar, P.; Kazy, S.K. Genome analysis of crude oil degrading Franconibacter pulveris strain DJ34 revealed its genetic basis for hydrocarbon degradation and survival in oil contaminated environment. Genomics 2017, 109, 374–382. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- Hussain, Z.; Arslan, M.; Malik, M.H.; Mohsin, M.; Iqbal, S.; Afzal, M. Integrated perspectives on the use of bacterial endophytes in horizontal flow constructed wetlands for the treatment of liquid textile effluent: Phytoremediation advances in the field. J. Environ. Manag. 2018, 224, 387–395. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, J.; Lou, L.; Zhu, L. Solubilization properties of polycyclic aromatic hydrocarbons by saponin, a plant-derived biosurfactant. Environ. Pollut. 2011, 159, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Gogosz, A.; Bona, C.; Santos, G.; Botosso, P. Germination and initial growth of Campomanesia xanthocarpa O. Berg.(Myrtaceae), in petroleum-contaminated soil and bioremediated soil. Braz. J. Biol 2010, 70, 977–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. Ecology of Alkane-Degrading Bacteria and Their Interaction with the Plant. Mol. Microb. Ecol. Rhizosphere 2013, 975–989. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant–endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Mesa, V.; Navazas, A.; González-Gil, R.; González, A.; Weyens, N.; Lauga, B.; Gallego, J.L.R.; Sánchez, J.; Peláez, A.I. Use of endophytic and rhizosphere bacteria to improve phytoremediation of arsenic-contaminated industrial soils by autochthonous Betula celtiberica. Appl. Environ. Microbiol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, A.; Shabir, G.; Khan, Q.M.; Afzal, M. Enhanced remediation of sewage effluent by endophyte-assisted floating treatment wetlands. Ecol. Eng. 2015, 84, 58–66. [Google Scholar] [CrossRef]
- Bai, H.; Wu, M.; Zhang, H.; Tang, G. Chronic polycyclic aromatic hydrocarbon exposure causes DNA damage and genomic instability in lung epithelial cells. Oncotarget 2017, 8, 79034. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment | Days | Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| pH | EC (ms/cm) | TDS (mg/L) | TS (mg/L) | TSS (mg/L) | DO (mg/L) | Phenol (mg/L) | ||
| Control | 0 | 8.7ab(0.13) | 3.3ab(0.01) | 1918a(175) | 2274a(232) | 356b(13) | 5.5ef(0.13) | 0.35a(0.01) |
| 15 | 8.5abc(0.12) | 3.3ab(0.01) | 1912ab(197) | 2270a(215) | 350bcd(25) | 5.2fg(0.14) | 0.34b(0.01) | |
| 30 | 8.3def(0.16) | 3.2ab(0.03) | 1908ab(178) | 2268a(234) | 346bcde(16) | 5.0ghi(0.12) | 0.32c(0.01) | |
| 45 | 8.2efg(0.15) | 3.2ab(0.07) | 1906ab(183) | 2255a(196) | 336cdef(24) | 5.1gh(0.13) | 0.32cd(0.06) | |
| 60 | 8.1fgh(0.11) | 3.2b(0.01) | 1903ab(192) | 2245a(234) | 331def(26) | 4.9hij(0.14) | 0.32cd(0.01) | |
| 75 | 8.0fgh(0.19) | 3.2b(0.08) | 1902ab(125) | 2239a(266) | 329ef(16) | 4.7ij(0.16) | 0.32cd(0.01) | |
| 90 | 8.0fgh(0.14) | 3.1b(0.01) | 1900ab(147) | 2225a(297) | 317f(24) | 4.5jk(0.15) | 0.31d(0.07) | |
| T1 | 0 | 8.8a(0.14) | 3.3ab(0.01) | 1921a(115) | 2276a(195) | 355bc(15) | 5.3fg(0.15) | 0.35ab(0.01) |
| 15 | 8.7ab(0.15) | 2.3c(0.04) | 1615d(156) | 1811c(176) | 296g(18) | 5.1gh(0.14) | 0.17f(0.03) | |
| 30 | 8.6abc(0.12) | 2.1d(0.05) | 1312g(169) | 1509g(197) | 197ijk(17) | 4.8jk(0.13) | 0.13i(0.00) | |
| 45 | 8.6abc(0.13) | 1.2fghij(0.05) | 1208h(149) | 1321h(144) | 143l(15) | 4.3kl(0.15) | 0.10j(0.00) | |
| 60 | 8.5bcd(0.15) | 1.1ghij(0.03) | 1129i(124) | 1217ij(105) | 117m(17) | 4.0lm(0.16) | 0.09k(0.00) | |
| 75 | 8.4cde(0.17) | 1.1hij(0.04) | 1078j(156) | 1161jk(108) | 103mn(18) | 3.8mn(0.17) | 0.08k(0.00) | |
| 90 | 8.2def(0.19) | 1.0ij(0.04) | 1011k(108) | 1117kl(145) | 92n(15) | 3.5n(0.13) | 0.08k(0.00) | |
| T2 | 0 | 8.5bcd(0.13) | 3.2ab(0.06) | 1924a(254) | 2275a(196) | 359b(15) | 5.6de(0.14) | 0.35ab(0.04) |
| 15 | 8.2efg(0.11) | 2.9b(0.04) | 1880b(147) | 1972b(162) | 388a(22) | 5.3fg(0.15) | 0.19e(0.01) | |
| 30 | 8.1fgh(0.16) | 2.3c(0.06) | 1505e(138) | 1681d(144) | 258h(23) | 5.9cd(0.16) | 0.13h(0.00) | |
| 45 | 8.0gh(0.17) | 1.5defgh(0.05) | 1008k(148) | 1514f(177) | 198i(12) | 6.3bc(0.14) | 0.03n(0.00) | |
| 60 | 7.5i(0.14) | 1.3efghij(0.06) | 937lm(168) | 1475fg(176) | 184ij(14) | 6.1c(0.16) | 0.03no(0.00) | |
| 75 | 7.2j(0.13) | 1.4efghi(0.04) | 929m(159) | 1357h(156) | 145l(15) | 5.9cd(0.13) | 0.02no(0.00) | |
| 90 | 7.1j(0.15) | 1.3efghij(0.01) | 916m(148) | 1253i(145) | 86n(15) | 6.3bc(0.16) | 0.020(0.00) | |
| T3 | 0 | 8.5cd(0.17) | 3.6a(0.05) | 1922a(138) | 2278a(184) | 356b(16) | 5.6de(0.17) | 0.35ab(0.01) |
| 15 | 8.1fgh(0.14) | 1.9de(0.04) | 1718c(129) | 1921b(192) | 317f(18) | 5.7de(0.14) | 0.14g(0.00) | |
| 30 | 7.8h(0.16) | 1.6def(0.05) | 1408f(127) | 1611e(147) | 193i(15) | 6.5b(0.16) | 0.12hi(0.00) | |
| 45 | 7.5i(0.15) | 1.5defg(0.05) | 1211h(148) | 1438g(174) | 173jk(19) | 6.9a(0.15) | 0.06l(0.00) | |
| 60 | 7.2j(0.17) | 1.2fghij(0.01) | 1179h(136) | 1338h(134) | 160kl(14) | 7.1a(0.16) | 0.05m(0.00) | |
| 75 | 7.1j(0.14) | 1.0ij(0.01) | 1029k(148) | 1138kl(136) | 112m(17) | 7.0a(0.14) | 0.02no(0.00) | |
| 90 | 6.9k(0.18) | 0.9j(0.01) | 968l(149) | 1089l(142) | 87n(13) | 7.1a(0.15) | 0.02o(0.00) | |
| Treatments | Cfu × 105 | ||||||
|---|---|---|---|---|---|---|---|
| 0 d | 15 d | 30 d | 45 d | 60 d | 75 d | 90 d | |
| Water (Cfu/mL) | 27.8f(1.5) | 26.3h(1.3) | 17.8j(0.9) | 16.5k(1.8) | 14.4l(1.6) | 12.3m(1.5) | 10.1o(0.8) |
| Rhizoplane (Cfu/g) | 8.7p(0.8) | 12.3m(1.1) | 19.7i(1.2) | 27.5fg(1.9) | 34.2d(2.1) | 39.6c(2.4) | 45.8a(2.7) |
| Root (Cfu/g) | 0.4v(0.05) | 5.4s(1.5) | 11.7n(1.8) | 19.9i(1.5) | 27.3g(1.3) | 33.8e(1.4) | 40.5b(1.3) |
| Shoot (Cfu/g) | 0.1v(0.01) | 1.2u(1.4) | 3.5t(0.1) | 5.9r(0.3) | 7.0q(1.1) | 9.8o(1.3) | 12.3m(1.6) |
| Treatments | Fresh Biomass (g) | Dry Biomass (g) | Length (cm) | |||
|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | |
| C2 | 302b(7.6) | 454a(33.7) | 191b(7.8) | 306a(11.8) | 31.4c(1.1) | 56.2a(2.1) |
| T2 | 91f(3.8) | 198d(6.3) | 46f(1.3) | 68e(3.1) | 14.4f(0.8) | 28.6d(1.8) |
| T3 | 109e(6.7) | 218c(13.8) | 78d(4.9) | 93c(4.2) | 18.5e(1.2) | 34.9b(2.2) |
| Treatments | Fish Death Time | Total Death | Detoxification Position | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |||
| Control | 10 | 0 | 0 | 0 | 10/10a | Negligible |
| T1 | 2 | 1 | 1 | 1 | 5/10b | Partial |
| T2 | 1 | 1 | 1 | 1 | 3/10c | Partial |
| T3 | 1 | 1 | 0 | 0 | 2/10d | Complete |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahid, M.; Ali, S.; Shabir, G.; Rashid Ahmad, S.; Yasmeen, T.; Afzal, M.; Arslan, M.; Hussain, A.; Hashem, A.; Abd Allah, E.F.; et al. Cyperus laevigatus L. Enhances Diesel Oil Remediation in Synergism with Bacterial Inoculation in Floating Treatment Wetlands. Sustainability 2020, 12, 2353. https://doi.org/10.3390/su12062353
Fahid M, Ali S, Shabir G, Rashid Ahmad S, Yasmeen T, Afzal M, Arslan M, Hussain A, Hashem A, Abd Allah EF, et al. Cyperus laevigatus L. Enhances Diesel Oil Remediation in Synergism with Bacterial Inoculation in Floating Treatment Wetlands. Sustainability. 2020; 12(6):2353. https://doi.org/10.3390/su12062353
Chicago/Turabian StyleFahid, Muhammad, Shafaqat Ali, Ghulam Shabir, Sajid Rashid Ahmad, Tahira Yasmeen, Muhammad Afzal, Muhammad Arslan, Afzal Hussain, Abeer Hashem, Elsayed Fathi Abd Allah, and et al. 2020. "Cyperus laevigatus L. Enhances Diesel Oil Remediation in Synergism with Bacterial Inoculation in Floating Treatment Wetlands" Sustainability 12, no. 6: 2353. https://doi.org/10.3390/su12062353
APA StyleFahid, M., Ali, S., Shabir, G., Rashid Ahmad, S., Yasmeen, T., Afzal, M., Arslan, M., Hussain, A., Hashem, A., Abd Allah, E. F., Alyemeni, M. N., & Ahmad, P. (2020). Cyperus laevigatus L. Enhances Diesel Oil Remediation in Synergism with Bacterial Inoculation in Floating Treatment Wetlands. Sustainability, 12(6), 2353. https://doi.org/10.3390/su12062353









