Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology
Abstract
1. Introduction
2. Disadvantages in Conventional Extraction Technology
3. Supercritical Fluid Extraction Technology
3.1. Selection of Extractive Solvent
3.2. Development of Supercritical CO2 Technology
3.2.1. Conventional SFE Technology Design
- Single extraction concept—Extractions are taken place in an enclosed semi-batch or tubular reactor.
- Sequential extraction concept—Customized for efficient compound separation by fractionation in additional sequencing reactors [40].
- Co-integrated extraction system concept—The integration of ultrasonication-assisted extraction in SFE system to accelerate extraction kinetics [41].
3.2.2. Single and Sequential Extractions Concept
3.2.3. Co-Integrated Extraction System Concepts
4. Applications of SFE Technology on Tropical Biomass
4.1. Phenolic Compound Extraction
4.2. Biorepellent Compound Extraction
4.3. Biopesticidal Compound Extraction
4.4. Lipid Extraction
5. Considerations in Upscaling SFE Technology
6. Role of SFE in Circular Economy and Sustainable Development Approaches
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current Status, Challenges, and Future Direction. Energy Fuels 2006, 1727–1737. [Google Scholar] [CrossRef]
- Jarboe, P.J.; Candela, P.A.; Zhu, W.; Kaufman, A.J. Extraction of Hydrocarbons from High-Maturity Marcellus Shale Using Supercritical Carbon Dioxide. Energy Fuels 2015, 29, 7897–7909. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Queiroga, C.L.; Duarte, G.H.B.; Eberlin, M.N.; Kohn, L.K.; Arns, C.W.; Cabral, F.A. Sequential extraction of bioactive compounds from Melia azedarach L. in fixed bed extractor using CO2, ethanol and water. J. Supercrit. Fluids 2014, 95, 355–363. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae–An update. TrAC-Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Salinas-Salazar, C.; Saul Garcia-Perez, J.; Chandra, R.; Castillo-Zacarias, C.; Iqbal, H.M.N.; Parra-Saldívar, R. Methods for Extraction of Valuable Products from Microalgae Biomass. Microalgae Biotechnol. Dev. Biofuel Wastewater Treat. 2019, 245–263. [Google Scholar] [CrossRef]
- Lee, S.; Park, M.K.; Kim, K.H.; Kim, Y.S. Effect of supercritical carbon dioxide decaffeination on volatile components of green teas. J. Food Sci. 2007, 72, S497–S502. [Google Scholar] [CrossRef]
- Kurt, Z. Process for the Decaffeination of Coffee. U.S. Patent 4260639, 7 April 1981. [Google Scholar]
- Lack, E.; Seidlitz, H. Commercial scale decaffeination of coffee and tea using supercritical CO2. In Extraction of Natural Products Using Near-Critical Solvents; King, M.B., Bott, T.R., Eds.; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 1–22. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarcă, S.; Muntean, D.L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Med. (United Kingdom) 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Lee, N.Y.; Yunus, M.A.C.; Idham, Z.; Ruslan, M.S.H.; Aziz, A.H.A.; Irwansyah, N. Extraction and identification of bioactive compounds from agarwood leaves. IOP Conf. Ser. Mater. Sci. Eng. 2016, 162, 012028. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, D.; Indira, G. A comparative evaluation of maceration, soxhlation and ultra sound assisted extraction for the phytochemical screening of the leaves of Nephelium lappaceum. L. (Sapindaceae). J. Pharmacogn. Phytochem. 2016, 5, 386–389. [Google Scholar]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, G.; Wang, X.; Hu, X.; Chen, L. The Study on Optimization of Soxhlet Extraction Process for Ursolic Acid from Cynomorium. Food Res. Devellopment 2013, 7. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef]
- Knez, Ž; Markočič, E.; Leitgeb, M.; Primožič, M.; Hrnčič, M.K.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Ali-Nehari, A.; Kim, S.B.; Lee, Y.B.; Lee, H.Y.; Chun, B.S. Characterization of oil including astaxanthin extracted from krill (Euphausia superba) using supercritical carbon dioxide and organic solvent as comparative method. Korean J. Chem. Eng. 2012, 29, 329–336. [Google Scholar] [CrossRef]
- Ekinci, M.S.; Gürü, M. Extraction of oil and β-sitosterol from peach (Prunus persica) seeds using supercritical carbon dioxide. J. Supercrit. Fluids 2014, 92, 319–323. [Google Scholar] [CrossRef]
- Abbas, K.; Mohamed, A.; Abdulamir, A.; Abas, H. A Review on Supercritical Fluid Extraction as New Analytical Method. Am. J. Biochem. Biotechnol. 2008, 4, 345–353. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Loh, S.K. Fractionation of pyrolysis oil via supercritical carbon dioxide extraction: Optimization study using response surface methodology (RSM). Biomass Bioenergy 2017, 107, 155–163. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Raynie, D.E. Warning Concerning the Use of Nitrous Oxide in Supercritical Fluid Extractions. Anal. Chem. 1993, 65, 3127–3128. [Google Scholar] [CrossRef]
- Akgerman, A. Supercritical Fluids in Environmental Remediation and Pollution Prevention. ACS Symp. Ser. 1997, 670, 208–231. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Coaquira-Quispe, J.J.; Meireles, M.A.A. Supercritical fluid extraction of polyphenols from lees: Overall extraction curve, kinetic data and composition of the extracts. Bioresour. Bioprocess. 2015, 2, 45. [Google Scholar] [CrossRef]
- Vatansever, S.; Hall, C. Flavor modification of yellow pea flour using supercritical carbon dioxide + ethanol extraction and response surface methodology. J. Supercrit. Fluids 2020, 156, 104659. [Google Scholar] [CrossRef]
- Campone, L.; Celano, R.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Ibañez, E.; Cifuentes, A.; Rastrelli, L. Response surface methodology to optimize supercritical carbon dioxide/co-solvent extraction of brown onion skin by-product as source of nutraceutical compounds. Food Chem. 2018, 269, 495–502. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Cahyadi, J.; Guigard, S.E.; Saldaña, M.D.A. Optimization of artemisinin extraction from Artemisia annua L. with supercritical carbon dioxide + ethanol using response surface methodology. Electrophoresis 2018, 39, 1926–1933. [Google Scholar] [CrossRef]
- Yoswathana, N. Optimization of ScCO2 Extraction of Rambutan Seed Oil Using Response Surface Methodology. Int. J. Chem. Eng. Appl. 2013, 4, 187–190. [Google Scholar] [CrossRef]
- Asep, E.K.; Jinap, S.; Jahurul, M.H.A.; Zaidul, I.S.M.; Singh, H. Effects of polar cosolvents on cocoa butter extraction using supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2013, 20, 152–160. [Google Scholar] [CrossRef]
- De Lucas, A.; Gracia, I.; Rincón, J.; García, M.T. Solubility determination and model prediction of olive husk oil in supercritical carbon dioxide and cosolvents. Ind. Eng. Chem. Res. 2007, 46, 5061–5066. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Kroon, M.C.; Peters, C.J. Supercritical Fluids in Ionic Liquids. In Ionic Liquids Further UnCOILed: Critical Expert Overviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 9781118438, pp. 39–57. ISBN 9781118839706. [Google Scholar]
- Feng, Y.; Meier, D. Supercritical carbon dioxide extraction of fast pyrolysis oil from softwood. J. Supercrit. Fluids 2017, 128, 6–17. [Google Scholar] [CrossRef]
- López-Padilla, A.; Ruiz-Rodriguez, A.; Reglero, G.; Fornari, T. Supercritical carbon dioxide extraction of Calendula officinalis: Kinetic modeling and scaling up study. J. Supercrit. Fluids 2017, 130, 292–300. [Google Scholar] [CrossRef]
- Hoshino, Y.; Ota, M.; Sato, Y.; Smith, R.L.; Inomata, H. Fractionation of hops-extract–ethanol solutions using dense CO2 with a counter-current extraction column. J. Supercrit. Fluids 2018, 136, 37–43. [Google Scholar] [CrossRef]
- Soares, J.F.; Prá, V.D.; Barrales, F.M.; Dos Santos, P.; Kuhn, R.C.; Rezende, C.A.; Martínez, J.; Mazutti, M.A. Extraction of rice bran oil using supercritical CO2 combined with ultrasound. Brazilian J. Chem. Eng. 2018, 35, 785–794. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Guerrero-Beltrán, J. Supercritical extraction of essential oils of Piper auritum and Porophyllum ruderale. J. Supercrit. Fluids 2017, 127, 97–102. [Google Scholar] [CrossRef]
- Aydi, A.; Zibetti, W.; Al-khazaal, A.Z.; Eladeb, A. Supercritical CO2 Extraction of Extracted Oil from Pistacia lentiscus L.: Mathematical Modeling, Economic Evaluation and Scale-Up Abdelkarim. Molecules 2020, 25, 199. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Chai, Y.H.; Uemura, Y.; Loh, S.K. Extraction of palm kernel shell derived pyrolysis oil by supercritical carbon dioxide: Evaluation and modeling of phenol solubility. Biomass Bioenergy 2018, 116, 106–112. [Google Scholar] [CrossRef]
- Elst, K.; Maesen, M.; Jacobs, G.; Bastiaens, L.; Voorspoels, S.; Servaes, K. Supercritical CO2 Extraction of nannochloropsis sp.: A lipidomic study on the influence of pretreatment on yield and composition. Molecules 2018, 23, 1854. [Google Scholar] [CrossRef] [PubMed]
- Jafarian Asl, P.; Niazmand, R.; Yahyavi, F. Extraction of phytosterols and tocopherols from rapeseed oil waste by supercritical CO2 plus co-solvent: A comparison with conventional solvent extraction. Heliyon 2020, 6, e03592. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Vardanega, R.; Nogueira, G.C.; Nascimento, C.D.O.; Faria-Machado, A.F.; Meireles, M.A.A. Selective extraction of bioactive compounds from annatto seeds by sequential supercritical CO2 process. J. Supercrit. Fluids 2019, 150, 122–127. [Google Scholar] [CrossRef]
- Fraga, S.; Gonçalves, D.; Nasário, F.; Pereira, E.; Pontes, P.; Ribas, L.; Barbeiro, L.B.; de Almeida Meirelles, A.J.; Cabral, F.; Sampaio, K.A. Sequential high-pressure extraction of caffeine and bioactive compounds from caferana seeds (Bunchosia glandulifera). J. Supercrit. Fluids 2020, 165, 1–9. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Siti Shuhadah, M.S. Co-Solvent Selection for Supercritical Fluid Extraction (SFE) of Phenolic Compounds from Labisia pumila. Molecules 2020, 25, 5859. [Google Scholar] [CrossRef]
- Garmus, T.T.; Paviani, L.C.; Queiroga, C.L.; Cabral, F.A. Extraction of phenolic compounds from pepper-rosmarin (Lippia sidoides Cham.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids 2015, 99, 68–75. [Google Scholar] [CrossRef]
- Cristianini, M.; Guillén Sánchez, J.S. Extraction of bioactive compounds from purple corn using emerging technologies: A review. J. Food Sci. 2020, 85, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Döker, O.; Salgin, U.; Şanal, I.; Mehmetoǧlu, Ü.; Çalimli, A. Modeling of extraction of β-carotene from apricot bagasse using supercritical CO2 in packed bed extractor. J. Supercrit. Fluids 2004, 28, 11–19. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Hasan, M. Supercritical Carbon Dioxide Extraction of Andrographolide from Andrographis paniculata: Effect of the Solvent Flow Rate, Pressure, and Temperature. Chin. J. Chem. Eng. 2007, 15, 877–883. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Mathematical modeling of mass transfer in supercritical fluid extraction of patchouli oil. Eng. Rep. 2019, 1–11. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E.; Lubián, L.M.; Montero, O. Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta 2009, 77, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Kentish, S.E.; Mawson, R.; Ashokkumar, M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef]
- Dassoff, E.S.; Li, Y.O. Mechanisms and effects of ultrasound-assisted supercritical CO2 extraction. Trends Food Sci. Technol. 2019, 86, 492–501. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C. A combined procedure of ultrasound-assisted and supercritical carbon dioxide for extraction and quantitation oleanolic and ursolic acids from Hedyotis corymbosa. Ind. Crops Prod. 2016, 79, 7–17. [Google Scholar] [CrossRef]
- Riera, E.; Blanco, A.; García, J.; Benedito, J.; Mulet, A.; Gallego-Juárez, J.A.; Blasco, M. High-power ultrasonic system for the enhancement of mass transfer in supercritical CO2 extraction processes. Phys. Procedia 2010, 3, 141–146. [Google Scholar] [CrossRef]
- Pasquel Reátegui, J.L.; Machado, A.P.D.F.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233. [Google Scholar] [CrossRef]
- Wei, M.C.; Hong, S.J.; Yang, Y.C. Isolation of triterpenic acid-rich extracts from Hedyotis corymbosa using ultrasound-assisted supercritical carbon dioxide extraction and determination of their fictitious solubilities. J. Ind. Eng. Chem. 2017, 48, 202–211. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Food Waste-Extent, Causes and Prevention; FAO: Dusseldorf, Germany, 2011; ISBN 9789251072059. [Google Scholar]
- Vieira, A.D.S.; Bedani, R.; Albuquerque, M.A.C.; Biscola, V.; Saad, S.M.I. The impact of fruit and soybean by-products and amaranth on the growth of probiotic and starter microorganisms. Food Res. Int. 2017, 97, 356–363. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Albuquerque, M.A.C.D.; Levit, R.; Beres, C.; Bedani, R.; de Moreno, M.A.; Isay Saad, S.M.; Leblanc, J.G.J. Tropical fruit by-products water extracts of tropical fruit by-products as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Stathopoulos, C.E. Extraction, Isolation and Utilization of Bioactive Compounds from Fruit Juice Industry Waste; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498741316. [Google Scholar]
- Balasundram, N. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Viganó, J.; Martinez, J. Trends for the Application of Passion Fruit Industrial By-Products: A Review on the Chemical Composition and Extraction Techniques of Phytochemicals. Food Public Heal. 2015, 5, 164–173. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Ferreira, S.R.S.; Parada-Alfonso, F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2010, 51, 319–324. [Google Scholar] [CrossRef]
- Palanisamy, U.; Cheng, H.M.; Masilamani, T.; Subramaniam, T.; Ling, L.T.; Radhakrishnan, A.K. Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants. Food Chem. 2008, 109, 54–63. [Google Scholar] [CrossRef]
- Trabelsi, D.; Aydi, A.; Zibetti, A.W.; Della Porta, G.; Scognamiglio, M.; Cricchio, V.; Langa, E.; Abderrabba, M.; Mainar, A.M. Supercritical extraction from Citrus aurantium amara peels using CO2with ethanol as co-solvent. J. Supercrit. Fluids 2016, 117, 33–39. [Google Scholar] [CrossRef]
- Silva, L.D.O.; Ranquine, L.G.; Monteiro, M.; Torres, A.G. Pomegranate (Punica granatum L.) seed oil enriched with conjugated linolenic acid (cLnA), phenolic compounds and tocopherols: Improved extraction of a specialty oil by supercritical CO2. J. Supercrit. Fluids 2019, 147, 126–137. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Angonese, M.; Gomes, C.; Ferreira, S.R.S. Valorization of passion fruit (Passiflora edulis sp.) by-products: Sustainable recovery and biological activities. J. Supercrit. Fluids 2016, 111, 55–62. [Google Scholar] [CrossRef]
- Chhouk, K.; Quitain, A.T.; Gaspillo, P.A.D.; Maridable, J.B.; Sasaki, M.; Shimoyama, Y.; Goto, M. Supercritical carbon dioxide-mediated hydrothermal extraction of bioactive compounds from Garcinia Mangostana pericarp. J. Supercrit. Fluids 2016, 110, 167–175. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Casu, R.; Pierucci, P. Comparative analysis of supercritical CO2 Extract and oil of Pimenta dioica leaves. J. Essent. Oil Res. 2005, 17, 530–532. [Google Scholar] [CrossRef]
- Maia, M.; Moore, S.J. Plant-based insect repellents: A reivew of their efficacy, development and testing. Malar. J. 2011, 10, S11. [Google Scholar] [CrossRef]
- Chang, L.H.; Jong, T.T.; Huang, H.S.; Nien, Y.F.; Chang, C.M.J. Supercritical carbon dioxide extraction of turmeric oil from Curcuma longa Linn and purification of turmerones. Sep. Purif. Technol. 2006, 47, 119–125. [Google Scholar] [CrossRef]
- Müller, G.C.; Junnila, A.; Butler, J.; Kravchenko, V.D.; Revay, E.E.; Weiss, R.W.; Schlein, Y. Efficacy of the botanical repellents geraniol, linalool, and citronella against mosquitoes. J. Vector Ecol. 2009, 34, 2–8. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Silva, C.F.; Moura, F.C.; Mendes, M.F.; Pessoa, F.L.P. Extraction of citronella (Cymbopogon nardus) essential oil using supercritical CO2: Experimental data and mathematical modeling. Brazilian J. Chem. Eng. 2011, 28, 343–350. [Google Scholar] [CrossRef]
- Restrepo Osorio, J.; Colmenares Dulcey, A.J.; Mora, L.E.; Sánchez Andica, R.A. Extraction, Chemical Composition and Antimicrobial Activity of the Essential Oils of Pipilongo (Piper Tuberculatum) Using Supercritical Carbon Dioxide. Rev. Ciencias 2014, 17, 45–56. [Google Scholar] [CrossRef]
- Chassagnez-Méndez, A.L.; Machado, N.T.; Araujo, M.E.; Maia, J.G.; Meireles, M.A.A. Supercritical CO2 extraction of curcumins and essential oil from the rhizomes of turmeric (Curcuma longa L.). Ind. Eng. Chem. Res. 2000, 39, 4729–4733. [Google Scholar] [CrossRef]
- Carvalho, P.I.N.; Osorio-Tobón, J.F.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Techno-economic evaluation of the extraction of turmeric (Curcuma longa L.) oil and ar-turmerone using supercritical carbon dioxide. J. Supercrit. Fluids 2015, 105, 44–54. [Google Scholar] [CrossRef]
- Ambrosino, P.; Fresa, R.; Fogliano, V.; Monti, S.M.; Ritieni, A. Extraction of azadirachtin a from neem seed kernels by supercritical fluid and its evaluation by HPLC and LC/MS. J. Agric. Food Chem. 1999, 47, 5252–5256. [Google Scholar] [CrossRef] [PubMed]
- Melwita, E.; Ju, Y.H. Separation of azadirachtin and other limonoids from crude neem oil via solvent precipitation. Sep. Purif. Technol. 2010, 74, 219–224. [Google Scholar] [CrossRef]
- Johnson, S.; Morgan, E.D. Supercritical fluid extraction of oil and triterpenoids from Neem seeds. Phytochem. Anal. 1997, 8, 228–232. [Google Scholar] [CrossRef]
- Ismadji, S.; Kurniawan, A.; Ju, Y.H.; Soetaredjo, F.E.; Ayucitra, A.; Ong, L.K. Solubility of azadirachtin and several triterpenoid compounds extracted from neem seed kernel in supercritical CO2. Fluid Phase Equilib. 2012, 336, 9–15. [Google Scholar] [CrossRef][Green Version]
- Wu, H.; Zhang, G.A.; Zeng, S.; Lin, K.C. Extraction of allyl isothiocyanate from horseradish (Armoracia rusticana) and its fumigant insecticidal activity on four stored-product pests of paddy. Pest Manag. Sci. 2009, 65, 1003–1008. [Google Scholar] [CrossRef]
- Zubairi, S.I.; Othman, Z.S.; Sarmidi, M.R.; Aziz, R.A. Environmental friendly bio-pesticide Rotenone extracted from Derris sp.: A review on the extraction method, toxicity and field effectiveness. J. Teknol. 2016, 8, 47–69. [Google Scholar] [CrossRef]
- Martín, L.; Marqués, J.L.; González-Coloma, A.; Mainar, A.M.; Palavra, A.M.F.; Urieta, J.S. Supercritical methodologies applied to the production of biopesticides: A review. Phytochem. Rev. 2012, 11, 413–431. [Google Scholar] [CrossRef]
- D’Andrea, A.; Aliboni, A.; De Santis, A.; Mariani, S.; Gorgoglione, D.; Ritieni, A. SFE of Derris elliptica (Wallich) Benth. roots: Influence of process parameters on yield and purity of rotenone. J. Supercrit. Fluids 2007, 42, 330–333. [Google Scholar] [CrossRef]
- Baldino, L.; Della Porta, G.; Reverchon, E. Supercritical CO2 processing strategies for pyrethrins selective extraction. J. CO2 Util. 2017, 20, 14–19. [Google Scholar] [CrossRef]
- Ramli, N.H.; Yusup, S.; Quitain, A.T.; Johari, K.; Kueh, B.W. Bin Optimization of saponin extracts using microwave-assisted extraction as a sustainable biopesticide to reduce Pomacea canaliculata population in paddy cultivation. Sustain. Chem. Pharm. 2019, 11, 23–35. [Google Scholar] [CrossRef]
- Mongkholkhajornsilp, D.; Douglas, S.; Douglas, P.L.; Elkamel, A.; Teppaitoon, W.; Pongamphai, S. Supercritical CO2 extraction of nimbin from neem seeds—A modelling study. J. Food Eng. 2005, 71, 331–340. [Google Scholar] [CrossRef]
- Tonthubthimthong, P.; Chuaprasert, S.; Douglas, P.; Luewisutthichat, W. Supercritical CO2 extraction of nimbin from neem seeds—An experimental study. J. Food Eng. 2001, 47, 289–293. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Extraction of rotenoids from Derris elliptica using supercritical CO2. J. Chem. Technol. Biotechnol. 2018, 93, 3656–3660. [Google Scholar] [CrossRef]
- Gallo, M.; Formato, A.; Ianniello, D.; Andolfi, A.; Conte, E.; Ciaravolo, M.; Varchetta, V.; Naviglio, D. Supercritical fluid extraction of pyrethrins from pyrethrum flowers (Chrysanthemum cinerariifolium) compared to traditional maceration and cyclic pressurization extraction. J. Supercrit. Fluids 2017, 119, 104–112. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Soh, L.; Zimmerman, J. Biodiesel production: The potential of algal lipids extracted with supercritical carbon dioxide. Green Chem. 2011, 13, 1422–1429. [Google Scholar] [CrossRef]
- Santana, A.; Jesus, S.; Larrayoz, M.A.; Filho, R.M. Supercritical carbon dioxide extraction of algal lipids for the biodiesel production. Procedia Eng. 2012, 42, 1755–1761. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.A.A.; Badens, E. Supercritical CO2 extraction of neutral lipids from microalgae: Experiments and modelling. J. Supercrit. Fluids 2013, 77, 7–16. [Google Scholar] [CrossRef]
- Liu, S.; Abu Hajar, H.A.; Riefler, G.; Stuart, B.J. Lipid Extraction from Spirulina sp. And Schizochytrium sp. Using Supercritical CO2 with Methanol. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.; Igl, N.; Tippelt, M.; Stege, A.; Qoura, F.; Sohling, U.; Brück, T. Extraction of microalgae derived lipids with supercritical carbon dioxide in an industrial relevant pilot plant. Bioprocess Biosyst. Eng. 2017, 40, 911–918. [Google Scholar] [CrossRef]
- Fukuzato, R. Current status of supercritical fluid technology in the East Asia. Hydrothermal React. Tech. 2003. [Google Scholar] [CrossRef]
- Prado, J.M.; Veggi, P.C.; Meireles, M.A.A. Scale-Up Issues and Cost of Manufacturing Bioactive Compounds by Supercritical Fluid Extraction and Ultrasound Assisted Extraction. In Global Food Security and Wellness; Springer: New York, NY, USA, 2017; pp. 377–433. ISBN 9781493964963. [Google Scholar]
- Hassim, N.; Markom, M.; Rosli, M.I.; Harun, S. Scale-up criteria and economic analysis for supercritical fluid extraction of Phyllanthus niruri. Chem. Eng. Process. Process Intensif. 2019, 139, 14–22. [Google Scholar] [CrossRef]
- Prado, J.M.; Prado, G.H.C.; Meireles, M.A.A. Scale-up study of supercritical fluid extraction process for clove and sugarcane residue. J. Supercrit. Fluids 2011, 56, 231–237. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Petenate, A.J.; Meireles, M.A.A. Influence of the bed geometry on the kinetics of the extraction of clove bud oil with supercritical CO2. J. Supercrit. Fluids 2014, 93, 56–66. [Google Scholar] [CrossRef]
- Moura, L.S.; Carvalho, R.N.; Stefanini, M.B.; Ming, L.C.; Meireles, M.A.A. Supercritical fluid extraction from fennel (Foeniculum vulgare): Global yield, composition and kinetic data. J. Supercrit. Fluids 2005, 35, 212–219. [Google Scholar] [CrossRef]
- Carvalho, R.N.; Moura, L.S.; Rosa, P.T.V.; Meireles, M.A.A. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity. J. Supercrit. Fluids 2005, 35, 197–204. [Google Scholar] [CrossRef]
- Wüst Zibetti, A.; Aydi, A.; Arauco Livia, M.; Bolzan, A.; Barth, D. Solvent extraction and purification of rosmarinic acid from supercritical fluid extraction fractionation waste: Economic evaluation and scale-up. J. Supercrit. Fluids 2013, 83, 133–145. [Google Scholar] [CrossRef]
- Veggi, P.C.; Cavalcanti, R.N.; Meireles, M.A.A. Production of phenolic-rich extracts from Brazilian plants using supercritical and subcritical fluid extraction: Experimental data and economic evaluation. J. Food Eng. 2014, 131, 96–109. [Google Scholar] [CrossRef]
- Núñez, G.A.; del Valle, J.M.; Navia, D. Supercritical CO2 oilseed extraction in multi-vessel plants. 3. Effect of extraction pressure and plant size on production cost. J. Supercrit. Fluids 2017, 122, 109–118. [Google Scholar] [CrossRef]
- Viganó, J.; Zabot, G.L.; Martínez, J. Supercritical fluid and pressurized liquid extractions of phytonutrients from passion fruit by-products: Economic evaluation of sequential multi-stage and single-stage processes. J. Supercrit. Fluids 2017, 122, 88–98. [Google Scholar] [CrossRef]
- Temelli, F.; Ciftci, O.N. Developing an integrated supercritical fluid biorefinery for the processing of grains. J. Supercrit. Fluids 2015, 96, 77–85. [Google Scholar] [CrossRef]
- Albarelli, J.Q.; Santos, D.T.; Cocero, M.J.; Meireles, M.A.A. Perspectives on the integration of a supercritical fluid extraction plant to a sugarcane biorefinery: Thermo-economical evaluation of CO2 recycle systems. Food Sci. Technol. 2018, 38, 13–18. [Google Scholar] [CrossRef]
- Schievano, A.; Adani, F.; Buessing, L.; Botto, A.; Casoliba, E.N.; Rossoni, M.; Goldfarb, J.L. An integrated biorefinery concept for olive mill waste management: Supercritical CO2 extraction and energy recovery. Green Chem. 2015, 17, 2874–2887. [Google Scholar] [CrossRef]
- Boulding, K. The economy of the coming spaceship earth. In Environmental Quality in a Growing Economy; Johns Hopkins University: Baltimore, MD, USA, 1996. [Google Scholar]
- Daly, H. Beyond growth: The economics of sustainable development. In Contemporary Sociology; American Sociological Association: Paris, France, 1973. [Google Scholar]
- Domenech, T.; Bleischwitz, R.; Doranova, A.; Panayotopoulos, D.; Roman, L. Mapping Industrial Symbiosis Development in Europe_typologies of networks, characteristics, performance and contribution to the Circular Economy. Resour. Conserv. Recycl. 2019, 141, 76–98. [Google Scholar] [CrossRef]
- McDonough, W.; Braungart, M. Remaking the Way we Make Things: Cradle to Cradle; North Point Press: New York, NY, USA, 2002. [Google Scholar]
- Charonis, G.-K. Degrowth, steady state economics and the circular economy: Three distinct yet increasingly converging alternative discourses to economic growth for achieving environmental sustainability and social equity. In Proceedings of the World Economic Association Sustainability Conference, Bristol, UK, 24 September–21 October 2012; Available online: http://www.rrojasdatabank.info/Charonis1.pdf (accessed on 11 October 2020).
- Tcvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. The changing role of CO2 in the transition to a circular economy: Review of carbon sequestration projects. Sustainability 2019, 11, 5834. [Google Scholar] [CrossRef]
- Eren, H.A.; Avinc, O.; Eren, S. Supercritical carbon dioxide for textile applications and recent developments. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 6–10. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, C.L.; Nagarajan, D.; Chang, J.S.; Hu, J.; Lee, D.J. Carbon capture and utilization of fermentation CO2: Integrated ethanol fermentation and succinic acid production as an efficient platform. Appl. Energy 2017, 206, 364–371. [Google Scholar] [CrossRef]
- Liu, X.; Elgowainy, A.; Wang, M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green Chem. 2020, 22, 5751–5761. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Gong, J. Alternative Strategies Toward Sustainable Ammonia Synthesis. Trans. Tianjin Univ. 2020, 26, 67–91. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Pacheco, R.; Silva, C. Global warming potential of biomass-to-ethanol: Review and sensitivity analysis through a case study. Energies 2019, 12, 2535. [Google Scholar] [CrossRef]
- Ping, L.; Zhao, G.; Lin, X.; Gu, Y.; Liu, W.; Cao, H.; Huang, J.; Xu, J. Feasibility and carbon footprint analysis of lime-dried sludge for cement production. Sustainability 2020, 12, 2500. [Google Scholar] [CrossRef]
- IEA Global Energy & CO2 Status Report 2019. Available online: https://www.iea.org/reports/global-energy-co2-status-report-2019 (accessed on 30 November 2020).
- Ferronato, N.; Rada, E.C.; Gorritty Portillo, M.A.; Cioca, L.I.; Ragazzi, M.; Torretta, V. Introduction of the circular economy within developing regions: A comparative analysis of advantages and opportunities for waste valorization. J. Environ. Manag. 2019, 230, 366–378. [Google Scholar] [CrossRef]
- Ngan, S.L.; How, B.S.; Teng, S.Y.; Promentilla, M.A.B.; Yatim, P.; Er, A.C.; Lam, H.L. Prioritization of sustainability indicators for promoting the circular economy: The case of developing countries. Renew. Sustain. Energy Rev. 2019, 111, 314–331. [Google Scholar] [CrossRef]
- Moreau, V.; Sahakian, M.; van Griethuysen, P.; Vuille, F. Coming Full Circle: Why Social and Institutional Dimensions Matter for the Circular Economy. J. Ind. Ecol. 2017, 21, 497–506. [Google Scholar] [CrossRef]
- Dani, S.; Wibawa, A. Challenges and policy for biomass energy in Indonesia. Int. J. Business Econ. Law 2018, 15, 41–47. [Google Scholar]
- Singh, R.; Setiawan, A.D. Biomass energy policies and strategies: Harvesting potential in India and Indonesia. Renew. Sustain. Energy Rev. 2013, 22, 332–345. [Google Scholar] [CrossRef]
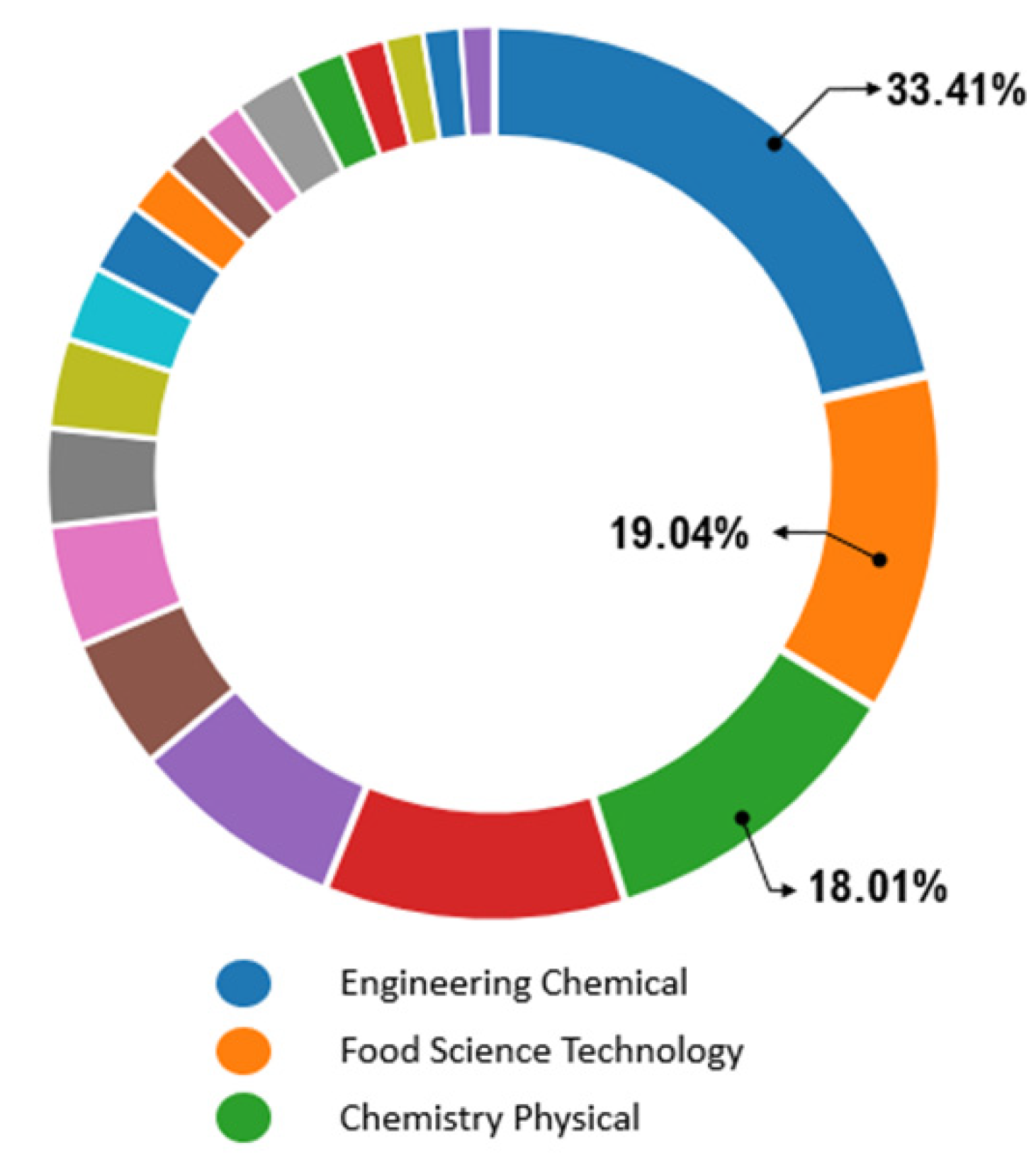

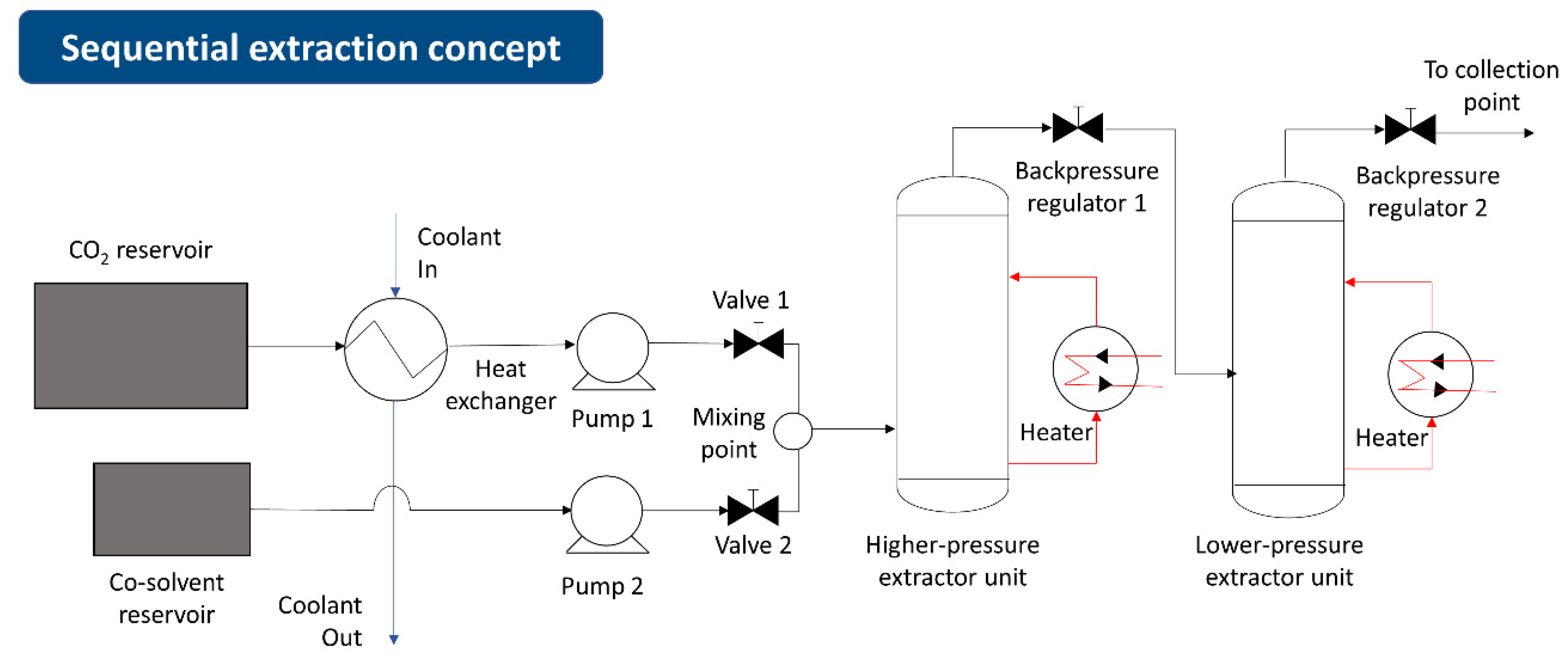

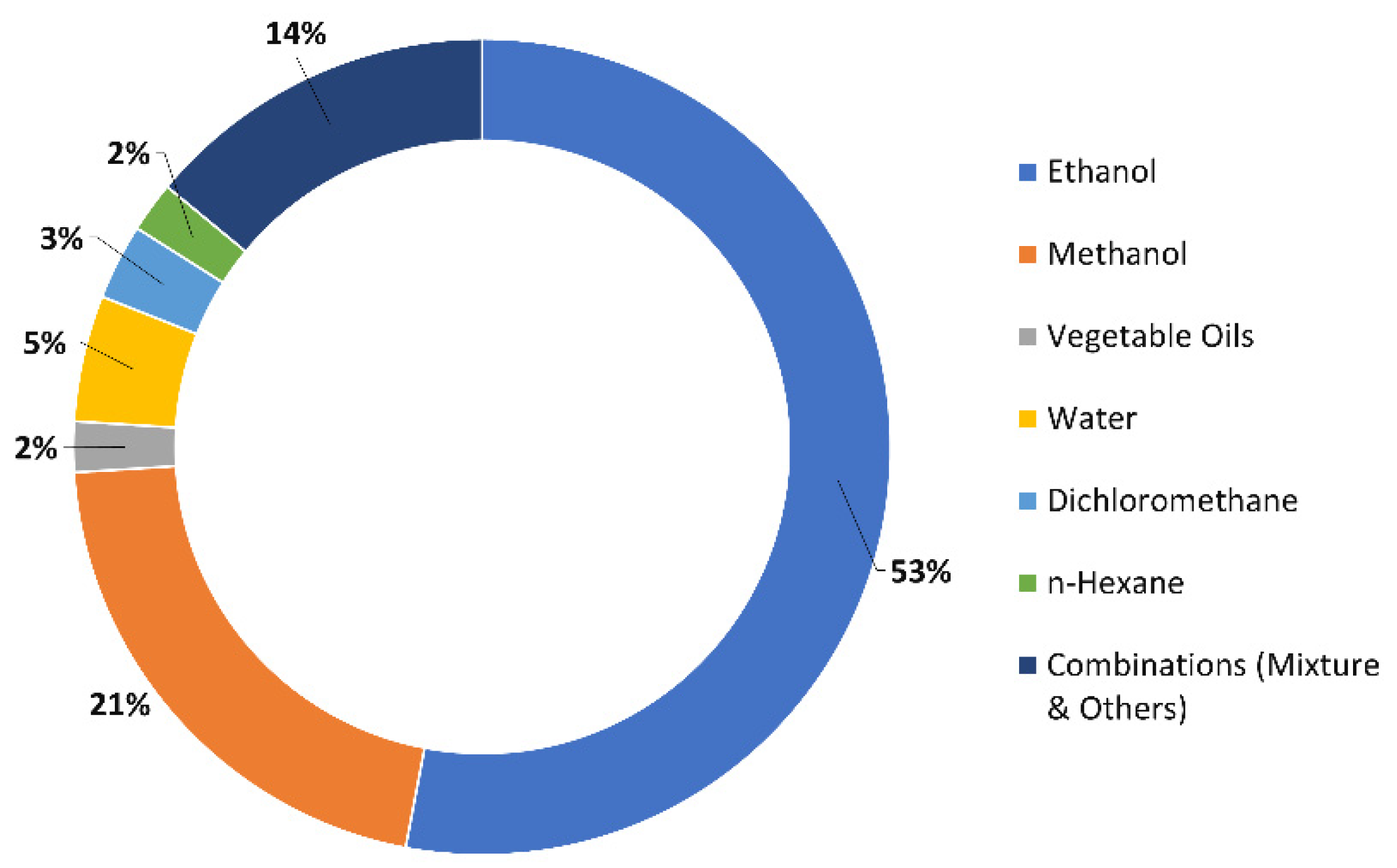
| Method | Solvent | Temperature | Pressure | Time | Volume of Organic Solvent Consumed | Polarity of Natural Products Extracted |
|---|---|---|---|---|---|---|
| Maceration | Water, and aqueous and nonaqueous solvents | Room temperature | Atmospheric | Long | Large | Dependent on extracting solvent |
| Soxhlet extraction | Organic solvents | Under heat | Atmospheric | Long | Moderate | Dependent on extracting solvent |
| Hydro-distillation and steam distillation | Water | Under heat | Atmospheric | Long | None | Essential oil (usually nonpolar) |
| Percolation | Water, and aqueous and nonaqueous solvents | Room temperature, occasionally under heat | Atmospheric | Long | Large | Dependent on extracting solvent |
| Reflux extraction | Aqueous and nonaqueous solvents | Under heat | Atmospheric | Moderate | Moderate | Dependent on extracting solvent |
| Raw Material | Phenolic Compound(s) | Potential Application/Industry | Extraction Conditions | Type of SFE Technology | Reference |
|---|---|---|---|---|---|
| Sour orange peel | Osthol | Medicinal application | Temperature: 90 °C Pressure: 17 MPa Flowrate: 2.7 kg/h Time: 120 min | Sequential extraction | [76] |
| Pomegranate seed | Ferulic acid, trans-cinnamic acid, vanillic acid, naringenin | Antioxidant agent, perfume industry | Temperature: 40–60 °C Pressure: 20–30 MPa Flowrate: 2 mL/min Co-solvent: Ethanol, methanol, iso-propanol | Single extraction | [77] |
| Mango | Mangiferin, quercetin, gallic acid, gallotannins, iriflophenones | Antioxidant agent, pharmaceutical application, cosmetic application | Temperature: 55 °C Pressure: 10 MPa Flowrate: 40–60 g/min Co-solvent: Ethanol (20%) Time: 20–360 min | Single and sequential extraction | [78] |
| Passion fruit seeds | Resveratrol, piceatannol, scirpusin B | Antioxidant agent, medical applications, pharmaceutical application | Temperature: 40–50 °C Pressure: 15–30 MPa Flowrate: 0.5 kg CO2/hour Time: 240–270 min | Single extraction | [79] |
| Mangosteen pericarp | Protocatechuic acid, p-hydroxybenzoic acid, | Pharmaceutical application, antioxidant agent | Temperature: 60 °C Pressure: 5–30 MPa Flowrate: 2 mL/min Time: 420 min | Single extraction, co-integrated extraction | [80] |
| Raw Material | Biorepellent Compound(s) | Potential Application | |||
|---|---|---|---|---|---|
| Allspice | Eugenol, myrcene, caryophyllene, methyl eugenol | Mosquito repellent, insect repellent | |||
| Turmeric (rhizome) | ar-turmerone | Insect-control application, larvicidal application, mosquito repellent | Temperature: 40–45 °C Pressure: 25–30 MPa Flowrate 6.54–14.65 kg/min | Single extraction | [88] |
| Temperature: 40–60 °C Pressure: 10–35 MPa Flowrate 8.6 g/min STF: 12.1 | Single extraction, sequential extraction | [89] | |||
| Citronella grass | Citronellal, citronellol, geraniol, eugenol, β-elemene | Insect-control application, mosquito repellent | Temperature: 40–80 °C Pressure: 6.2–18 MPa | Single extraction | [86] |
| Pipilongo seeds | Piperine | Bacterial growth inhibitor, fly repellent | Temperature: 40 °C Pressure: 13.8–17.2 MPa Flowrate: 5 mL/min Time: 2 h | Sequential extraction | [87] |
| Raw Material | Biopesticidal Compound(s) | Potential Application | Extraction Conditions | Type of SFE Technology | Reference |
|---|---|---|---|---|---|
| Neem seeds | Nimbin, nimbinin, nimbidin, nimbidiol | Antimicrobial properties, antifungal properties, antiviral properties | Temperature: 35–60 °C Pressure: 10–26 MPa Flowrate: 0.24–1.24 mL/min Loading: 1.0–2.5 g | Single extraction | [100] |
| Temperature: 35–55 °C Pressure: 10–26 MPa Flowrate: 0.62–1.24 mL/min Loading: 1.0–2.5 g | Single extraction | [101] | |||
| Derris elliptica (roots) | Rotenoids | Insecticidal properties | Temperature: 40 °C Pressure: 10–20 MPa Flowrate: 0.8–1.2 kg/h Time: 370–800 min | Sequential extraction | [102] |
| Pyrethrum flowers | Pyrethrin, cinerin | Insecticidal properties, mosquito repellent | Temperature: 40 °C Pressure: 40 MPa Flowrate: 0.019 kg/min Time: 210 min | Single extraction | [103] |
| Furcrae leaves | Saponin | Pesticidal properties | Temperature: 45 °C Pressure: 21.6 MPa Flowrate: 0.23 kg/h Time: 210 min | Single extraction | [99] |
| Country | Strategy/Approach/ Policy | Description | Reference |
|---|---|---|---|
| Malaysia | Valorization of organic waste and biomass |
| [140] |
| Indonesia | National Energy Policy (2006) Ministerial regulation No. 27/2014 |
| [142] |
| India | National Policy on Biofuels (2008) Renewable Power Policies Program-Wise |
| [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, Y.H.; Yusup, S.; Kadir, W.N.A.; Wong, C.Y.; Rosli, S.S.; Ruslan, M.S.H.; Chin, B.L.F.; Yiin, C.L. Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology. Sustainability 2021, 13, 233. https://doi.org/10.3390/su13010233
Chai YH, Yusup S, Kadir WNA, Wong CY, Rosli SS, Ruslan MSH, Chin BLF, Yiin CL. Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology. Sustainability. 2021; 13(1):233. https://doi.org/10.3390/su13010233
Chicago/Turabian StyleChai, Yee Ho, Suzana Yusup, Wan Nadiah Amalina Kadir, Chung Yiin Wong, Siti Suhailah Rosli, Muhammad Syafiq Hazwan Ruslan, Bridgid Lai Fui Chin, and Chung Loong Yiin. 2021. "Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology" Sustainability 13, no. 1: 233. https://doi.org/10.3390/su13010233
APA StyleChai, Y. H., Yusup, S., Kadir, W. N. A., Wong, C. Y., Rosli, S. S., Ruslan, M. S. H., Chin, B. L. F., & Yiin, C. L. (2021). Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology. Sustainability, 13(1), 233. https://doi.org/10.3390/su13010233








