Evidence That Forage-Fed Cows Can Enhance Milk Quality
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
2.2. Milk Analysis
2.3. Data Analysis
3. Results
3.1. Effect of Management on Composition
3.2. Effect of Sampling Month on Composition
3.3. Effect of Management and Month
4. Discussion
4.1. Milk Fat Composition
4.1.1. Omega-6 and Omega-3
4.1.2. Conjugated Linoleic Acid
4.2. Effect of Management and Season
4.3. Forage in the Diet
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Management | Month | ANOVA p-Value a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Conventional | Organic | PFLA b | April | July | October | Man | Mon | Man × Mon | |
| Fatty Acid | n = 15 | n = 15 | n = 15 | n = 18 | n = 18 | n = 18 | |||
| C4:0 | 3.0 ± 0.159 | 3.1 ± 0.127 | 3.0 ± 0.221 | 3.1 ± 0.171 | 3.1 ± 0.151 | 3.0 ± 0.235 | ns | ns | ns |
| C5:0 | 0.13 ± 0.084 | 0.13 ± 0.124 | 0.09 ± 0.060 | 0.11 ± 0.072 | 0.10 ± 0.055 | 0.13 ± 0.126 | ns | ns | ns |
| C6:0 | 2.5 ± 0.096 | 2.5 ± 0.092 | 2.5 ± 0.218 | 2.6 a ± 0.154 | 2.5 b ± 0.151 | 2.5 b ± 0.113 | ns | *** | t |
| C7:0 | 0.08 ± 0.066 | 0.08 ± 0.049 | 0.06 ± 0.046 | 0.06 ± 0.051 | 0.07 ± 0.050 | 0.08 ± 0.059 | ns | ns | ns |
| C8:0 | 1.3 ± 0.085 | 1.3 ± 0.093 | 1.3 ± 0.144 | 1.4 a ± 0.109 | 1.3 b ± 0.115 | 1.3 b ± 0.078 | ns | *** | ns |
| C9:0 | 0.08 a ± 0.041 | 0.08 a ± 0.044 | 0.06 b ± 0.024 | 0.06 b ± 0.030 | 0.07 b ± 0.024 | 0.09 a ± 0.045 | * | ** | ** |
| C10:0 | 2.8 ± 0.191 | 2.7 ± 0.097 | 2.9 ± 0.495 | 3.0 a ± 0.422 | 2.7 b ± 0.299 | 2.7 b ± 0.241 | ns | ** | t |
| C11:0 | 0.30 ± 0.029 | 0.31 ± 0.040 | 0.31 ± 0.054 | 0.29 ± 0.031 | 0.31 ± 0.058 | 0.31 ± 0.040 | ns | ns | ns |
| C12:0 | 3.5 ± 0.230 | 3.3 ± 0.146 | 3.4 ± 0.568 | 3.6 a ± 0.485 | 3.3 b ± 0.344 | 3.3 b ± 0.329 | ns | ** | ns |
| C13:0 | 0.22 ± 0.038 | 0.23 ± 0.052 | 0.21 ± 0.050 | 0.23 ± 0.039 | 0.20 ± 0.046 | 0.22 ± 0.054 | ns | ns | ns |
| C14:0 | 11.0 ± 0.383 | 11.0 ± 0.325 | 11.1 ± 1.179 | 11.2 ± 0.947 | 11.0 ± 0.887 | 11.0 ± 0.610 | ns | ns | ns |
| c9 C14:1 | 1.0 a ± 0.069 | 0.9 b ± 0.085 | 0.9 b ± 0.193 | 0.9 c ± 0.148 | 0.9 b ± 0.135 | 1.0 a ± 0.131 | ** | ** | ns |
| C15:0 | 1.1 b ± 0.062 | 1.2 b ± 0.117 | 1.4 a ± 0.208 | 1.3 ± 0.242 | 1.3 ± 0.176 | 1.3 ± 0.200 | *** | ns | ns |
| c9 C15:1 | 0.05 ± 0.034 | 0.07 ± 0.050 | 0.06 ± 0.036 | 0.06 ± 0.038 | 0.05 ± 0.029 | 0.07 ± 0.050 | ns | ns | t |
| C16:0 | 31.1 a ± 1.17 | 28.6 b ± 1.23 | 28.0 b ± 3.92 | 29.0 ± 3.97 | 28.6 ± 2.70 | 29.5 ± 2.29 | * | ns | ns |
| t9 C16:1 | 0.41 b ± 0.091 | 0.52 a ± 0.091 | 0.59 a ± 0.096 | 0.51 ± 0.118 | 0.52 ± 0.101 | 0.52 ± 0.139 | ** | ns | ns |
| C16:1 | 1.9 ± 0.151 | 1.8 ± 0.146 | 1.7 ± 0.315 | 1.7 ± 0.330 | 1.8 ± 0.163 | 1.8 ± 0.225 | ns | ns | ns |
| C17:0 | 0.55 b ± 0.080 | 0.61 b ± 0.089 | 0.68 a ± 0.129 | 0.61 ± 0.122 | 0.67 ± 0.113 | 0.59 ± 0.111 | ** | t | ns |
| c9 C17:1 | 0.23 ± 0.045 | 0.27 ± 0.058 | 0.29 ± 0.095 | 0.25 ± 0.057 | 0.26 ± 0.070 | 0.30 ± 0.096 | t | t | ns |
| C18:0 | 9.5 b ± 0.619 | 10.5 a ± 0.609 | 11.2 a ± 1.892 | 10.6 ± 1.708 | 11.0 ± 1.445 | 10.1 ± 1.272 | ** | ns | ns |
| t6,7,8 C18:1 | 0.27 ± 0.084 | 0.30 ± 0.075 | 0.23 ± 0.123 | 0.25 ± 0.123 | 0.25 ± 0.105 | 0.28 ± 0.082 | ns | ns | ns |
| t9 C18:1 | 0.20 ± 0.089 | 0.20 ± 0.086 | 0.18 ± 0.106 | 0.17 ± 0.093 | 0.17 ± 0.097 | 0.23 ± 0.085 | ns | ns | ns |
| t10 C18:1 | 0.37 a ± 0.099 | 0.26 b ± 0.131 | 0.19 b ± 0.115 | 0.22 ± 0.125 | 0.29 ± 0.160 | 0.26 ± 0.119 | ** | ns | ns |
| t11 C18:1 | 1.1 c ± 0.234 | 1.9 b ± 0.284 | 2.5 a ± 1.091 | 2.0 ± 1.369 | 1.8 ± 0.601 | 1.9 ± 0.809 | *** | ns | ns |
| t12,13,14 C18:1 | 0.42 ± 0.065 | 0.36 ± 0.101 | 0.32 ± 0.110 | 0.38 ± 0.097 | 0.36 ± 0.126 | 0.35 ± 0.090 | t | ns | ns |
| c9 C18:1 | 19.0 ± 0.676 | 19.3 ± 0.752 | 18.4 ± 2.931 | 18.5 ± 2.582 | 19.3 ± 1.898 | 18.7 ± 1.521 | ns | ns | ns |
| t15 C18:1 | 0.27 ± 0.094 | 0.25 ± 0.081 | 0.25 ± 0.112 | 0.26 ± 0.110 | 0.24 ± 0.069 | 0.27 ± 0.113 | ns | ns | * |
| c11 C18:1 | 0.53 a ± 0.177 | 0.36 b ± 0.155 | 0.41 b ± 0.137 | 0.38 ± 0.151 | 0.46 ± 0.176 | 0.44 ± 0.167 | * | ns | ns |
| c12 C18:1 | 0.26 a ± 0.096 | 0.17 b ± 0.116 | 0.11 b ± 0.064 | 0.16 ± 0.102 | 0.18 ± 0.115 | 0.16 ± 0.111 | ** | ns | ns |
| c13 C18:1 | 0.10 ± 0.064 | 0.10 ± 0.092 | 0.10 ± 0.055 | 0.09 ± 0.070 | 0.10 ± 0.065 | 0.11 ± 0.072 | ns | ns | ns |
| c14, t16 C18:1 | 0.33 ± 0.084 | 0.35 ± 0.104 | 0.36 ± 0.120 | 0.34 ± 0.119 | 0.35 ± 0.111 | 0.36 ± 0.089 | ns | ns | ns |
| c15 C18:1 | 0.10 ± 0.044 | 0.17 ± 0.120 | 0.13 ± 0.071 | 0.16 ± 0.111 | 0.13 ± 0.076 | 0.12 ± 0.059 | ns | ns | ns |
| t11,15 C18:2 (n3) | 0.05 ± 0.041 | 0.05 ± 0.033 | 0.05 ± 0.026 | 0.04 ± 0.026 | 0.05 ± 0.032 | 0.05 ± 0.038 | ns | ns | ns |
| t10,14 C18:2 | 0.06 ± 0.046 | 0.06 ± 0.046 | 0.07 ± 0.049 | 0.05 ± 0.019 | 0.08 ± 0.048 | 0.08 ± 0.058 | ns | t | ns |
| c9 t13, C18:2 | 0.08 ± 0.036 | 0.09 ± 0.040 | 0.11 ± 0.070 | 0.10 ± 0.068 | 0.09 ± 0.054 | 0.09 ± 0.043 | ns | ns | ns |
| t9,12 C18:2 (n6) | 0.11 ± 0.059 | 0.10 ± 0.039 | 0.08 ± 0.047 | 0.09 ± 0.049 | 0.09 ± 0.046 | 0.10 ± 0.056 | ns | ns | ns |
| t8 c13 C18:2 | 0.14 ± 0.067 | 0.09 ± 0.057 | 0.11 ± 0.097 | 0.09 ± 0.058 | 0.12 ± 0.070 | 0.14 ± 0.102 | ns | ns | ns |
| c9 t12 C18:2 (n6) | 0.10 ± 0.061 | 0.11 ± 0.044 | 0.10 ± 0.066 | 0.09 ± 0.038 | 0.11 ± 0.060 | 0.11 ± 0.072 | ns | ns | ns |
| t9 c12 C18:2 (n6) | 0.05 ± 0.033 | 0.07 ± 0.059 | 0.06 ± 0.044 | 0.05 ± 0.041 | 0.07 ± 0.048 | 0.06 ± 0.048 | ns | ns | * |
| ctmix10,14,12,16 C18:2 | 0.09 ± 0.056 | 0.06 ± 0.030 | 0.06 ± 0.039 | 0.07 ± 0.042 | 0.07 ± 0.047 | 0.07 ± 0.047 | t | ns | ns |
| t11 c15 C18:2 (n3) | 0.16 b ± 0.097 | 0.25 b ± 0.112 | 0.40 a ± 0.133 | 0.27 ± 0.164 | 0.28 ± 0.156 | 0.32 ± 0.152 | ** | ns | ns |
| c9,12 C18:2 (n6) (LA) | 1.8 a ± 0.168 | 1.6 a ± 0.232 | 0.9 b ± 0.321 | 1.4 a ± 0.548 | 1.4 a ± 0.430 | 1.3 b ± 0.441 | *** | * | *** |
| C19:1 | 0.09 ± 0.033 | 0.10 ± 0.054 | 0.10 ± 0.074 | 0.08 ± 0.029 | 0.11 ± 0.054 | 0.11 ± 0.080 | ns | ns | t |
| Unknown LA2 | 0.09 ± 0.046 | 0.09 ± 0.052 | 0.11 ± 0.066 | 0.08 b ± 0.046 | 0.09 b ± 0.047 | 0.13 a ± 0.066 | ns | * | ns |
| Unknown LA3 | 0.05 ± 0.025 | 0.08 ± 0.068 | 0.07 ± 0.068 | 0.06 ± 0.030 | 0.08 ± 0.062 | 0.08 ± 0.077 | ns | ns | t |
| c9,15 C18:2 (n3) | 0.03 ± 0.029 | 0.04 ± 0.024 | 0.04 ± 0.040 | 0.03 ± 0.041 | 0.04 ± 0.030 | 0.04 ± 0.029 | ns | ns | ns |
| c12,15 C18:2 (n3) | 0.05 ± 0.029 | 0.06 ± 0.045 | 0.05 ± 0.041 | 0.05 ± 0.041 | 0.06 ± 0.043 | 0.06 ± 0.034 | ns | ns | ns |
| C20:0 | 0.15 b ± 0.034 | 0.18 b ± 0.054 | 0.22 a ± 0.058 | 0.18 ± 0.046 | 0.20 ± 0.064 | 0.18 ± 0.062 | ** | ns | ns |
| c6,9,12 C18:3 (n6) | 0.04 ± 0.029 | 0.07 ± 0.046 | 0.05 ± 0.032 | 0.04 b ± 0.027 | 0.05 ab ± 0.031 | 0.07 a ± 0.043 | ns | * | ** |
| c8 C20:1 | 0.08 ± 0.033 | 0.11 ± 0.034 | 0.11 ± 0.038 | 0.09 ± 0.037 | 0.10 ± 0.039 | 0.11 ± 0.034 | t | ns | ns |
| C18:3 (n3) (ALA) | 0.47 c ± 0.079 | 0.79 b ± 0.125 | 1.06 a ± 0.274 | 0.78 ± 0.279 | 0.82 ± 0.331 | 0.86 ± 0.353 | *** | ns | ns |
| c9 t11 C18:2 (CLA9) | 0.62 c ± 0.071 | 0.92 b ± 0.138 | 1.16 a ± 0.431 | 0.95 ± 0.502 | 0.90 ± 0.238 | 0.98 ± 0.348 | *** | ns | ns |
| t11 c13 CLA | 0.05 ± 0.031 | 0.10 ± 0.053 | 0.09 ± 0.064 | 0.08 ± 0.062 | 0.07 ± 0.048 | 0.09 ± 0.059 | t | ns | ns |
| Unknown CLA1 | 0.05 ± 0.034 | 0.08 ± 0.055 | 0.08 ± 0.054 | 0.06 ± 0.035 | 0.07 ± 0.052 | 0.08 ± 0.062 | ns | ns | ns |
| Unknown CLA2tt | 0.07 ± 0.022 | 0.08 ± 0.052 | 0.07 ± 0.049 | 0.07 ± 0.038 | 0.08 ± 0.041 | 0.07 ± 0.053 | ns | ns | ns |
| Unknown CLA3tt | 0.06 ± 0.043 | 0.11 ± 0.069 | 0.11 ± 0.081 | 0.08 ± 0.069 | 0.10 ± 0.072 | 0.10 ± 0.076 | ns | ns | ns |
| Unknown CLA6tt | 0.06 ± 0.055 | 0.07 ± 0.065 | 0.07 ± 0.065 | 0.07 ± 0.058 | 0.06 ± 0.053 | 0.08 ± 0.072 | ns | ns | ns |
| c9,13,15 C18:3 (n3) | 0.05 ± 0.032 | 0.06 ± 0.027 | 0.06 ± 0.028 | 0.05 ± 0.036 | 0.07 ± 0.020 | 0.06 ± 0.026 | ns | ns | * |
| c11,14 C20:2 (n6) | 0.05 ± 0.028 | 0.09 ± 0.058 | 0.06 ± 0.032 | 0.06 ± 0.024 | 0.07 ± 0.054 | 0.07 ± 0.042 | ns | ns | ns |
| c9,11,15 C18:3 (n3) | 0.08 b ± 0.040 | 0.13 a ± 0.068 | 0.10 ab ± 0.043 | 0.10 ± 0.047 | 0.11 ± 0.067 | 0.10 ± 0.046 | * | ns | ns |
| C22:0 | 0.11 ± 0.057 | 0.13 ± 0.019 | 0.12 ± 0.040 | 0.12 ± 0.041 | 0.12 ± 0.031 | 0.12 ± 0.053 | ns | ns | ns |
| c8,11,14 C20:3 (n6) | 0.14 a ± 0.052 | 0.10 ab ± 0.030 | 0.08 b ± 0.036 | 0.10 ± 0.048 | 0.10 ± 0.040 | 0.11 ± 0.051 | * | ns | ns |
| c13 C22:1 | 0.10 ± 0.059 | 0.08 ± 0.052 | 0.07 ± 0.047 | 0.06 ± 0.039 | 0.09 ± 0.057 | 0.10 ± 0.054 | ns | t | ns |
| c11,14,17 C20:3 (n3) | 0.07 ± 0.061 | 0.08 ± 0.058 | 0.07 ± 0.052 | 0.07 ± 0.046 | 0.05 ± 0.059 | 0.09 ± 0.057 | ns | ns | ns |
| c5,8,11,14 C20:4 (n6) | 0.21 ± 0.122 | 0.19 ± 0.090 | 0.14 ± 0.074 | 0.17 ± 0.089 | 0.16 ± 0.096 | 0.19 ± 0.110 | ns | ns | ns |
| C23:0 | 0.06 b ± 0.033 | 0.10 a ± 0.033 | 0.08 b ± 0.023 | 0.07 ± 0.038 | 0.08 ± 0.033 | 0.08 ± 0.025 | * | ns | ns |
| c13,16 C22:2 (n6) | 0.08 ± 0.046 | 0.10 ± 0.041 | 0.10 ± 0.030 | 0.10 ± 0.039 | 0.08 ± 0.035 | 0.10 ± 0.039 | ns | ns | ns |
| c5,8,11,14,17 C20:5 (n3) (EPA) | 0.09 b ± 0.044 | 0.17 a ± 0.089 | 0.16 a ± 0.052 | 0.14 ± 0.082 | 0.14 ± 0.070 | 0.15 ± 0.061 | ** | ns | ns |
| C24:0 | 0.09 ± 0.054 | 0.15 ± 0.117 | 0.14 ± 0.094 | 0.12 ± 0.089 | 0.14 ± 0.107 | 0.14 ± 0.088 | ns | ns | ns |
| c15 C24:1 | 0.06 ± 0.062 | 0.07 ± 0.042 | 0.05 ± 0.019 | 0.06 ± 0.023 | 0.06 ± 0.057 | 0.05 ± 0.039 | ns | ns | ns |
| c13,16,19 C22:3 (n3) | 0.04 ± 0.029 | 0.04 ± 0.025 | 0.04 ± 0.022 | 0.04 ± 0.028 | 0.03 ± 0.019 | 0.04 ± 0.026 | ns | ns | * |
| c7,10,13,16 C22:4 (n6) | 0.05 ± 0.024 | 0.06 ± 0.031 | 0.05 ± 0.036 | 0.05 b ± 0.023 | 0.04 b ± 0.018 | 0.07 a ± 0.041 | ns | ** | t |
| c7,10,13,16,19 C22:5 (n3) (DPA) | 0.12 b ± 0.030 | 0.16 a ± 0.067 | 0.18 a ± 0.046 | 0.17 ± 0.057 | 0.15 ± 0.054 | 0.15 ± 0.056 | ** | ns | ns |
| c4,7,10,13,16,19 C22:5 (n3) (DHA) | 0.05 ± 0.062 | 0.06 ± 0.037 | 0.04 ± 0.023 | 0.05 ± 0.034 | 0.05 ± 0.058 | 0.05 ± 0.025 | ns | ns | ns |
| SFA c | 67.7 ± 0.868 | 66.3 ± 1.487 | 66.9 ± 4.536 | 67.7 ± 4.123 | 66.6 ± 2.948 | 66.6 ± 2.159 | ns | ns | ns |
| MUFA d | 27.0 ± 0.800 | 27.6 ± 0.972 | 27.1 ± 3.636 | 26.6 ± 3.353 | 27.6 ± 2.295 | 27.3 ± 1.522 | ns | ns | ns |
| PUFA e | 5.3 ± 0.412 | 6.2 ± 0.797 | 6.0 ± 1.522 | 5.7 ± 1.130 | 5.8 ± 1.091 | 6.1 ± 1.261 | ns | ns | ns |
| n-3 f | 1.2 c ± 0.197 | 1.9 b ± 0.374 | 2.3 a ± 0.480 | 1.8 ± 0.539 | 1.8 ± 0.590 | 2.0 ± 0.596 | *** | ns | ns |
| n-6 g | 2.7 a ± 0.217 | 2.4 a ± 0.234 | 1.6 b ± 0.455 | 2.1 ± 0.633 | 2.1 ± 0.552 | 2.2 ± 0.573 | *** | ns | ns |
| n3n6 | 0.47 c ± 0.083 | 0.77 b ± 0.136 | 1.40 a ± 0.202 | 0.96 ± 0.488 | 0.94 ± 0.394 | 1.01 ± 0.448 | *** | ns | t |
| n-6/n-3 | 2.2 a ± 0.377 | 1.3 b ± 0.230 | 0.7 c ± 0.102 | 1.4 ± 0.727 | 1.3 ± 0.665 | 1.2 ± 0.587 | *** | t | ns |
| EPA + DPA + DHA | 0.25 b ± 0.086 | 0.39 a ± 0.147 | 0.38 a ± 0.080 | 0.36 ± 0.136 | 0.33 ± 0.119 | 0.35 ± 0.105 | ** | ns | ns |
References
- Lorenz, H.; Reinsch, T.; Hess, S.; Taube, F. Is low-input dairy farming more climate friendly? A meta-analysis of the carbon footprints of different production systems. J. Clean. Product. 2019, 211, 161–170. [Google Scholar] [CrossRef]
- Mills, S.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Stanton, C. Milk intelligence: Mining milk for bioactive substances associated with human health. Int. Dairy J. 2011, 21, 377–401. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Davis, D.R.; Heins, B.J.; Latif, M.A.; Leifert, C.; Peterman, L.; Butler, G.; Faergeman, O.; Abel-Caines, S.; Baranski, M. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modeling of diet outcomes. Food Sci. Nutr. 2018, 6, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Stergiadis, S.; Leifert, C.; Seal, C.J.; Eyre, M.D.; Larsen, M.K.; Slots, T.; Nielsen, J.H.; Butler, G. A 2-year study on milk quality from three pasture-based dairy systems of contrasting production intensities in Wales. J. Agric. Sci. 2015, 153, 708–731. [Google Scholar] [CrossRef] [Green Version]
- Givens, D.I.; Gibbs, R.A. Very long chain n-3 polyunsaturated fatty acids in the food chain in the UK and the potential of animal-derived foods to increase intake. Nutr. Bull. 2006, 31, 104–110. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Janssen, C.I.F.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Wahle, K.W.J.; Heys, S.D.; Rotondo, D. Conjugated linoleic acids: Are they beneficial or detrimental to health? Prog. Lipid Res. 2004, 43, 553–587. [Google Scholar] [CrossRef]
- Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 2016, 785, 87–95. [Google Scholar] [CrossRef]
- Su, K.P.; Huang, S.Y.; Chiu, T.H.; Huang, K.C.; Huang, C.L.; Chang, H.C.; Pariante, C.M. Omega-3 fatty acids for major depressive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatr. 2008, 69, 644. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Hostmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.M.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the ω-6 and ω-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907. [Google Scholar]
- Simopoulos, A. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A. Dietary Omega-3 Fatty Acid Deficiency and High Fructose Intake in the Development of Metabolic Syndrome, Brain Metabolic Abnormalities, and Non-Alcoholic Fatty Liver Disease. Nutrients 2013, 5, 2901. [Google Scholar] [CrossRef] [Green Version]
- Thorsdottir, I.; Hill, J.; Ramel, A. Omega-3 fatty acid supply from milk associates with lower type 2 diabetes in men and coronary heart disease in women. Prev. Med. 2004, 39, 630–634. [Google Scholar] [CrossRef]
- Butler, G.; Nielsen, J.H.; Slots, T.; Seal, C.; Eyre, M.D.; Sanderson, R.; Leifert, C. Fatty acid and fat-soluble antioxidant concentrations in milk from high- and low-input conventional and organic systems: Seasonal variation. J. Sci. Food Agric. 2008, 88, 1431–1441. [Google Scholar] [CrossRef]
- Ellis, K.A.; Innocent, G.; Grove-White, D.; Cripps, P.; McLean, W.G.; Howard, C.V.; Mihm, M. Comparing the fatty acid composition of organic and conventional milk. J. Dairy Sci. 2006, 89, 1938–1950. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Barański, M.; Seal, C.J.; Sanderson, R.; Benbrook, C.; Steinshamn, H.; Gromadzka-Ostrowska, J.; Rembiałkowska, E.; Skwarło-Sońta, K.; Eyre, M. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: A systematic literature review and meta-and redundancy analyses. Br. J. Nutr. 2016, 115, 1043–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stergiadis, S.; Leifert, C.; Seal, C.J.; Eyre, M.D.; Nielsen, J.H.; Larsen, M.K.; Slots, T.; Steinshamn, H.; Butler, G. Effect of Feeding Intensity and Milking System on Nutritionally Relevant Milk Components in Dairy Farming Systems in the North East of England. J. Agric. Food Chem. 2012, 60, 7270–7281. [Google Scholar] [CrossRef] [PubMed]
- PFLA. The Pasture for Life Standards. Available online: https://www.pastureforlife.org/certification/the-pasture-for-life-standards/ (accessed on 28 June 2018).
- Markey, O.; Vasilopoulou, D.; Givens, D.I.; Lovegrove, J.A. Dairy and cardiovascular health: Friend or foe? Nutr. Bull. 2014, 39, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Martin, C.; Rouel, J.; Doreau, M. Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output1. J. Dairy Sci. 2009, 92, 5199–5211. [Google Scholar] [CrossRef]
- Stergiadis, S.; Bieber, A.; Franceschin, E.; Isensee, A.; Eyre, M.D.; Maurer, V.; Chatzidimitriou, E.; Cozzi, G.; Bapst, B.; Stewart, G. Impact of US Brown Swiss genetics on milk quality from low-input herds in Switzerland: Interactions with grazing intake and pasture type. Food Chem. 2015, 175, 609–618. [Google Scholar] [CrossRef]
- Stergiadis, S.; Leifert, C.; Seal, C.J.; Eyre, M.D.; Steinshamn, H.; Butler, G. Improving the fatty acid profile of winter milk from housed cows with contrasting feeding regimes by oilseed supplementation. Food Chem. 2014, 164, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Butler, G.; Stergiadis, S.; Seal, C.; Eyre, M.; Leifert, C. Fat composition of organic and conventional retail milk in northeast England. J. Dairy Sci. 2011, 94, 24–36. [Google Scholar] [CrossRef]
- Herzallah, S.M.; Humeid, M.A.; Al-Ismail, K.M. Effect of Heating and Processing Methods of Milk and Dairy Products on Conjugated Linoleic Acid and Trans Fatty Acid Isomer Content. J. Dairy Sci. 2005, 88, 1301–1310. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Donahue, S.M.A.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stergiadis, S.; Berlitz, C.B.; Hunt, B.; Garg, S.; Ian Givens, D.; Kliem, K.E. An update to the fatty acid profiles of bovine retail milk in the United Kingdom: Implications for nutrition in different age and gender groups. Food Chem. 2019, 276, 218–230. [Google Scholar] [CrossRef]
- Slots, T.; Butler, G.; Leifert, C.; Kristensen, T.; Skibsted, L.H.; Nielsen, J.H. Potentials to differentiate milk composition by different feeding strategies. J. Dairy Sci. 2009, 92, 2057–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, J.J.; Coakley, M.; Stanton, C. Supplementation of dairy cows with a fish oil containing supplement and sunflower oil to increase the CLA content of milk produced at pasture. Livest. Sci. 2008, 116, 332–337. [Google Scholar] [CrossRef]
- Kelly, M.L.; Kolver, E.S.; Bauman, D.E.; Van Amburgh, M.E.; Muller, L.D. Effect of Intake of Pasture on Concentrations of Conjugated Linoleic Acid in Milk of Lactating Cows1. J. Dairy Sci. 1998, 81, 1630–1636. [Google Scholar] [CrossRef]
- Kay, J.K.; Roche, J.R.; Kolver, E.S.; Thomson, N.A.; Baumgard, L.H. A comparison between feeding systems (pasture and TMR) and the effect of vitamin E supplementation on plasma and milk fatty acid profiles in dairy cows. J. Dairy Res. 2005, 72, 322–332. [Google Scholar] [CrossRef]
- Glasser, F.; Ferlay, A.; Chilliard, Y. Oilseed Lipid Supplements and Fatty Acid Composition of Cow Milk: A Meta-Analysis. J. Dairy Sci. 2008, 91, 4687–4703. [Google Scholar] [CrossRef] [Green Version]
- Kliem, K.E.; Shingfield, K.J.; Livingstone, K.M.; Givens, D.I. Seasonal variation in the fatty acid composition of milk available at retail in the United Kingdom and implications for dietary intake. Food Chem. 2013, 141, 274–281. [Google Scholar] [CrossRef]
- Kalač, P.; Samková, E. The effects of feeding various forages on fatty acid composition of bovine milk fat: A review. Czech. J. Anim. Sci. 2010, 55, 521–537. [Google Scholar] [CrossRef] [Green Version]
- PFLA. The Farm Business Case for Feeding Ruminants Just on Pasture. Available online: https://www.pastureforlife.org/media/2018/10/Pasture-for-Life-It-can-be-done-e-version-Oct-18.pdf (accessed on 23 November 2018).
- Google. Map of PFLA Dairy Farmers and Supermarkets Visited in Study. Available online: https://maps.google.com/ (accessed on 25 April 2020).
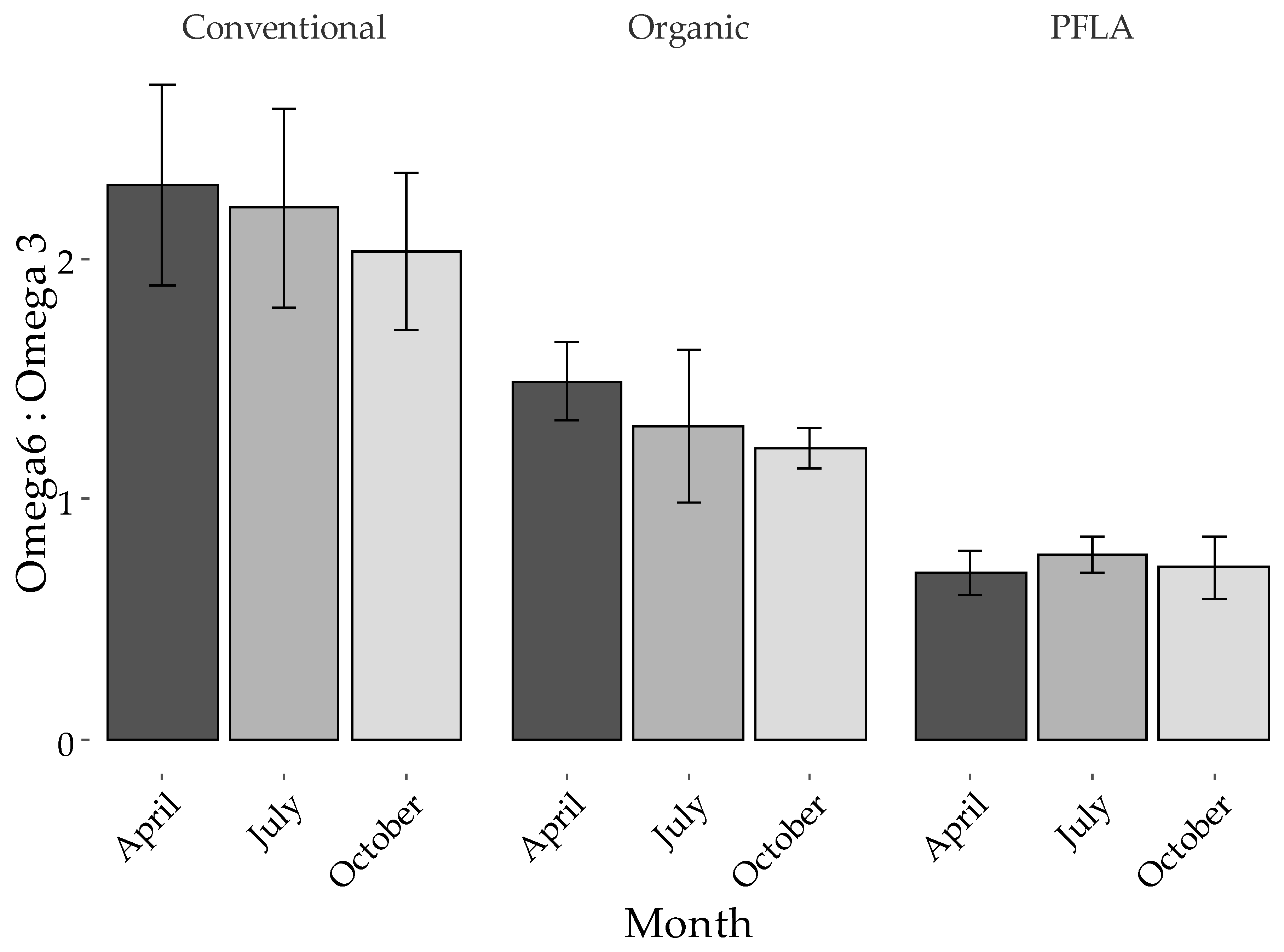
| Management | Month | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Conv. | Org. | PFLA | April | July | October | Man | Mon | Man × Mon † | |
| Fatty Acids | n = 15 | n = 15 | n = 24 | n = 18 | n = 18 | n = 18 | |||
| ALA ‡ | 0.47c ± 0.020 | 0.79b ± 0.032 | 1.1a ± 0.06 | 0.79 ± 0.066 | 0.82 ± 0.078 | 0.86 ± 0.083 | *** | ns | ns |
| CLA9 | 0.62c ± 0.018 | 0.92b ± 0.036 | 1.2a ± 0.09 | 0.95 ± 0.118 | 0.90 ± 0.056 | 0.98 ± 0.082 | *** | ns | ns |
| n-3 | 1.2c ± 0.05 | 1.9 b ± 0.10 | 2.3a ± 0.10 | 1.8 ± 0.13 | 1.8 ± 0.14 | 2.00 ± 0.14 | *** | ns | ns |
| EPA + DHA + DPA | 0.25b ± 0.022 | 0.39 a ± 0.038 | 0.38 a ± 0.016 | 0.36 ± 0.032 | 0.34 ± 0.028 | 0.35 ± 0.025 | ** | ns | ns |
| C12:0 | 3.5 ± 0.06 | 3.3 ± 0.04 | 3.4 ± 0.12 | 3.6a ± 0.11 | 3.3b ± 0.08 | 3.3b ± 0.08 | ns | ** | ns |
| C14:0 | 11.0 ± 0.10 | 11.0 ± 0.08 | 11.1 ± 0.24 | 11.2 ± 0.22 | 11.0 ± 0.21 | 11.0 ± 0.14 | ns | ns | ns |
| C16:0 | 31.1a ± 0.30 | 28.6 b ± 0.32 | 28.0b ± 0.80 | 29.0 ± 0.94 | 28.6 ± 0.64 | 29.5 ± 0.54 | * | ns | ns |
| n-6 | 2.7a ± 0.06 | 2.5a ± 0.06 | 1.6b ± 0.09 | 2.1 ± 0.15 | 2.1 ± 0.13 | 2.2 ± 0.14 | *** | ns | ns |
| n-6/n-3 | 2.2a ± 0.10 | 1.3b ± 0.06 | 0.73c ± 0.021 | 1.4 ± 0.17 | 1.3 ± 0.16 | 1.2 ± 0.14 | *** | t | ns |
| Source | Location | Management | Omega-6/Omega-3 | Reference |
|---|---|---|---|---|
| Retail studies | NE England | Conventional | 3.8 | [28] |
| Organic | 2.6 | |||
| USA | Conventional | 5.8 | [3] | |
| Organic | 2.3 | |||
| Grassmilk® | 0.95 | |||
| England | Conventional | 2.2 | Current study | |
| Organic | 1.3 | |||
| South England | Conventional | 2.6 | [34] | |
| Organic | 1.7 | |||
| Farm surveys | NW England and Wales | Conventional | 2.5 | [20] |
| Organic | 1.5 | |||
| UK | Conventional | 2.7 | [19] | |
| Organic | 1.3 | |||
| Conventional Low-Input † | 1.1 | |||
| Denmark UK | Conventional | 4.7 | [35] | |
| Organic | 1.9 | |||
| Low-Input | 1.0 | |||
| Wales | Conventional | 3.1 | [4] | |
| Organic | 1.7 | |||
| Conventional Low-Input | 1.4 | |||
| USA | Grassmilk | 0.95 | [3] | |
| England | PFLA | 0.73 | Current study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, H.; Chatzidimitriou, E.; Leifert, C.; Butler, G. Evidence That Forage-Fed Cows Can Enhance Milk Quality. Sustainability 2020, 12, 3688. https://doi.org/10.3390/su12093688
Davis H, Chatzidimitriou E, Leifert C, Butler G. Evidence That Forage-Fed Cows Can Enhance Milk Quality. Sustainability. 2020; 12(9):3688. https://doi.org/10.3390/su12093688
Chicago/Turabian StyleDavis, Hannah, Eleni Chatzidimitriou, Carlo Leifert, and Gillian Butler. 2020. "Evidence That Forage-Fed Cows Can Enhance Milk Quality" Sustainability 12, no. 9: 3688. https://doi.org/10.3390/su12093688
APA StyleDavis, H., Chatzidimitriou, E., Leifert, C., & Butler, G. (2020). Evidence That Forage-Fed Cows Can Enhance Milk Quality. Sustainability, 12(9), 3688. https://doi.org/10.3390/su12093688






