Techno-Economic Analysis of Electrocoagulation on Water Reclamation and Bacterial/Viral Indicator Reductions of a High-Strength Organic Wastewater—Anaerobic Digestion Effluent
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed
2.2. Electrocoagulation (EC)
2.3. Technoeconomic Analysis
2.4. Parameter Analysis
2.5. Statistical Analysis
3. Results and Discussions
3.1. Effects of Metal Combinations on EC of AD Effluent
3.2. EC Treatment of Water Reclamation and Microorganism Removal
3.3. Technoeconomic Analysis
3.3.1. Mass and Energy Balance
3.3.2. Economic Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dean, R.B. Process for water reclamation. Waste Manag. Res. 1991, 9, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Khan, A.A.; Kazmi, A.A.; Chopra, A.K. Enhancement of Coagulation Flocculation Process Using Anionic Polymer for the Post Treatment of UASB Reactor Effluent. Sep. Sci. Technol. 2010, 45, 626–634. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M.; Patureau, D. Digestate mechanical separation: Efficiency profiles based on anaerobic digestion feedstock and equipment choice. Bioresour. Technol. 2019, 274, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, W.; Drosg, B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Omidinasab, M.; Ghanbari, F. Combined electrocoagulation and UV-based sulfate radical oxidation processes for treatment of pulp and paper wastewater. Process Saf. Environ. Prot. 2016, 102, 462–472. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Argyriou, R.; Economou, C.N.; Charalampous, N.; Dailianis, S.; Tatoulis, T.I.; Tekerlekopoulou, A.G.; Vayenas, D.V. Treatment of printing ink wastewater using electrocoagulation. J. Environ. Manag. 2019, 237, 442–448. [Google Scholar] [CrossRef]
- Changmai, M.; Pasawan, M.; Purkait, M.K. Treatment of oily wastewater from drilling site using electrocoagulation followed by microfiltration. Sep. Purif. Technol. 2019, 210, 463–472. [Google Scholar] [CrossRef]
- Heidmann, I.; Calmano, W. Removal of Ni, Cu and Cr from a galvanic wastewater in an electrocoagulation system with Fe-and Al-electrodes. Sep. Purif. Technol. 2010, 71, 308–314. [Google Scholar] [CrossRef]
- Liu, Z.; Stromberg, D.; Liu, X.; Liao, W.; Liu, Y. A new multiple-stage electrocoagulation process on anaerobic digestion effluent to simultaneously reclaim water and clean up biogas. J. Hazard. Mater. 2015, 285, 483–490. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Ilhan, F.; Sapci-Zengin, Z.; Sakar, S.; Gonullu, M.T. Decolorization and COD reduction of UASB pretreated poultry manure wastewater by electrocoagulation process: A post-treatment study. J. Hazard. Mater. 2009, 162, 120–132. [Google Scholar] [CrossRef]
- Kobya, M.; Senturk, E.; Bayramoglu, M. Treatment of poultry slaughterhouse wastewaters by electrocoagulation. J. Hazard. Mater. 2006, 133, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Ricordel, C.; Darchen, A.; Hadjiev, D. Electrocoagulation-electroflotation as a surface water treatment for industrial uses. Sep. Purif. Technol. 2010, 74, 342–347. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009, 90, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D.; Badis, A.; Kellil, A.; Ghernaout, B. Application of electrocoagulation in Escherichia coli culture and two surface waters. Desalination 2008, 219, 118–125. [Google Scholar] [CrossRef]
- Anfruns-Estrada, E.; Bruguera-Casamada, C.; Salvado, H.; Brillas, E.; Sires, I.; Araujo, R.M. Inactivation of microbiota from urban wastewater by single and sequential electrocoagulation and electro-Fenton treatments. Water Res. 2017, 126, 450–459. [Google Scholar] [CrossRef]
- Butler, E.; Hung, Y.-T.; Yeh, R.Y.-L.; Al Ahmad, M.S. Electrocoagulation in Wastewater Treatment. Water 2011, 3, 495–525. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 2nd ed.; IWA Publishing: London, UK, 2006; pp. 1–407. [Google Scholar]
- Crittenden, J.C.; Harza, B.M.W. Water Treatment: Principles and Design; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Paulista, L.O.; Presumido, P.H.; Peruco Theodoro, J.D.; Novaes Pinheiro, A.L. Efficiency analysis of the electrocoagulation and electroflotation treatment of poultry slaughterhouse wastewater using aluminum and graphite anodes. Environ. Sci. Pollut. Res. 2018, 25, 19790–19800. [Google Scholar] [CrossRef]
- Djerroud, N.; Adjeroud, N.; Felkai-Haddache, L.; Hammoui, Y.; Remini, H.; Dahmoune, F.; Merzouk, B.; Madani, K. Enhanced electrocoagulation-electroflotation for turbidity removal by Opuntia ficus indica cladode mucilage. Water Environ. J. 2018, 32, 321–332. [Google Scholar] [CrossRef]
- Bassala, H.D.; Dedzo, G.K.; Bememba, C.B.N.; Seumo, P.M.T.; Dazie, J.D.; Nanseu-Njiki, C.P.; Ngameni, E. Investigation of the efficiency of a designed electrocoagulation reactor: Application for dairy effluent treatment. Process Saf. Environ. Prot. 2017, 111, 122–127. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Askari, M.; Dehghani, M.H.; Dalvand, A.; Saeedi, R.; Yetilmezsoy, K.; Heibati, B.; McKay, G. Elimination of natural organic matter by electrocoagulation using bipolar and monopolar arrangements of iron and aluminum electrodes. Int. J. Environ. Sci. Technol. 2017, 14, 2125–2134. [Google Scholar] [CrossRef]
- Daniel, R.; Rao, P.A.V.S. An Efficient Removal of Arsenic from Industrial Effluents using Electro-coagulation as Clean Technology Option. Int. J. Environ. Res. 2012, 6, 711–718. [Google Scholar]
- Aoudj, S.; Khelifa, A.; Drouiche, N. Removal of fluoride, SDS, ammonia and turbidity from semiconductor wastewater by combined electrocoagulation-electroflotation. Chemosphere 2017, 180, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Greenburg, A. Standard Methods for the Examination of Water and Wastewater, 16th ed.; American Public Health Asscociation: Washington, DC, USA, 1985. [Google Scholar]
- Clancy, J.L.; Bukhari, Z.; McCuin, R.M.; Matheson, Z.; Fricker, C.R. USEPA method 1622. Am. Water Work. Assoc. J. 1999, 91, 60–68. [Google Scholar] [CrossRef]
- ASTM: D6503-99, Standard Test Method for Enterococci in Water Using Enterolert; ASTM International: West Conshohocken, PA, USA, 2005.
- USEPA. Method 1602: Male-specific (F+) and Somatic Coliphage in Water by Single Agar Layer (SAL) Procedure; USEPA: Washington, DC, USA, 2001.
- Picard, T.; Cathalifaud-Feuillade, G.; Mazet, M.; Vandensteendam, C. Cathodic dissolution in the electrocoagulation process using aluminium electrodes. J. Environ. Monit. 2000, 2, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L. Aluminum and Alzheimer’s Disease: After a Century of Controversy, Is there a Plausible Link? J. Alzheimers Dis. 2011, 23, 567–598. [Google Scholar] [CrossRef]
- Moeller, K.; Mueller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Guerrini, A.; Romano, G.; Indipendenza, A. Energy Efficiency Drivers in Wastewater Treatment Plants: A Double Bootstrap DEA Analysis. Sustainability 2017, 9, 1126. [Google Scholar] [CrossRef]
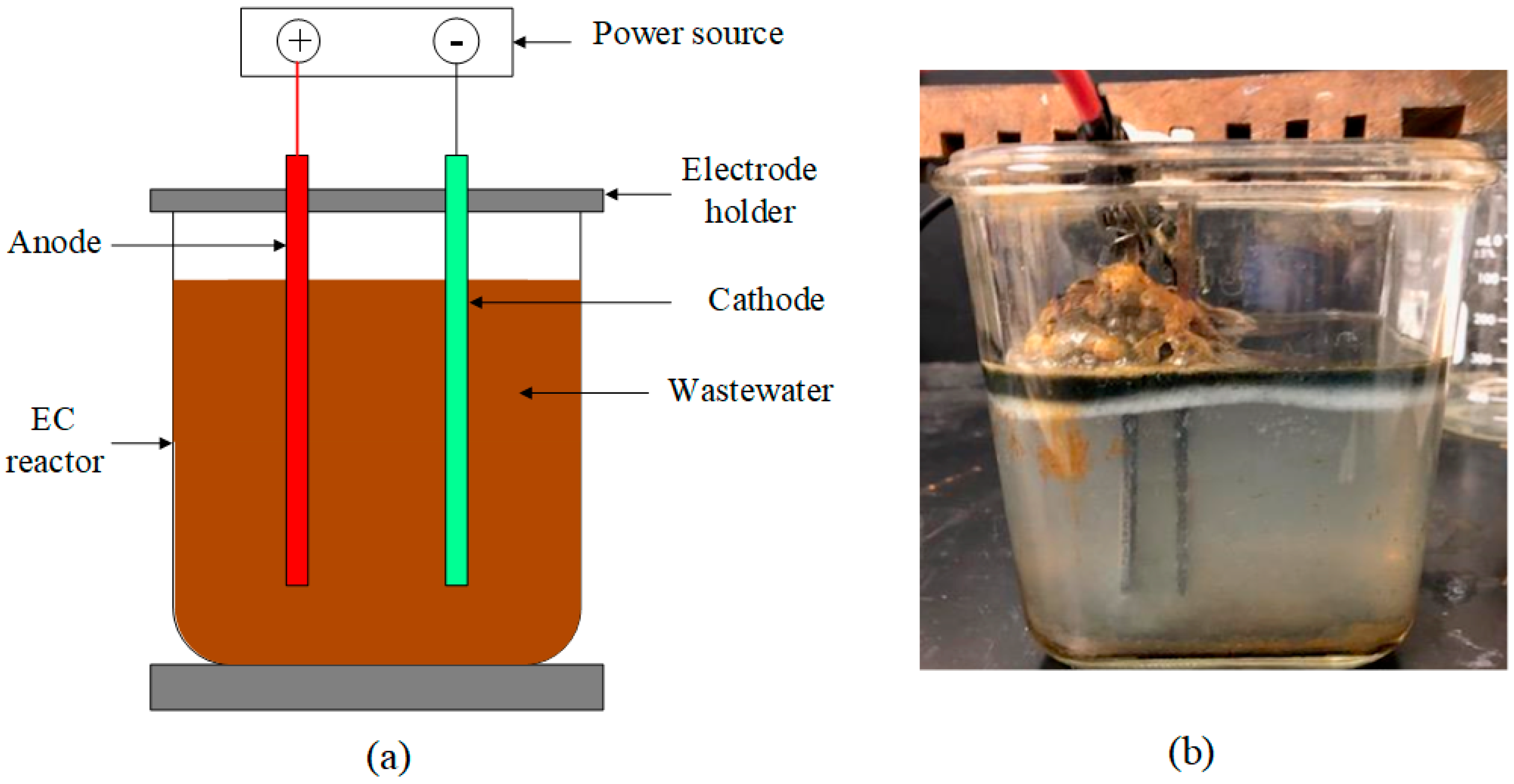



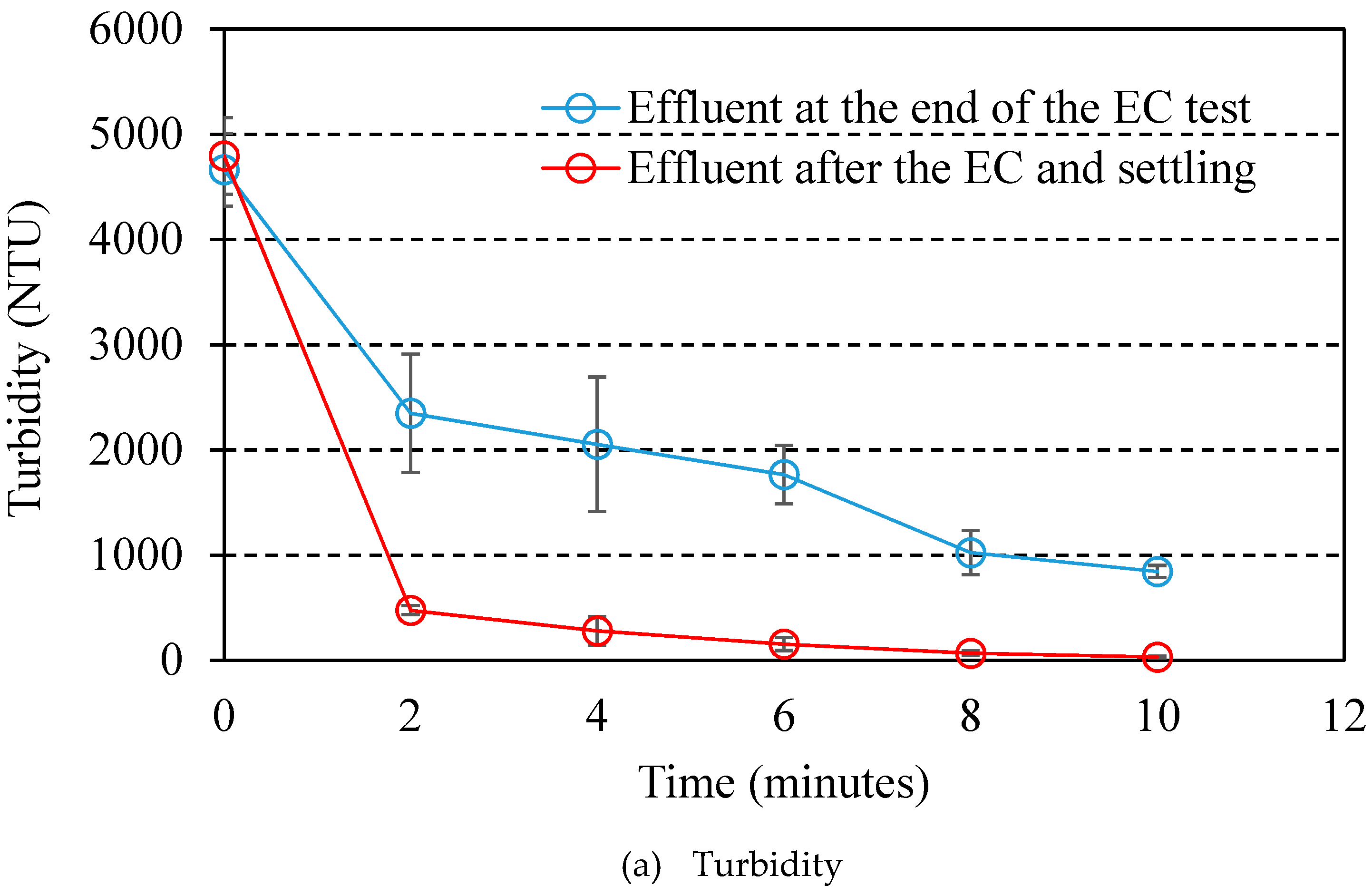

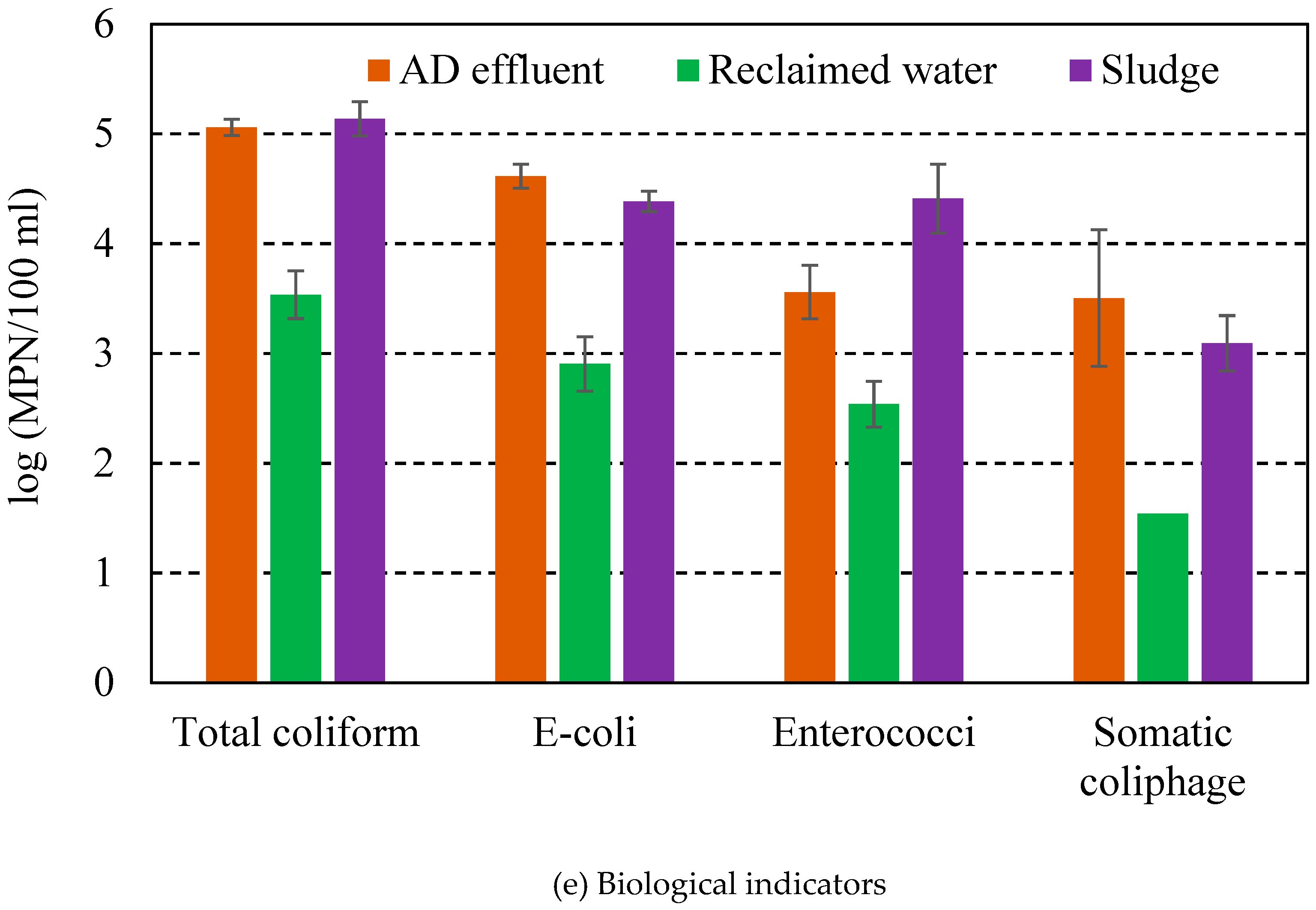

| Parameters | Value |
|---|---|
| pH | 7–8 |
| sCOD (mg/L) | 2243.3 ± 220.5 |
| tCOD (mg/L) | 3853.3 ± 587.1 |
| TAN (mg N/L) | 690 ± 30 |
| TN (mg N/L) | 5246.7 ± 450.1 |
| PO4-P (mg P/L) | 28.7 ± 1.56 |
| TP (mg P/L) | 487.6 ± 17.4 |
| Turbidity (NTU) | 4663.3 ± 345.3 |
| TS (g/L) | 3.39 ± 0.27 |
| Conductivity (mS/cm) | 6.83 ± 0.06 |
| Electrode Combination (Anode-cathode) | pH | Turbidity (NTU) | tCOD (mg/L) | TP (mg PO4/L) | Anode consumed (g/L) | Cathode consumed (g/L) |
|---|---|---|---|---|---|---|
| Fe-Fe | 7.46 | 32.6 ± 6.8 | 206.7 ± 34.0 | 0.85 ± 0.04 | 0.72 ± 0.04 | 0.05 ± 0.08 |
| Al-Al | 8.62 | 284 ± 164 | 313.3 ± 89.6 | 5.6 ± 3.2 | 0.26 ± 0.02 | 0.08 ± 0.01 |
| Fe-Al | 7.82 | 287.3 ± 116.4 | 256.7 ± 34.0 | 0.1 ± 0.1 | 0.70 ± 0.03 | 0 |
| Al-Fe | 8.17 | 30.9 ± 11.6 | 156.7 ± 5.8 | 0 | 0.25 ± 0.03 | 0 |
| Unit Operations | Electricity Demand (Wh/day) |
|---|---|
| Electrocoagulation a | 39,000 |
| Brushing electrodes b | 19 |
| Sludge pumps c | 1312 |
| Total energy demand | 40,331 |
| Unit | Unit Description | Cost ($) | |
|---|---|---|---|
| Capital expenditure (CapEx) a | |||
| EC reactor vessel | 1 | 60 L effective volume, PVC material | $200 |
| Iron electrodes b | 10 | 4200 cm2 surface area each with a thickness of 0.5 cm and the mass of 1.7 kg each | $10 |
| Brushes with gearbox and motor | 2 | Nylon brush with 50 rpm high torque turbine worm gear box with 12 V DC motor | $200 |
| Settling tank with baffles | 1 | 60 L effective volume, PVC material | $200 |
| Sludge discharging pumps with an air compressor | 2 | 12 L/min diaphragm pump with 0.82 kw 16 L air compressor | $1000 |
| Timers | 2 | Controlling feeding and discharging | $100 |
| Pipelines and other miscellaneous items | 1 | PVC pipes, fittings and valves | $300 |
| Total CapEx cost | $2010 | ||
| Operational expenditure (OpEx)c | |||
| Energy cost d | $1884/year | ||
| Electrode replacement e | $499/year | ||
| Maintenance f | $500/year | ||
| Total OpEx cost | $2883/year | ||
| Year | Treatment Cost ($/1000 L AD Effluent) | Year | Treatment Cost ($/1000 L AD Effluent) |
|---|---|---|---|
| 1 | 2.72 | 11 | 3.01 |
| 2 | 2.77 | 12 | 3.04 |
| 3 | 2.79 | 13 | 3.06 |
| 4 | 2.82 | 14 | 3.09 |
| 5 | 2.84 | 15 | 3.12 |
| 6 | 2.87 | 16 | 3.15 |
| 7 | 2.90 | 17 | 3.18 |
| 8 | 2.92 | 18 | 3.22 |
| 9 | 2.95 | 19 | 3.25 |
| 10 | 2.98 | 20 | 3.28 |
| Item | Key Parameter | Values | Corresponding Average Cost to Treat the AD Effluent ($/1000 L) | Change on the Average Cost to Treat the AD Effluent (%) | |
|---|---|---|---|---|---|
| Base Value | Sensitivity Range | ||||
| EC reaction | Retention time (min) | 10 | 7.5–12.5 | 3 | ± 16 |
| EC process | CapEx ($) | 2010 | 1508–2513 | 3 | ± 0.7 |
| Lifespan of the process | 20 | 15-25 | 3 | ± 1.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uludag-Demirer, S.; Olson, N.; Ives, R.; Nshimyimana, J.P.; Rusinek, C.A.; Rose, J.B.; Liao, W. Techno-Economic Analysis of Electrocoagulation on Water Reclamation and Bacterial/Viral Indicator Reductions of a High-Strength Organic Wastewater—Anaerobic Digestion Effluent. Sustainability 2020, 12, 2697. https://doi.org/10.3390/su12072697
Uludag-Demirer S, Olson N, Ives R, Nshimyimana JP, Rusinek CA, Rose JB, Liao W. Techno-Economic Analysis of Electrocoagulation on Water Reclamation and Bacterial/Viral Indicator Reductions of a High-Strength Organic Wastewater—Anaerobic Digestion Effluent. Sustainability. 2020; 12(7):2697. https://doi.org/10.3390/su12072697
Chicago/Turabian StyleUludag-Demirer, Sibel, Nathan Olson, Rebecca Ives, Jean Pierre Nshimyimana, Cory A. Rusinek, Joan B. Rose, and Wei Liao. 2020. "Techno-Economic Analysis of Electrocoagulation on Water Reclamation and Bacterial/Viral Indicator Reductions of a High-Strength Organic Wastewater—Anaerobic Digestion Effluent" Sustainability 12, no. 7: 2697. https://doi.org/10.3390/su12072697
APA StyleUludag-Demirer, S., Olson, N., Ives, R., Nshimyimana, J. P., Rusinek, C. A., Rose, J. B., & Liao, W. (2020). Techno-Economic Analysis of Electrocoagulation on Water Reclamation and Bacterial/Viral Indicator Reductions of a High-Strength Organic Wastewater—Anaerobic Digestion Effluent. Sustainability, 12(7), 2697. https://doi.org/10.3390/su12072697






