Variation in Phytoplankton Community Due to an Autumn Typhoon and Winter Water Turbulence in Southern Korean Coastal Waters
Abstract
1. Introduction
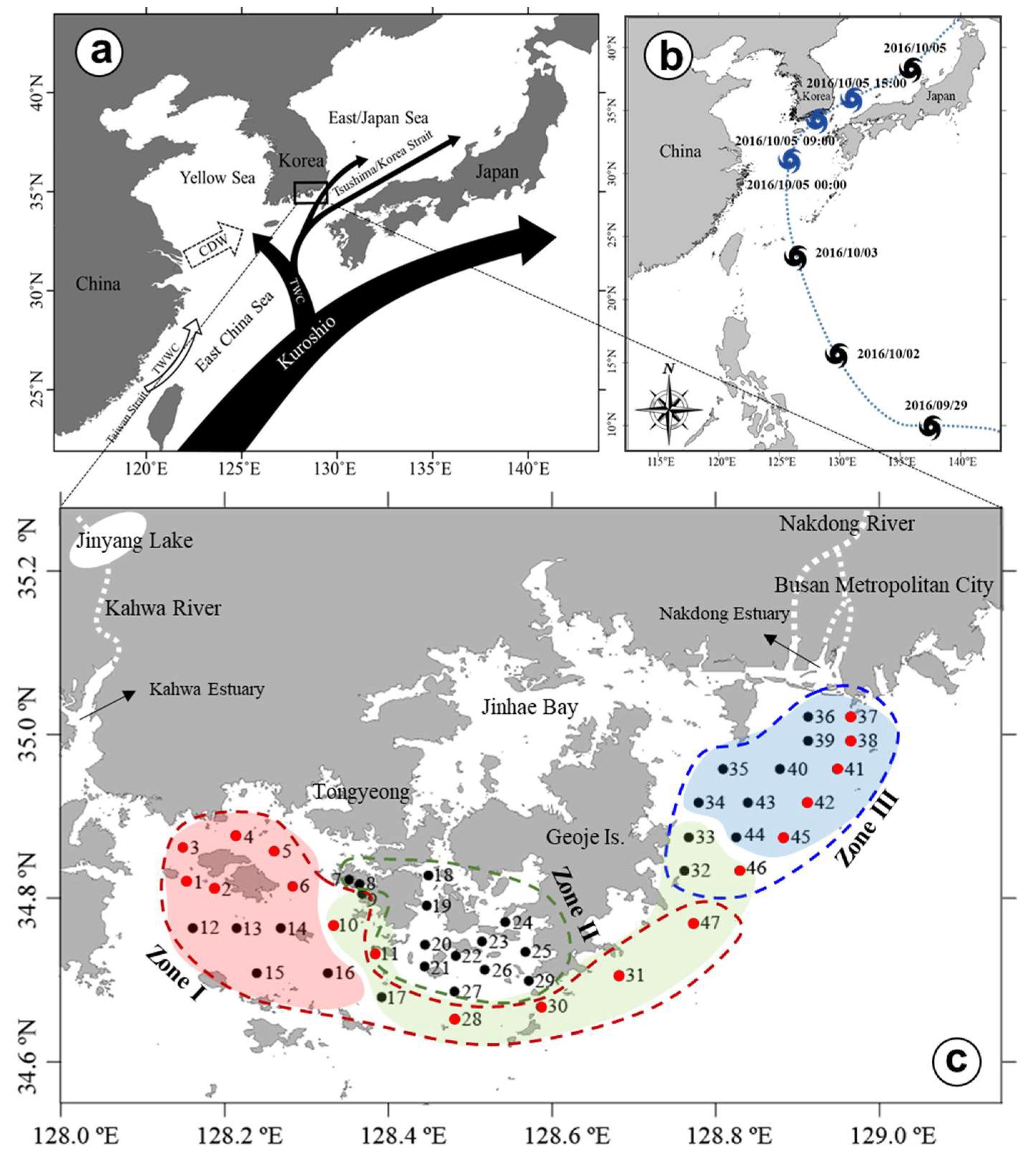
2. Materials and Methods
2.1. Field Sampling and Analysis
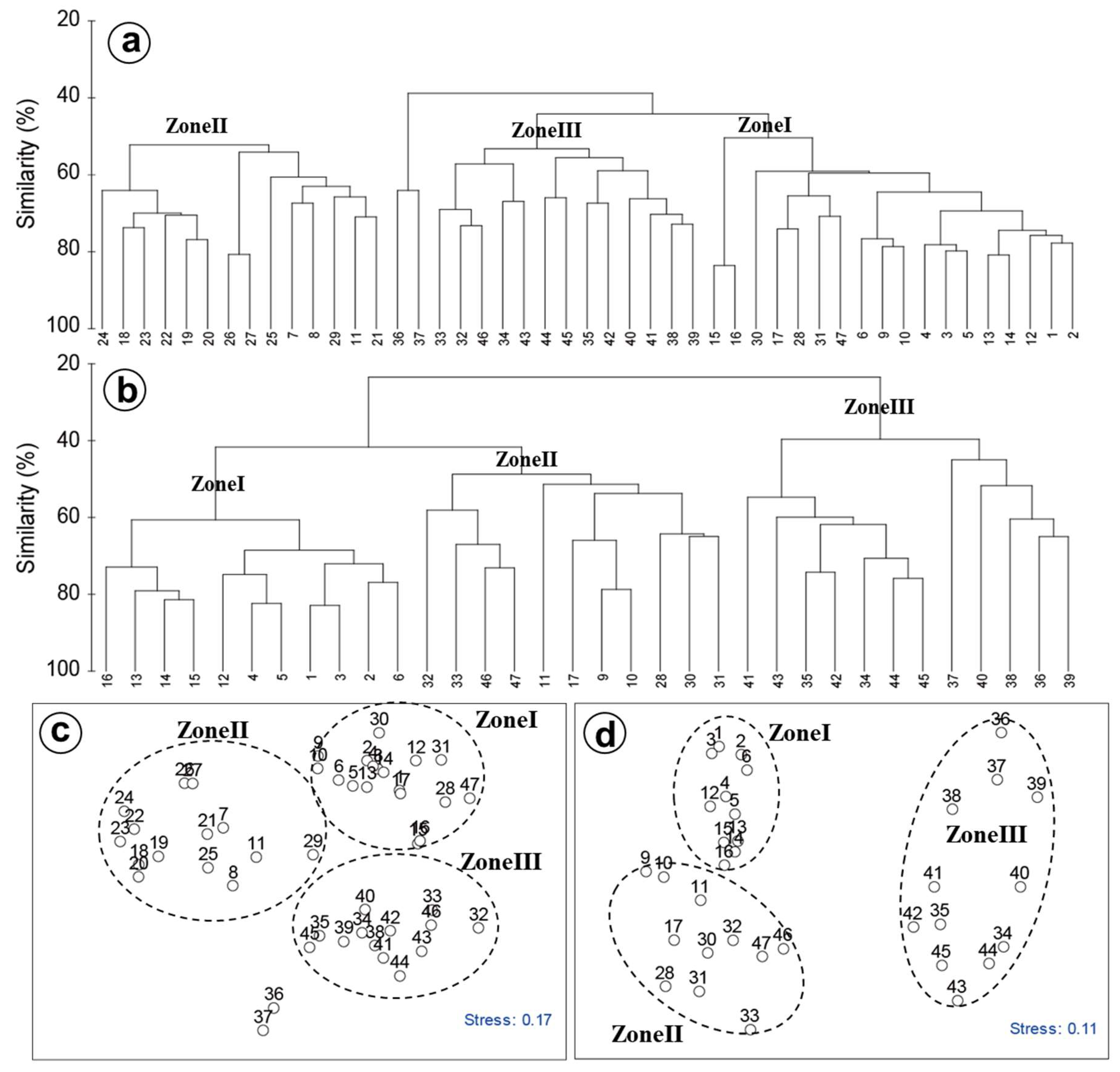
2.2. Statistical Analysis
3. Results
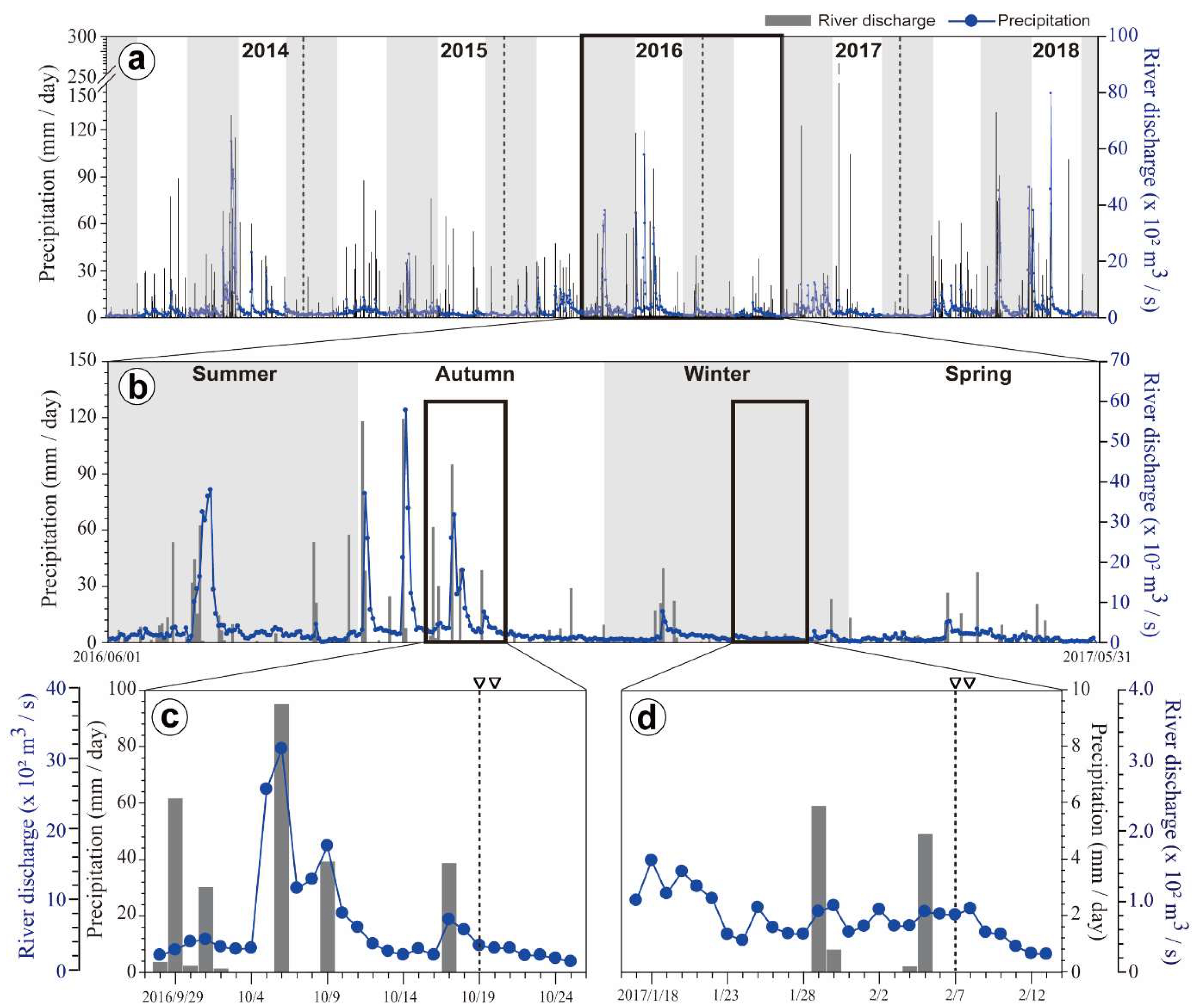
3.1. Precipitation and River Discharge
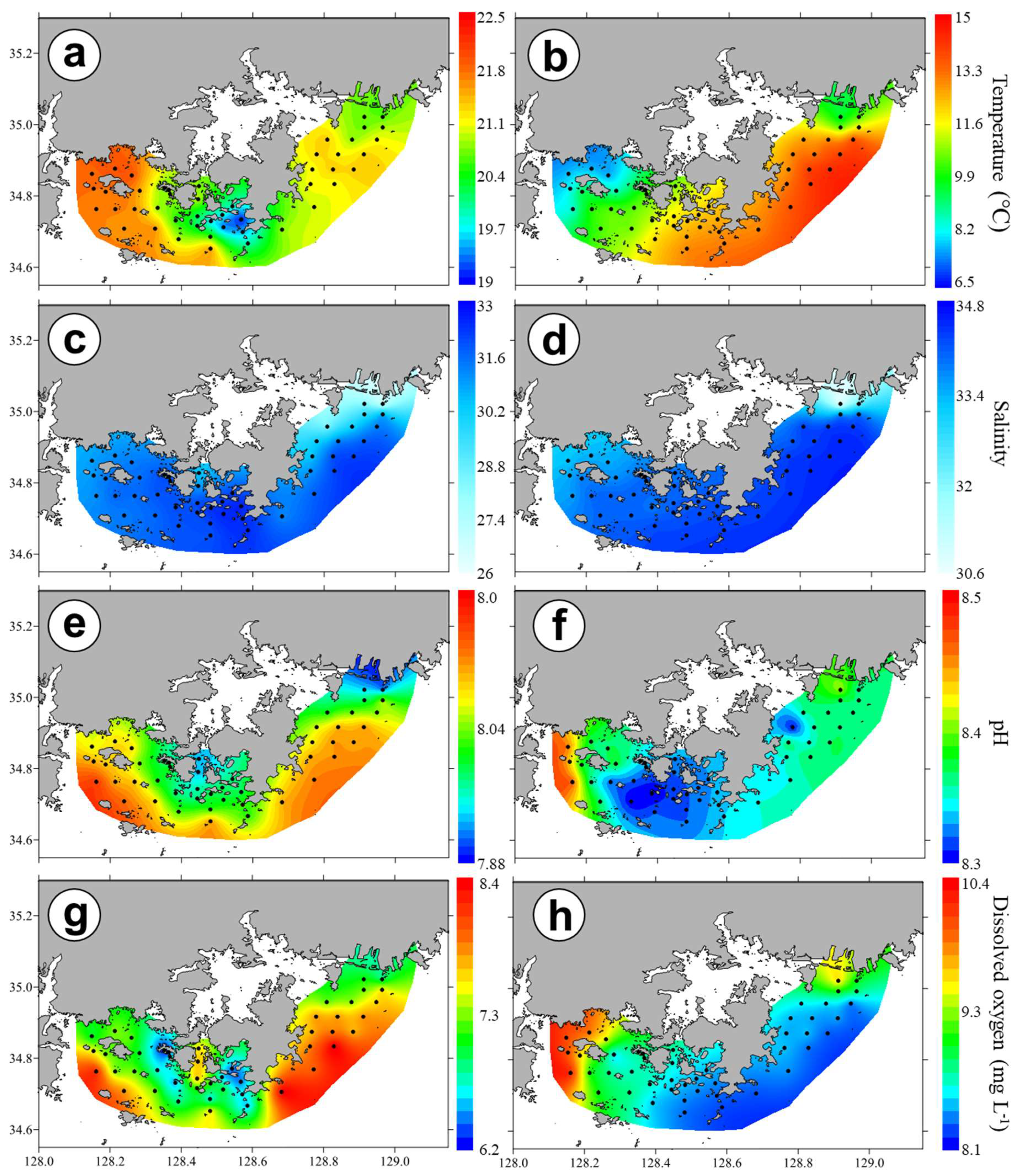
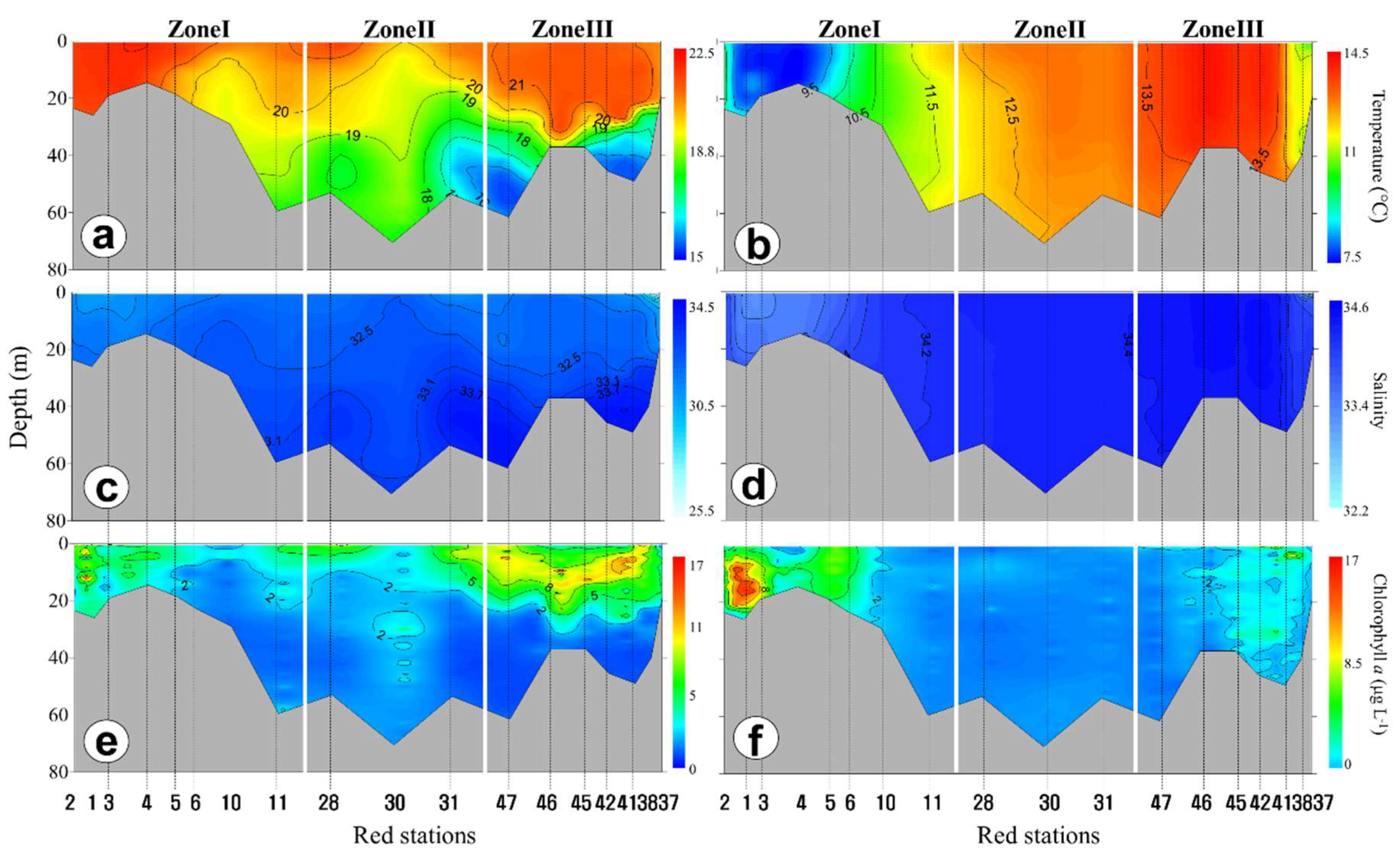
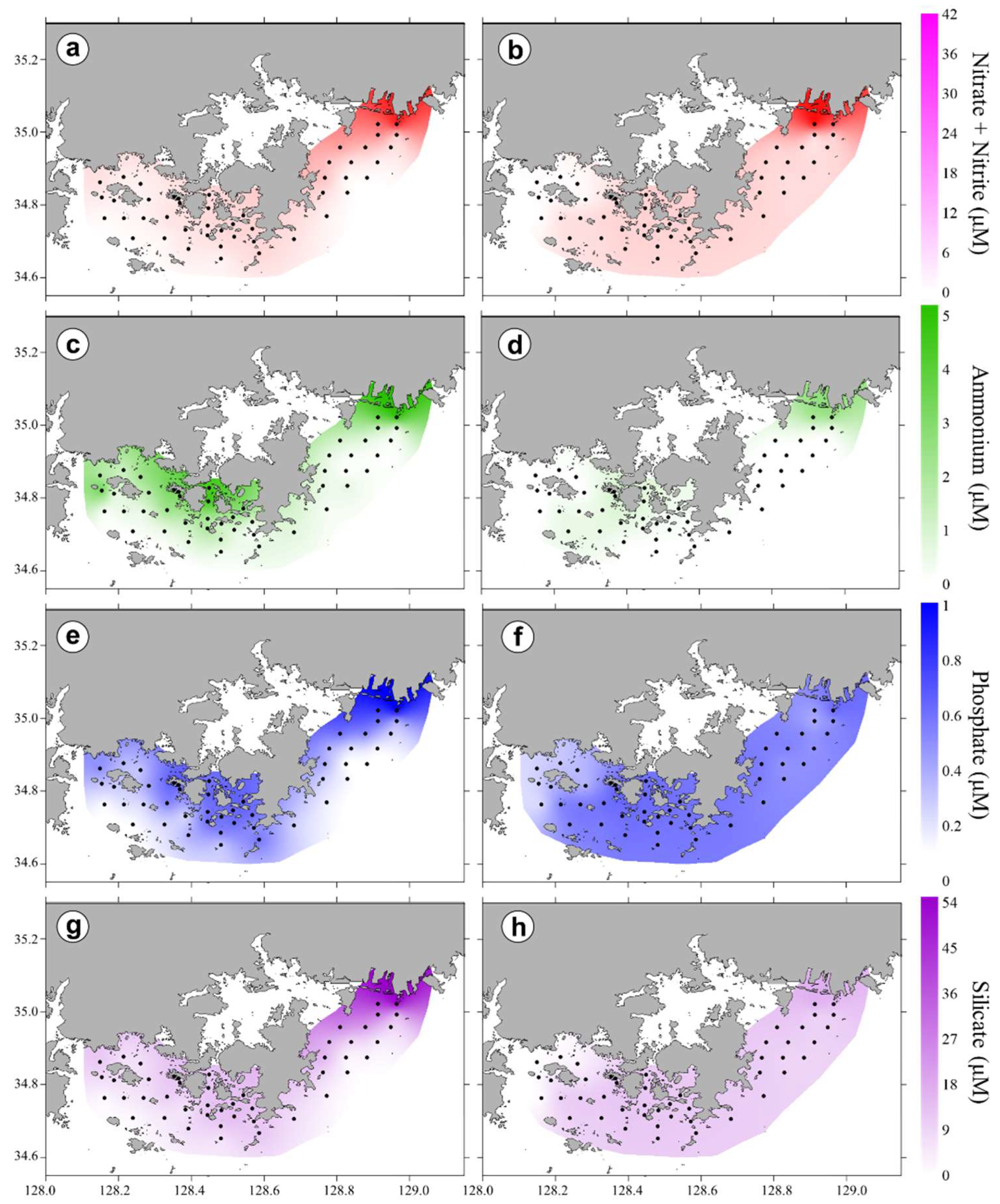
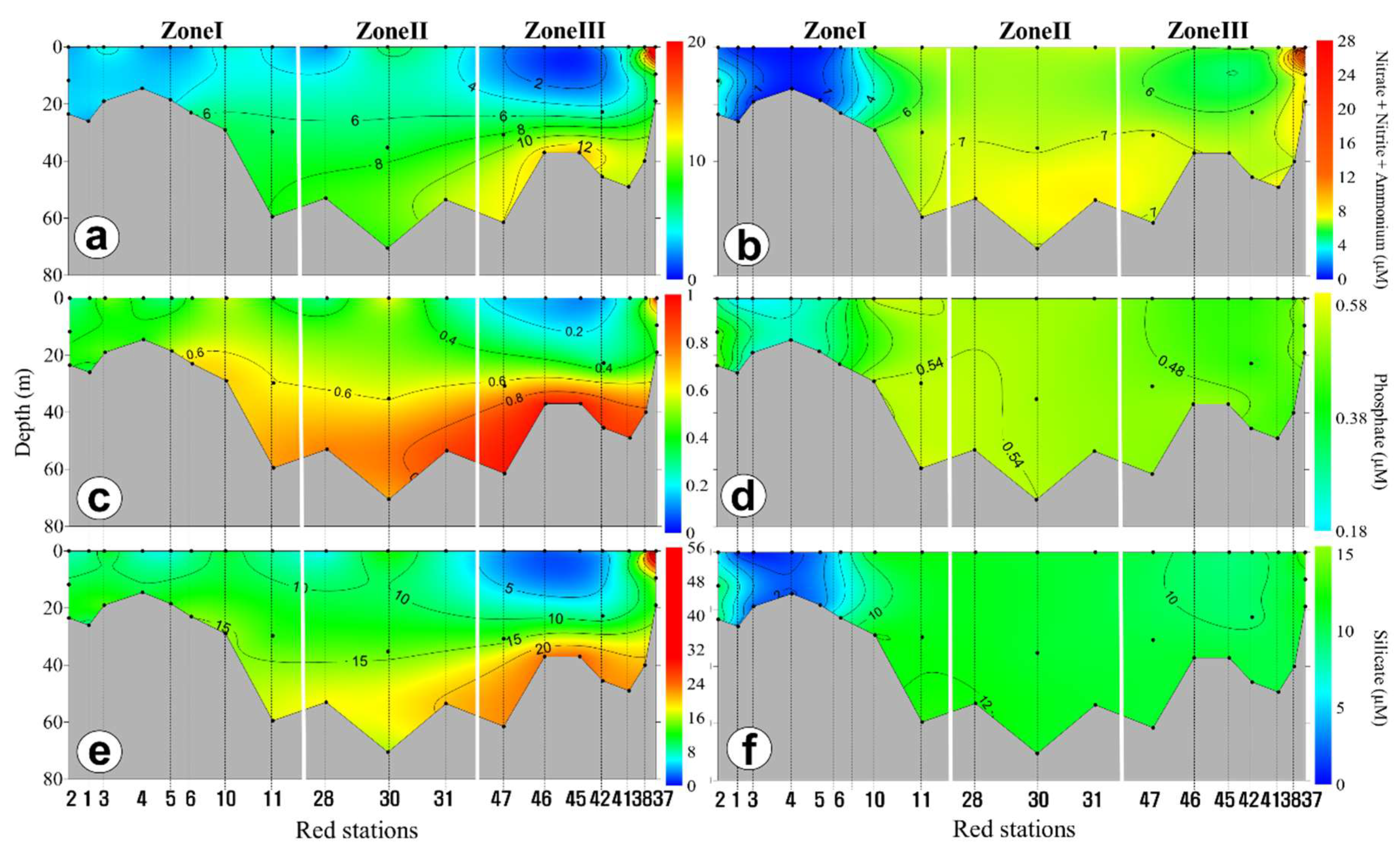
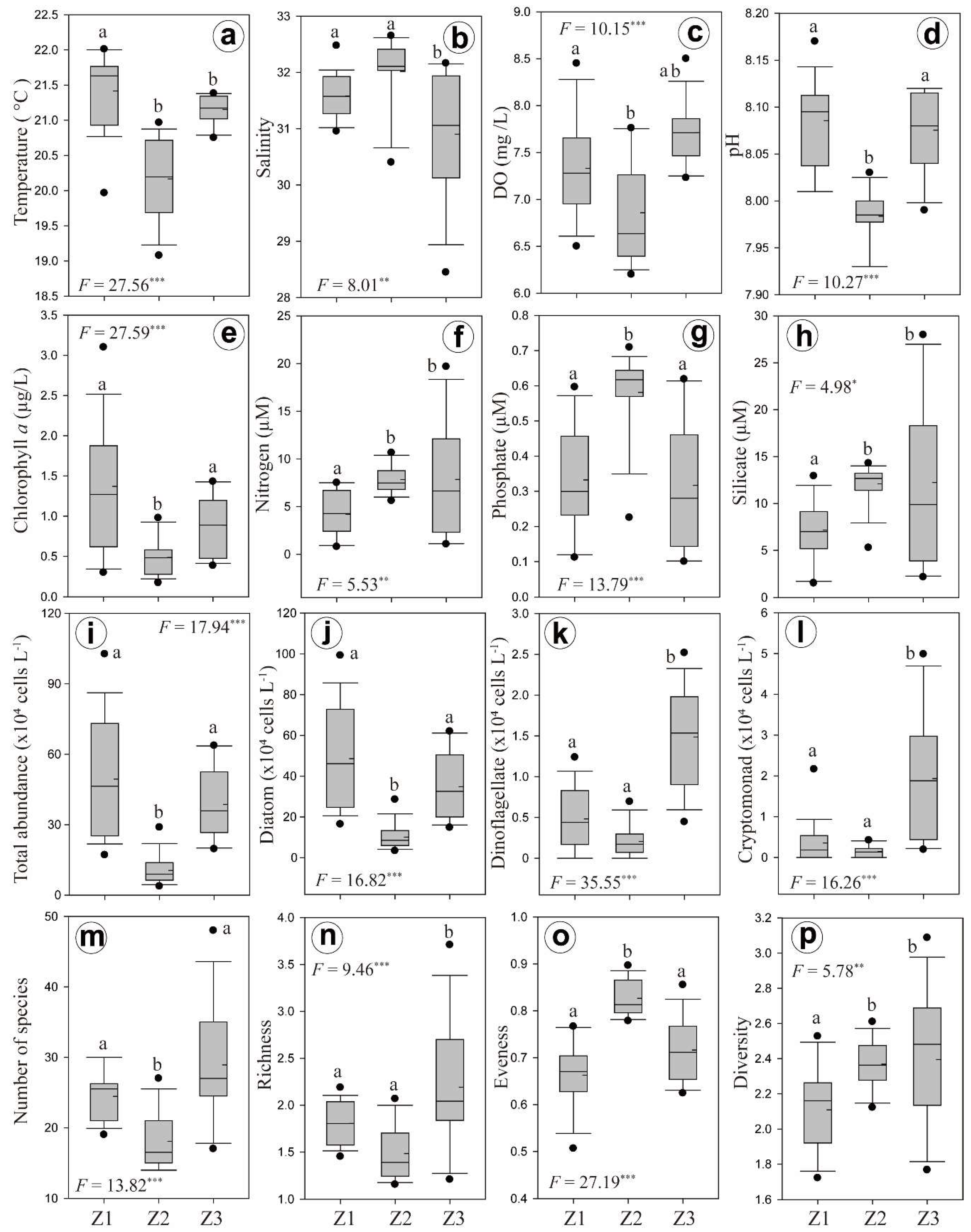
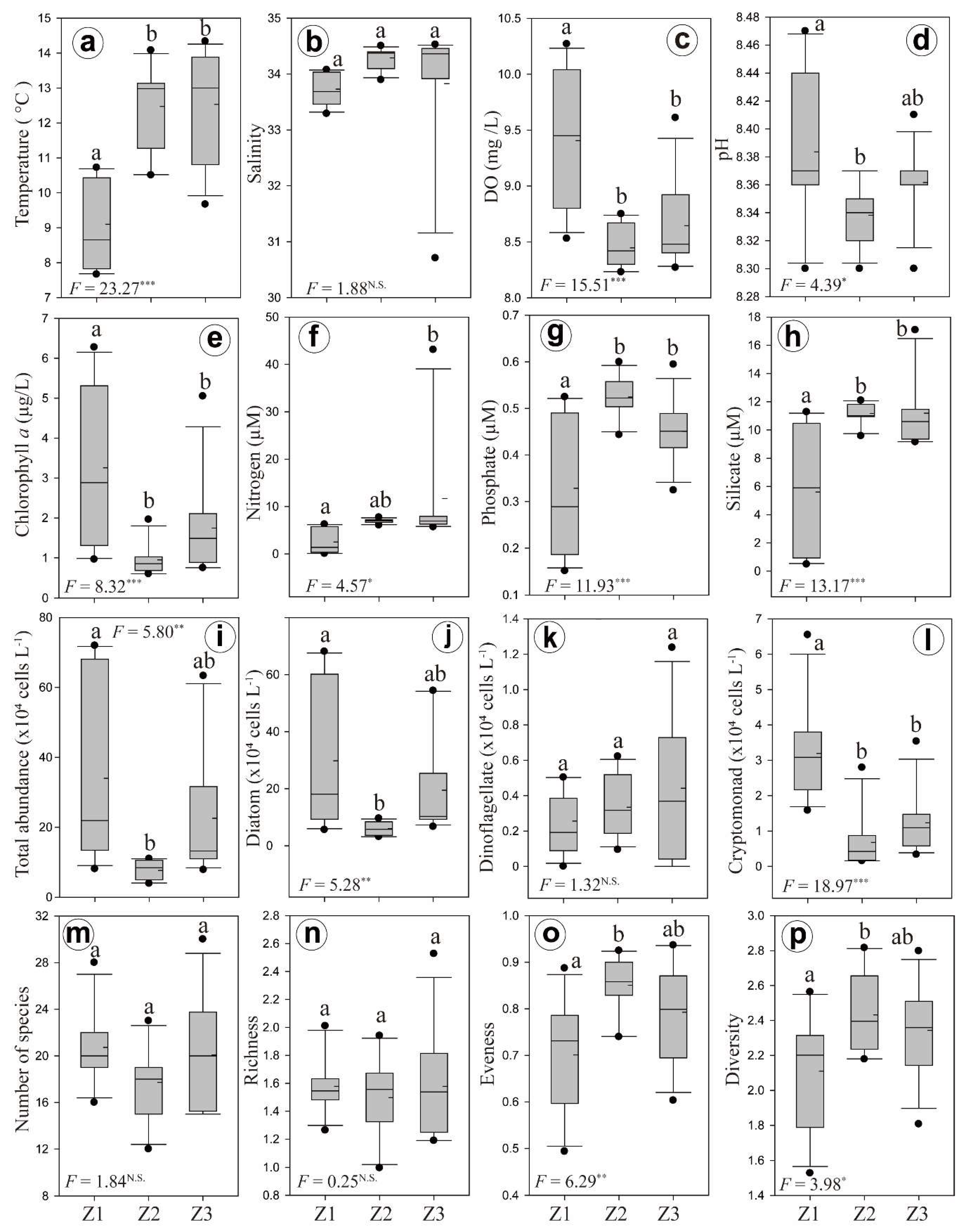
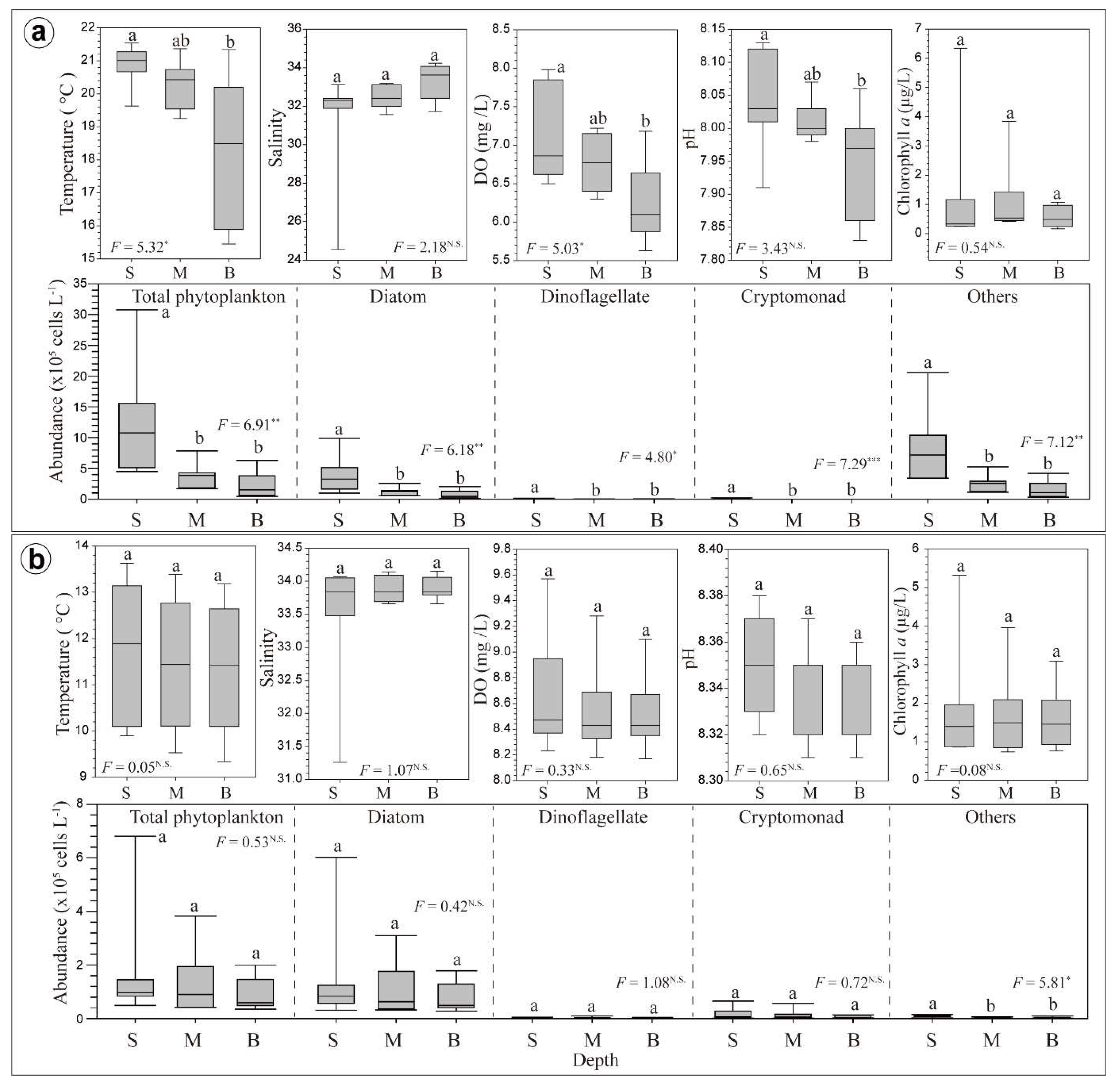
3.2. Horizontal and Vertical Distributions of Environmental Factors
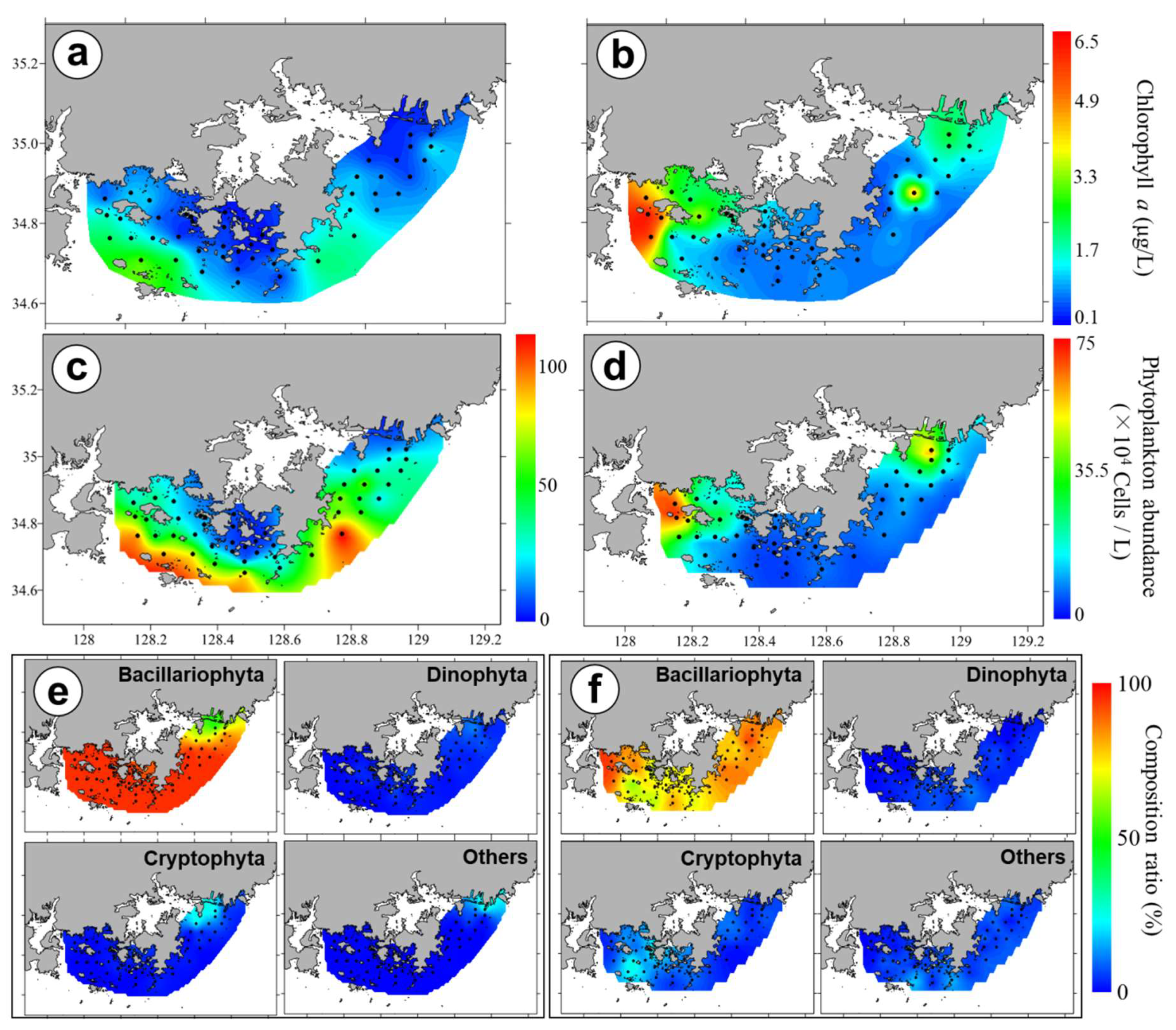
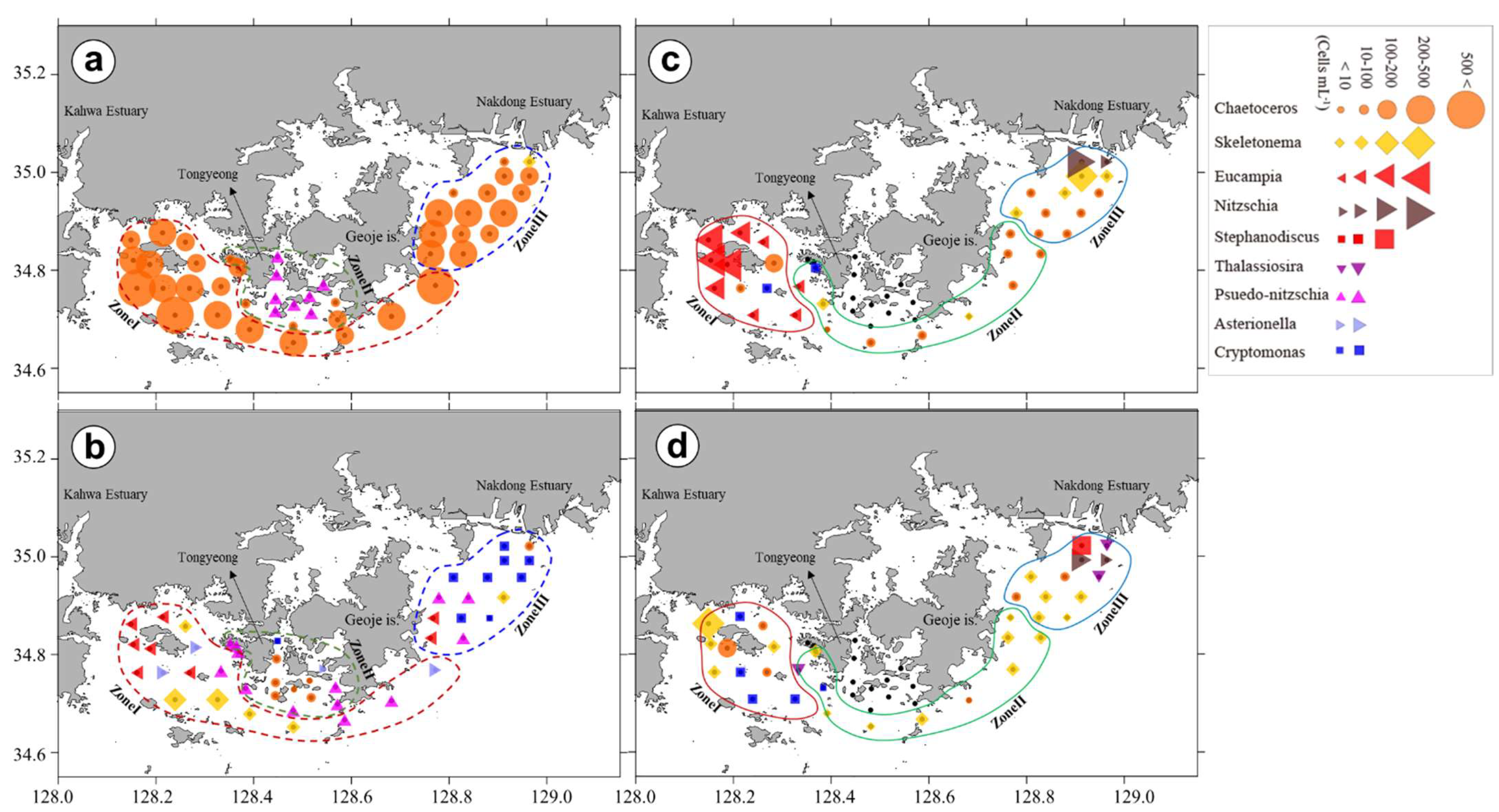
3.3. Chl-a Concentration and Presence of Different Phytoplankton
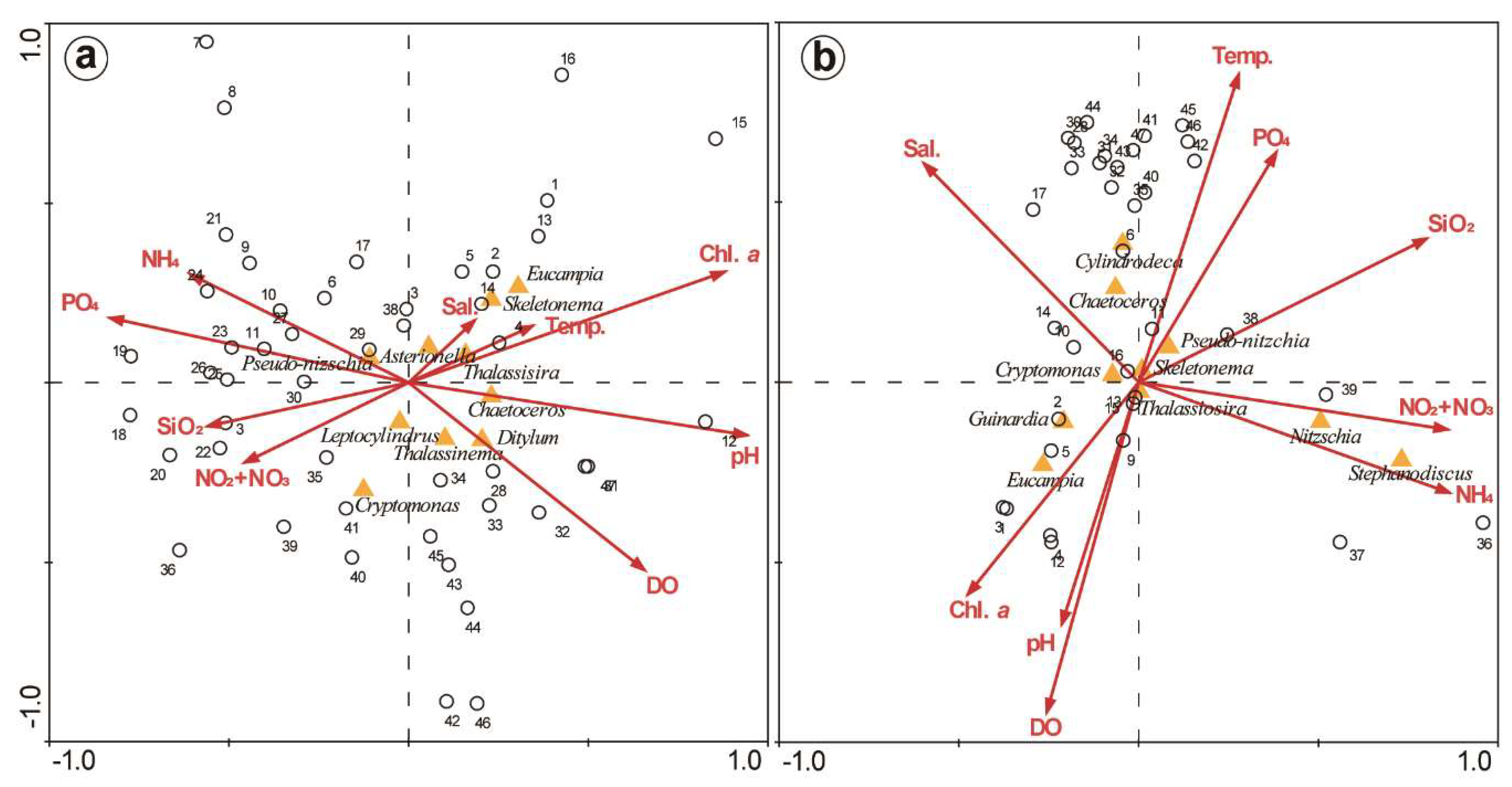
3.4. PCA and CCA of Factors Affecting Phytoplankton
4. Discussion
4.1. Relationship of Phytoplankton Bloom With High Nutrient Level After Typhoon Chaba
4.2. Spatial and Temporal Variations of Biotic and Abiotic Factors
4.3. Vertical Variations of Biotic and Abiotic Factors
4.4. Phytoplankton Community Structure and Characteristics of Phytoplankton Species
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, P.A.; Bonham, P.I.; Swadling, K.M. Phytoplankton blooms in the Huon Estuary, Tasmania: Top-down or bottom-up control? J. Plankton Res. 2008, 7, 735–753. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, D.S.; Son, M.H.; Yun, S.M.; Kim, Y.O. Seasonal distribution of phytoplankton assemblages and nutrient enriched bioassays as indicators of nutrient limitation of phytoplankton growth in Gwangyang Bay, Korea. Estuar. Coast. Shelf Sci. 2015, 163, 265–278. [Google Scholar] [CrossRef]
- Eppley, R.W.; Rogers, J.N.; McCarthy, J.J. Half-saturation constants for uptake of nitrate and ammonium by phytoplankton. Limnol. Oceanogr. 1969, 14, 912–920. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Baek, S.H.; Shimode, S.; Han, M.S.; Kikuchi, T. Growth of dinoflagellates Ceratium furca and Ceratium fusus in Sagami Bay, Japan: The role of nutrient. Harmful Algae 2008, 7, 729–739. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Thomas, W.H.; Gibson, C.H. Effects of small-scale turbulence on microalgae. J. Appl. Phycol. 1990, 2, 71–779. [Google Scholar] [CrossRef]
- Baek, S.H.; Shimode, S.; Kim, H.C.; Han, M.S.; Kikuchi, T. Strong bottom-up effects on phytoplankton community caused by a rainfall during spring and summer in Sagami Bay, Japan. J. Mar. Syst. 2009, 75, 253–264. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, D.; Kim, Y.O.; Son, M.; Kim, Y.J.; Lee, M.; Park, B.S. Seasonal changes in abiotic environmental conditions in the Busan coastal region (South Korea) due to the Nakdong River in 2013 and effect of these changes on phytoplankton communities. Cont. Shelf Res. 2019, 175, 116–126. [Google Scholar] [CrossRef]
- Chen, Y.I.; Chen, H.; Jan, S.; Tuo, S. Phytoplankton productivity enhancement and assemblage change in the upstream Kuroshio after typhoon. Mar. Ecol. Progr. Ser. 2009, 385, 111–126. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Yoshiki, T.; Nakajima, R.; Miyaguchi, H.; Kuwahara, V.S.; Taguchi, S.; Kikuchi, T.; Toda, T. Typhoon-driven variations in primary production and phytoplankton assemblages in Sagami Bay, Japan: A case study of typhoon Mawar (T0511). Plankton Benthos Res. 2013, 8, 74–87. [Google Scholar] [CrossRef][Green Version]
- Townsend, D.W.; Keller, M.D.; Sieracki, M.E.; Ackleson, S.G. Spring phytoplankton blooms in the absence of vertical water column stratification. Nature 1992, 360, 59–62. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, M.; Kim, Y.B. Spring phytoplankton community response to an episodic windstorm event in oligotrophic waters offshore from the Ulleungdo and Dokdo islands, Korea. J. Sea Res. 2018, 132, 1–14. [Google Scholar] [CrossRef]
- Isobe, A. On the origin of the Tsushima Warm Current and its seasonality. Cont. Shelf Res. 1999, 19, 117–133. [Google Scholar] [CrossRef]
- Lie, H.J.; Cho, C.H. On the origin of the Tsushima Warm Current. J. Geophys. Res. Oceans 1994, C12, 25081–25091. [Google Scholar] [CrossRef]
- Takikawa, T.; Yoon, J.H. Volume transport through the Tsushima Straits estimated from sea level difference. J. Oceanogr. 2005, 61, 699–708. [Google Scholar] [CrossRef]
- Hwang, C.S.; Kim, K.T.; Oh, C.Y.; Jin, C.G.; Choi, C.U. A study on correlation between RUSLE and estuary in Nakdong River watershed. J. Korean Soc. Geosp. Inform. Syst. 2010, 18, 3–10. [Google Scholar]
- Field, J.; Clarke, K.; Warwick, R. A practical strategy for analysing multispecies distribution patterns. Mar. Ecol. Prog. Ser. 1982, 8, 37–52. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kuwahara, V.S.; Yoshiki, T.; Nakajima, R.; Miyaguchi, H.; Kumekawa, N.; Kikuchi, T.; Toda, T. Phytoplankton community response and succession in relation to typhoon passages in the coastal waters of Japan. J. Plankton Res. 2014, 36, 424–438. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kuwahara, V.S.; Tomoko, M.; Yoshiki, T.; Nakajima, R.; Shimode, S.; Kikuchi, T.; Toda, T. Response of phytoplankton and enhanced biogeochemical activity to an episodic typhoon event in the coastal waters of Japan. Estuar. Coast. Shelf Sci. 2017, 194, 30–39. [Google Scholar] [CrossRef]
- Kunlasak, K.; Chitamanat, C.; Whangchai, N.; Promya, J.; Lebel, L. Relationships of dissolved oxygen with Chlorophyll a and phytoplankton composition in Tilapia Ponds. Int. J. Geosci. 2013, 4, 46–53. [Google Scholar] [CrossRef]
- Hinga, K.R. Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 2002, 238, 281–300. [Google Scholar] [CrossRef]
- Yin, K.; Harrison, P.J.; Goldblatt, R.H.; St. John, M.A.; Beamish, R.J. Factors controlling the timing of the spring bloom in the Strait of Georgia estuary, British Columbia, Canada. Can. J. Fish. Aquat. Sci. 1997, 54, 1985–1995. [Google Scholar] [CrossRef]
- Mikaelyan, A.S. Winter bloom of the diatom Nitzschia delicatula in the open waters of the Black Sea. Mar. Ecol. Prog. Ser. 1995, 129, 241–251. [Google Scholar] [CrossRef]
- Kakehi, S.; Ito, S.I.; Kuwata, A.; Saito, H.; Tadokoro, K. Phytoplankton distribution during the winter convective season in Sendai Bay, Japan. Cont. Shelf Res. 2015, 97, 43–53. [Google Scholar] [CrossRef]
- Klaveness, D. Biology and ecology of the cryptophyceae: Status and challenges. Biolog. Oceanogr. 1989, 6, 257–270. [Google Scholar]
- Ito, Y.; Katano, T.; Fujii, N.; Koriyama, M.; Yoshino, K.; Hayami, Y. Decreases in turbidity during neap tides initiate late winter blooms of Eucampia zodiacus in a macrotidal embayment. J. Oceanogr. 2013, 69, 467–479. [Google Scholar] [CrossRef]
- Nishikawa, T.; Hori, Y.; Tanida, K.; Imai, I. Population dynamics of the harmful diatom Eucampia zodiacus Ehrenberg causing bleaching of Porphyra thalli in aquaculture in Harima-Nada, the Seto Inland Sea, Japan. Harmful Algae 2007, 6, 763–773. [Google Scholar] [CrossRef]
- Jung, S.W.; Kwon, O.Y.; Lee, J.H. Effect of wter temperature and silicate on the winter blooming diatom Stephanodiscus hantzschii (Bacillariophyceae) growing in eutriphic conditions in the lower Han River, South Korea. J. Freshwater Ecol. 2009, 24, 219–226. [Google Scholar] [CrossRef]
| October | February | |||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| Temperature | –0.56 | –0.30 | –0.50 | –0.85 | –0.10 | 0.47 |
| Salinity | –0.32 | 0.73 | 0.45 | –0.53 | –0.80 | 0.10 |
| NO2 + NO3 | 0.75 | –0.59 | –0.16 | –0.17 | 0.96 | 0.12 |
| NH4 | 0.70 | 0.13 | –0.47 | 0.03 | 0.90 | –0.28 |
| PO4 | 0.94 | 0.08 | –0.11 | –0.88 | 0.24 | –0.25 |
| SiO2 | 0.82 | –0.49 | –0.17 | –0.74 | 0.61 | –0.02 |
| Chl-a | –0.77 | –0.17 | –0.42 | 0.86 | –0.15 | 0.21 |
| DO | –0.62 | –0.51 | 0.03 | 0.93 | 0.10 | –0.14 |
| pH | –0.93 | –0.17 | –0.02 | 0.78 | 0.01 | 0.40 |
| Total phytoplankton | –0.82 | –0.36 | –0.23 | 0.88 | 0.17 | 0.28 |
| Total diatom | –0.84 | –0.29 | –0.25 | 0.87 | 0.14 | 0.28 |
| Total dinoflagellate | –0.14 | –0.68 | 0.48 | –0.30 | –0.19 | 0.45 |
| Total cryptophyte | 0.18 | –0.72 | 0.33 | 0.73 | –0.07 | –0.16 |
| Others | 0.56 | –0.64 | –0.28 | 0.20 | 0.78 | 0.28 |
| Eigenvalue | 6.61 | 3.62 | 1.87 | 7.12 | 3.59 | 1.53 |
| Variability (%) | 44.05 | 24.15 | 12.45 | 47.47 | 23.96 | 10.21 |
| Cumulative % | 44.05 | 68.20 | 80.65 | 47.47 | 71.43 | 81.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.H.; Lee, M.; Park, B.S.; Lim, Y.K. Variation in Phytoplankton Community Due to an Autumn Typhoon and Winter Water Turbulence in Southern Korean Coastal Waters. Sustainability 2020, 12, 2781. https://doi.org/10.3390/su12072781
Baek SH, Lee M, Park BS, Lim YK. Variation in Phytoplankton Community Due to an Autumn Typhoon and Winter Water Turbulence in Southern Korean Coastal Waters. Sustainability. 2020; 12(7):2781. https://doi.org/10.3390/su12072781
Chicago/Turabian StyleBaek, Seung Ho, Minji Lee, Bum Soo Park, and Young Kyun Lim. 2020. "Variation in Phytoplankton Community Due to an Autumn Typhoon and Winter Water Turbulence in Southern Korean Coastal Waters" Sustainability 12, no. 7: 2781. https://doi.org/10.3390/su12072781
APA StyleBaek, S. H., Lee, M., Park, B. S., & Lim, Y. K. (2020). Variation in Phytoplankton Community Due to an Autumn Typhoon and Winter Water Turbulence in Southern Korean Coastal Waters. Sustainability, 12(7), 2781. https://doi.org/10.3390/su12072781





